Abstract
The major neural stem cell population in the developing cerebral cortex is composed of the radial glial cells, which generate glial cells and neurons. The mechanisms that modulate the maintenance of the radial glia (RG) stem cell phenotype, or its differentiation, are not yet completely understood. We previously demonstrated that the transforming growth factor-β1 (TGF-β1) promotes RG differentiation into astrocytes in vitro (Glia 2007; 55:1023-33) through activation of multiple canonical and non-canonical signaling pathways (Dev Neurosci 2012; 34:68-81). However, it remains unknown if TGF-β1 acts in RG-astrocyte differentiation in vivo. Here, we addressed the astrogliogenesis induced by TGF-β1 by using the intraventricular in utero injection in vivo approach. We show that injection of TGF-β1 in the lateral ventricles of E14,5 mice embryos resulted in RG fibers disorganization and premature gliogenesis, evidenced by appearance of GFAP positive cells in the cortical wall. These events were followed by decreased numbers of neurons in the cortical plate (CP). Together, we also described that TGF-β1 actions are region-dependent, once RG cells from dorsal region of the cerebral cortex demonstrated to be more responsive to this cytokine compared with RG from lateral cortex either in vitro as well as in vivo. Our work demonstrated that TGF-β1 is a critical cytokine that regulates RG fate decision and differentiation into astrocytes in vitro and in vivo. We also suggest that RG cells are heterogeneous population that acts as distinct targets of TGF-β1 during cerebral cortex development.
Keywords: radial glia, TGF-β, gliogenesis, neurogenesis, cerebral cortex
Introduction
Radial glia (RG) cells are considered the major progenitor cell population present in the developing cerebral cortex (Kriegstein and Alvarez-Buylla, 2009).These cells have a long radial fiber that elongate from its cell body, in the ventricular zone (VZ), through the entire developing cortical wall. During the initial steps of brain development, RG cells, which are derived from the neuroepithelium, are actively proliferative cells and, by asymmetric divisions, originate neurons that migrate along their radial fibers to their specific layers at the cortical plate (CP). By the end of the neuronal migratory period, RG cells arrest their cycle and differentiate into cortical astrocytes (Munoz-Garcia and Ludwin, 1986; Voigt, 1989; Culican et al., 1990; Bentivoglio and Mazzarello, 1999; Miyata et al., 2001; Noctor et al., 2001, 2002; Götz et al., 2002; Malatesta et al., 2003; Anthony et al., 2004).
Although characteristics of RG cells such as, self-renewal and progenitor capacity, have been assured, it is widely discussed if these features can be attributed to all RG cells of the embryonic brain, or if it is restricted to specific populations of these cells (Pinto and Götz, 2007). Heterogeneity in RG cells has been described along the telencephalon regions, revealed by distinct expression of the transcription factors Pax6, Emx2 and FoxG1, which confers to these cells their neurogenic or gliogenicprogenitor property (Kriegstein and Götz, 2003; Hevner et al., 2006; Pinto and Götz, 2007). These transcription factors have been reported to be under the control of a combination of morphogen gradients along the developing axes, which determines specific telencephalon region territories (O’Leary and Sahara, 2008).
RG-astrocyte differentiation is a well-recognized event, however the mechanisms and molecules that control generation of different pools of astrocytes and neurons are still elusive. Several lines of evidence suggest that increasing neuronal pools play essential role in the control of RG maintenance and/or differentiation (Hunter and Hatten, 1995; Anton et al., 1997; Nakashima et al., 1999; Mi et al., 2001; Takizawa et al., 2001; Uemura et al., 2002; Patten et al., 2003; Schmid et al., 2003; Nishino et al., 2004; Barnabé-Heider et al., 2005; He et al., 2005; Stipursky and Gomes, 2007; Stipursky et al., 2012a). Although several soluble factors were demonstrated to control astrocytogenesis during CNS development such as leukemia inhibitor factors (LIFs) of the interleukin-6 (IL-6) family, including CNTF, LIF, and Cardiotrophin-1 (CT-1) (for revision see Stipursky et al., 2009), the role of neuronal derived soluble factors on RG-astrocyte transformation is still poorly known.
We previously reported that cerebral cortex neurons induce RG-astrocyte differentiation in vitro through secretion of the transforming growth factor-β1 (TGF-β1; Stipursky and Gomes, 2007; Stipursky et al., 2012a).
TGF-β1 is a multifunctional cytokine, present virtually in all tissues, that controls multiple biological and pathological events such as embryogenesis, immune response, extracellular matrix protein (ECM) production, cell differentiation and cell-cycle control in different tissues (Massagué, 1998; Massagué and Gomis, 2006). In the CNS, TGF-β1 has been reported to play key function in neuronal generation, survival and migration (Brionne et al., 2003; Miller, 2003; Espósito et al., 2005), glial differentiation(Sousa Vde et al., 2004; Romão et al., 2008), and synapse formation (Diniz et al., 2012, 2014).
TGF-β1 signaling might be mediated by the canonical pathway that involves SMADs2/3 and SMAD4 transcription factors or non-canonical signaling pathways, that involve the RasGTPAses, mitogen-activated protein kinase (MAPK), or phosphatidylinositol-3 kinase (PI3K) proteins (Javelaud and Mauviel, 2005; Massagué and Gomis, 2006). We previously reported that TGF-β1 controls RG differentiation into neurons and astrocytes by activation of SMADs/PI3K and MAPK, respectively, in distinct RG subpopulations in vitro (Stipursky et al., 2012a).
Although the presence of different isoforms of TGF-β molecules have already been described in the proliferative zones of the embryonic cerebral cortex (Mecha et al., 2008), there are few data regarding the expression, modulation and distribution of TGF-β receptors in RG cells in vivo. Further, the mechanisms that modulate neurogenesis to gliogenesis switch of RG induced by TGF-β1 are still unknown.
Here, we investigated the role of TGF-β1 on RG-astrocyte switch in the developing cerebral cortex and the implications of RG heterogeneity to this event. We showed that TGF-β1 induces premature gliogenesis and disrupts RG polarity mainly in the dorsomedial area of the cerebral cortex. For the first time, we provide evidence that specific RG subpopulations distinctly respond to TGF-β1 in vivo.
Methods
Ethical approval
All animal protocols were approved by the Animal Research Committee of the Federal University of Rio de Janeiro (DAHEICB024).
RG cell cultures
Gestational day 14 Swiss mice embryos were collected and dissected for cerebral cortex separation. After dissection tissues were dissociated in DMEM/F12 (Invitrogen) medium and after cell counting, 105 cells were plated in 25 cm2 culture bottles in neurosphere growing media DMEM/F12 containing 1% glutamine, 0.1% de penicillin/streptomycin, 2% B27 (Invitrogen), 20 ng/mL EGF (Epidermal growth factor, Invitrogen) and 20 ng/mL FGFb (basic Fibroblast growth factor, R&D Systems), for 6 days, in vitro. The 2/3 of the media was changed every 2 days. After this period, neurospheres were enzymatically dissociated in 0.05% Trypsin/EDTA (Invitrogen), and 105 RG isolated cells were plated in glass coverslips previously coated with 50 μg/mL with poli-L-lisin (Invitrogen) and 10 μg/mL laminin (Invitrogen) in 24 wells culture plates. Cells were kept in DMEM/F12 containing 1% glutamine, 0.1% penicillin/streptomycin, 2% de B27 (Invitrogen), 20 ng/mL EGF (Invitrogen) and 20 ng/mL FGFb (R&D Systems) for 24 h. After this period, cells were treated with 10 ng/mL of TGF-β1 (R&D Systems) or 10 μm of SB431542 (Sigma Aldrich) in medium, without mitogenic factors, for 24 h.
In utero intraventricular injection
In utero intraventricular injections of E14 mice embryos were performed as described by Walantus et al. (2007). Pregnant Swiss mice in the 14 gestational day were anesthetized with intraperitoneal injection of 2-2-2 Tribromoethanol (Sigma Aldrich) 1 mg/g of body weight. After anesthesia, females were subjected to surgical procedure, in which the uterus was exposed. After visualization of the embryos, they were manually positioned to allow observation of brain hemispheres. Each embryo was subjected to intraventricular injection inside the lateral ventricles of 2 μl of control solution (PBS, 0.05% BSA, 0.025% Fast Green [Sigma Aldrich]), or solution containing 100 ng of TGF-β1 (R&D Systems) or 10 μM of SB431542 (Sigma Aldrich), using glass micropipettes. After injections, the uterus was repositioned inside abdominal cavity and abdominal muscle and skin layers sutured. Bromodeoxiridine (BrdU, Sigma Aldrich) was intraperitoneally injected in the preagnant mouse after 2 and 24 h of surgery, to follow cells generated from RG just after TGF-β1 stimulation and to analyze long lasting effects in RG population. Forty-eight hours after surgery, the female was sacrificed and embryos were perfused with ice cold 4% paraformaldehyde (PFA). Brains were collected and processed for immunohistochemistry and real time RT-PCR.
Immunocytochemistry and immunohistochemistry
After culture, cells were fixed in 4% PFA (Vetec) for 15 min. After this period, cells were extensively washed in PBS (phosphate buffered saline) and permeabilized with 0.2% Triton X-100 (Vetec) for 5 min at room temperature. Cells were then incubated with blocking buffer containing 3% serum bovine albumin (BSA), 5% normal goat serum (NGS) (Sigma Aldrich) diluted in PBS for 1 h, followed by 12 h incubation with primary antibodies at 4°C diluted in the same solution. After this period, cells were extensively washed in PBS and incubated with secondary antibodies for 2 h at room temperature. Nuclei were labeled with DAPI (4’, 6-Diamidino-2-phenylindole; Sigma Aldrich), or Draq5 (Pierce). Glass coverslips were mounted in glass slides with Faramount mounting media (DakoCytomation), and stained cells were visualized using a fluorescent optical microscope Nikon TE3000. For immunohistochemistry, brain were fixed in 4% PFA for 48 h, and subjected to vibratome sectioning, to obtain 40 μm sections. After sectioning, floating brain slices were incubated with blocking buffer for 1 h under shaking. After incubation with primary antibodies for 12 h at 4°C, followed by extensive washing in PBS, slices were incubated with secondary antibodies for 2 h at room temperature under shaking. Primary antibodies were: mouse anti-Nestin (Chemicon, 1:100), rabbit anti-BLBP (Chemicon, 1:200), rabbit anti-ErbB2 (Santa Cruz Biotechnology, 1:200), rabbit anti-Notch1 (Cell Signaling, 1:500), rabbi anti-TGFRII (Santa Cruz Biotechnology, 1:100), rabbit anti- phophoSmad 2/3 (Santa Cruz Biotechnology, 1:50), rabbit anti-Laminin (Sigma Aldrich, 1:100), rabbit anti-GFAP (Dakocytomation, 1:500), mouse anti-β TubulinIII (Promega, 1:1,000), rabbit anti-Doublecortin (Abcam, 1:200), rati anti-BrdU (Accurate, 1:1,000), rabbit anti-Foxg1 (Santa Cruz Biotechnology, 1:200). Secondary antibodies were conjugated to AlexaFluor 488, AlexaFluor 546, and AlexaFluor 633 (Invitrogen Molecular Probes). Nissl Trace Green (Molecular Probes, 1:1,000) staining was used to label neuronal cell soma. Images of labeled tissue were obtained using a Leica SP5confocal microscope.
Western blot
Protein levels were analyzed as previously described (Dezonne et al., 2013). After dissection, cerebral cortex tissues from Swiss mice embryos were lysed in RIPA buffer [20 mMTris-HCl (pH 7.5); 150 mMNaCl; 1 mM Na/EDTA; 1 mM EGTA; 1% NP-40; 1% sodium deoxycholate; 2.5 mM sodium pyrophosphate; 1 mMglycerophosphate; 1 mM Na3VO4; 1 μg/mLleupeptin]. Cell suspension was homogenized, sonicated, and centrifuged for 10 min at 14,000 rpm in a refrigerated centrifuge. Subsequently, the supernatant was collected and the protein dosage was performed using the BCA Protein Assay kit (Pierce, Rockford, Ill., USA). A total of 20 μg of protein was loaded per lane and submitted to electrophoretic separation in a 10% SDS-PAGE gel. After separation, proteins were electrically transferred onto a nitrocellulose transfer membrane (Protran, Dassel, Germany) for 1 h. The membrane was blocked in 5% BSA in Tris-buffered Tween 20 (TBS-T; Merck, Darmstadt, Germany) and primary antibody incubation was performed overnight at 4°C followed by peroxidase-conjugated secondary antibody incubation for 1 h at room temperature. Proteins were visualized using the enhancing chemiluminescence detection system (SuperSignal West Pico Chemiluminescent Substrate; Thermo Scientific, Rockford, Ill., USA) and nitrocellulose membranes were exposed to autoradiographic films (Kodak, São José dos Campos, Brazil). Primary antibodies were: mouse phosphoSmad2 (Cell Signaling; 1:1,000), rabbit anti-ErbB2 (Santa Cruz Biotechnology 1:200); rabbit anti-TGFRII (Santa Cruz Biotechnology; 1:200); mouse anti-α-tubulin (Sigma Aldrich; 1: 5,000). The secondary peroxidase-conjugated antibodies were: goat anti-rabbit IgG and goat anti-mouse IgG (Amersham Biosciences, Piscataway, N.J., USA; 1: 3,000). After protein detection, densitometric analysis of autoradiographic films was done using Image J 1.48 software. Each experiment was done in triplicate, and proteins were loaded in triplicate in SDS-PAGE gel.
Real time RT-PCR
Total RNA was isolated from embryonic mice cerebral cortex using Direct-zol™ RNA MiniPrep (ZymoReserch, USA) according to the protocol provided by the manufacturer, and quantified using NanoDrop ND-1000 Spectrophotometer ThermoFisherScientific, USA).Two micrograms of total RNA were reverse transcribed with RevertAid first Strand cDNA Synthesis Kit according to the manufacturer (Thermo Fisher Scientific, USA). Sense and antisense specific for FoxG1, and β-actin genes were used. β actin sense: TGG ATC GGT TCC ATC CTG G, anti-sense: GCA GCT CAG TAA CAG TCC GCC TAG A; FoxG1 sense: CGA CAA GAA GAA CGG CAA GTA CGA, anti-sense: AGC ACT TGT TGA GGG ACA GGT TGT. Sequences were verified to be specific using Gen Bank’s BLAST (Altschul et al., 1997). Quantitative real-time RT-PCR was performed using Maxima SYBR green qPCR Master Mix (Thermo Scientific, USA). Reactions were per formed on ABI PRISM 7500 Real Time PCR System (Applied Biosystems). The relative expression levels of genes were calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001). The amount of target genes expressed in a sample was normalized to the average of thee ndogenous control.
Statistical analysis
Statistical analyses were done using one-way non-parametric ANOVA coupled with Tukey post-test by GraphPad Prism 4.0 software, and P < 0.05 was considered statistically significant. The experiments were performed in triplicate, and each result represents the mean of at least 4–6 animals analyzed.
Results
RG cells are potential targets of TGF-β1 in vivo
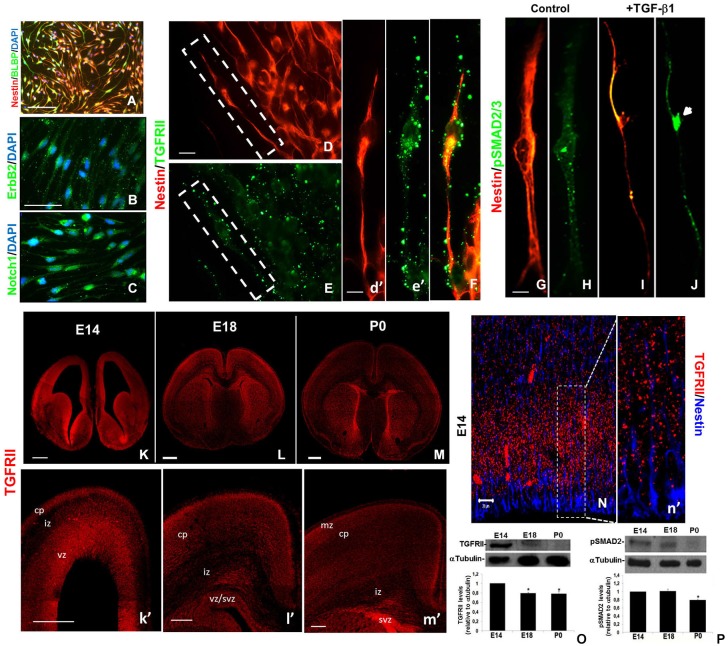
In order to investigate RG cells responsiveness to TGF-β1, we first identified the TGF-β receptor type II (TGFRII) in RG cells in vitro and in vivo. To do that, we performed RG isolation from neurospheres derived E14 mice embryos cerebral cortex. Under this culture condition, these cells present a typical radial morphology and label for Nestin, BLPB, Notch1 and ErbB2 (Figures 1A–C), attesting their RG cells phenotype. We also detected high staining for TGFRII in their membranes (Figures 1D–F). Treatment of RG culture with TGF-β1 induced phosphorylation and nuclear translocation of Smads2/3, a hallmark of TGF-β1 signaling pathway activation (Figures 1G–J).
Figure 1.
RG cells express the TGF-β1 signaling pathway members. RG enriched cell cultures were isolated from cerebral cortex, grown into neurospheres and analyzed for specific cellular markers. Under these conditions the cells exhibit the typical RG cell elongated morphology and staining for specific markers: nestin/BLBP (A); ErbB2 (B); and Notch1 (C). RG also expresses TGFRII in their membranes (D–F). Treatment of these cells with 10 ng/mL of TGF-β1 induces phosphorylation and nuclear translocation of Smad2/3 (G–J). Note that TGFRII is expressed predominantly in VZ/CP (E14),VZ/SVZ/CP (E18), and SVZ/CP (P0) in mice (K–M, k’–m’). TGFRII is distributed as a punctate pattern all over nestin+ RG cell bodies and fibers (N, n’). Western blotting assays revealed that either TGFRII (O) as phosphorylated Smad2 (P) is down regulated during cerebral cortex development. vz:ventricular zone, svz: subventricular zone, cp:cortical plate. *P < 0.05. Scales: 50 μm (A), 20 μm (B), 10 μm (D, I) 500 μm (K–M); 200 μm (k’–m”) 20 μm (N).
Immunohistochemical assays of the mouse brain revealed that TGFRII is more robustly expressed in the VZ (ventricular zone) and CP (cortical plate) of E14 and in the same layers as well as in SVZ (subventricular zone) of E18 and P0 mice cerebral cortex (Figures 1K–M,k′–m′). We identified a punctate TGFRII staining in RG cell body and processes in the E14 telencephalon (Figures 1N,n′). Western blotting analysis revealed that TGFRII is negatively modulated during development, since this protein is present at high levels in E14 telencephalon, is slightly detectable in E18 and tend to disappear in P0 (Figure 1O). The down regulation of TGFRII overlaps with the amount of phospho Smad2 at P0 (Figure 1P). Together, this data suggest that RG cells might be target of TGF-β1 actions in vitro, as well as in vivo.
Intraventricular injection of TGF-β1 disrupts polarity of RG cells
RG cell elongated morphology is a critic characteristic that allows neuronal migration and correct positioning in the CP within the different layers of the cerebral cortex (Rakic, 1971, 1995; Hatten, 1999; Yokota et al., 2007; Radakovits et al., 2009). Loss of this typical morphology is a hallmark of RG-astrocyte differentiation.
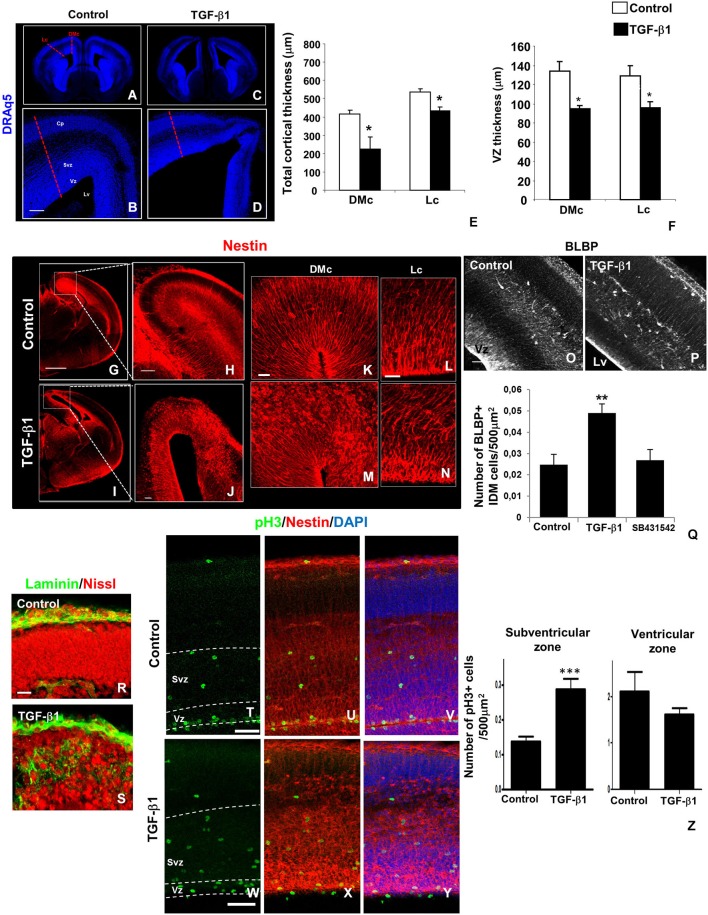
Intraventricular injection of TGF-β1 resulted in profound morphological alterations especially in the telencephalon, resulting in dilated lateral ventricles, and evident reduction of cortical wall thickness in dorsomedial (DMc) and lateral (Lc) areas of the cortex (Figures 2A–E).We also observed reduced VZ thickness in TGF-β-injected brains compared with vehicle solution injected brains (Figure 2F).These thickness reduction is observed along rostral to caudal regions of the cerebral cortex (data not shown).Interestingly, these morphological alterations were accompanied by severe disorganization of nestin labeled-RG fibers in TGF-β1-injected brains (Figures 2G,J). This disorganization characterized loss of polarity of the radial processes and was more prominently observed in DMc rather than in Lc areas of the cortex (Figures 2K–N).
Figure 2.
TGF-β1 injection affects cerebral cortex and RG morphology. In utero intraventricular injection of TGF-β1 in mouse embryos (injection at E14 and analysis at E16) promoted several morphological alterations in the cerebral cortex wall in lateral (Lc) and dorsomedial (DMc) cortex areas (A–D). TGF-β1 reduces the thickness of total cortical area (E) and VZ (F) in both regions. Note that TGF-β1 also disrupted nestin+ (red) radial fiber networks (G–J), an event more prominent at DMc than in Lc (K–N). RG loss of polarity induced by TGF-β1 is accompanied by increase in the numbers of BLBP+ cells (white) with intermediate differentiated morphology (IDM) across cortical wall (O–Q). SB431542 injection does not affect BLBP+ IDM cells numbers. These morphological alterations were followed by basal membrane laminin (green) and neuronal cell bodies (Nissl) ectopic distribution (R,S). TGF-β1 also disorganized pH3+ cells (green) distribution across cortical wall, especially VZ pH3+ cells’ nucleus alignment (T–Y), without affects its numbers (Z), however increased the numbers of pH3+ cells in the SVZ (Z). *P < 0.05, **P < 0,005, ***P < 0005. Scales: 500 μm (G), 100 μm (B,O), 50 μm (H,J,T,W), 20 μm (K,L,R). Cp: cortical plate, Vz: ventricular zone, Svz: subventricular zone, Cx: cortex, Lv: lateral ventricle.
In addition to RG fibers displacement, TGF-β1 also promoted an increment in approximately 98% on BLBP-labeled cells witha morphology similar to glial progenitors, in the midway of their differentiation path, which we called RG intermediate differentiation morphology (IDM; Figures 2O–Q). Injection with pharmacological inhibitor of TGF-β1 signaling pathway SB431542 did not affect BLBP+ IDM cells generation (Figure 2Q). We also observed that TGF-β1 caused ectopic laminin distribution in the pial region of the cortical wall (Figures 2R,S).These phenotypes were also associated with increasing numbers of pH3+ cells in SVZ, but not in VZ (Figures 2T–Z). In addition RG fibers disorganization were also followed by displacement of pH3+ cells at VZ, leading to ectopic positioning of these proliferative cell’s nucleus (Figures 2T,W).
These data shows that TGF-β1 regulates cerebral cortex thickness, RG morphology and polarity and progenitor positioning, and suggest that these events might be associated to regulation of basal lamina structure, an issue clearly related to RG cell polarity.
TGF-β1 promotes premature gliogenesis in dorsomedial (DMc) area of the cerebral cortex
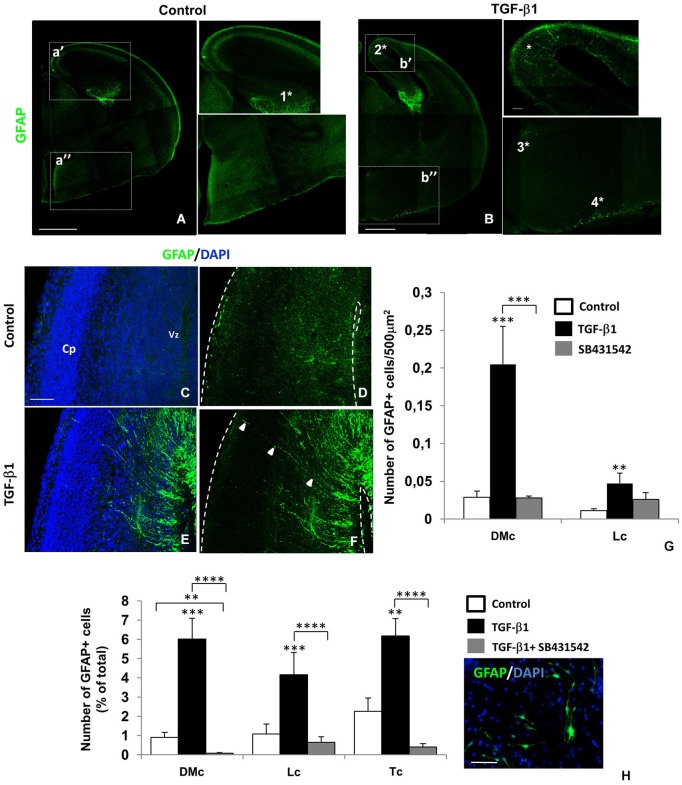
We previously demonstrated that TGF-β1 controls RG differentiation into astrocytes and neurons by distinct signaling pathways in vitro (Stipursky et al., 2012a). In order to assess the fate of RG under the influence of TGF-β1 in vivo, we took the advantage of in utero intraventricular injection technique. Injection of TGF-β1 inside the lateral ventricles of mouse embryos also caused robust premature astrocyte generation (Figure 3). In the telencephalon TGF-β1 injection caused appearance of GFAP+ cells in distinct regions compared with vehicle injected brains (Figures 3A,B), such as the cingulate cortex (2*) neuroepithelium related to the third ventricle associated with the ventral diencephalic sulcus (3*), and also at the pial region of the preoptic area (4*).In the evident hippocampal neuroepithelium there was no difference in GFAP labeling pattern in control and TGF-β1 injected brains (1*).
Figure 3.
TGF-β1 promotes premature gliogenesis in the cerebral cortex. Intraventricular injection of TGF-β1 in mouse embryos (injection at E14 and analysis at E16) caused premature appearance of GFAP+ cells (green) in different telencephalon regions: dorsomedial cortex/cingulate cortex (2*), neuroepithelium related to the third ventricule (3*) and pial surface of the preoptic area (4*). At the hippocampal formation (1*), GFAP labeling was not affected. TGF-β1 induced gliogenesis was more evident at the dorsomedial area of the cerebral cortex (DMc), than in lateral cortex (Lc) (C–G). Note the GFAP+ (green) radial fibers of differentiating cells (arrows, F). In radial glia (RG) isolated cultures, TGF-β1 also promoted appearance of GFAP+ cells in a greater extend in DMc than in Lc and total cortex (Tc) (H). ***P < 0.0005, *P < 0.005. Scales: 500 μm (A,B), 50 μm (C,H). Cp: cortical plate, Vz: ventricular zone.
Apart from other regions, we observed that in DMc area of the cerebral cortex (cingulate cortex) astrocytogenesis was more evident. The appearance of GFAP+ cells bearing a yet radial-like morphology in this area (Figures 3C–F) suggest that TGF-β1 induced RG cells to adopt an astrocyte phenotype.
Astrocyte differentiation was significantly increased by TGF-β1 in the DMc area in comparison to the lateral area of the cerebral cortex (15 X; Figure 3G). Injection of a pharmacological inhibitor of TGF-β receptor, SB431542, did not affect the gliogenesis in this area (Figure 3G).
In order to confirm the specificity of TGF-β1 actions in different cortical areas, we generated cultures of isolated RG cells from DMc and Lc areas and from total cortex (Tc). We observed that DMc cells were more responsive to TGF-β1 astrocytogenic induction, than Lc cells. The number of GFAP+ cells increased by 5 times in DMc cells treated with TGF-β1 whereas only 3 times in Lc cells. For Tc cells, the increasing in GFAP+ cell numbers was compared to those found in DMc-treated condition (Figure 3H).
Thus, RG from different cerebral cortexareas respond to TGF-β1 by acquiring the astrocytic phenotype.
TGF-β1 affects neurogenesis and neuronal positioning in cortical plate
Neurogenesis and neuronal migration are events that occur during specific time window in the developing cerebral cortex; both events directly dependent of RG cell stem cell and scaffold properties, respectively (Rakic, 1971; Costa et al., 2010; Vogel et al., 2010; Sild and Ruthazer, 2011; Stipursky et al., 2012b). We previously described that as well as astrocytogenesis, neurogenesis can be controlled by TGF-β1 by activation of canonical and non-canonical signaling pathways, respectively (Stipursky et al., 2012a). Although neurogenesis was reported to involve TGF-β1 action in vitro (Vogel et al., 2010), it is not known if this factor controls RG neurogenic potential in vivo. In order to address this question, we have performed intraventricular injection of TGF-β1.
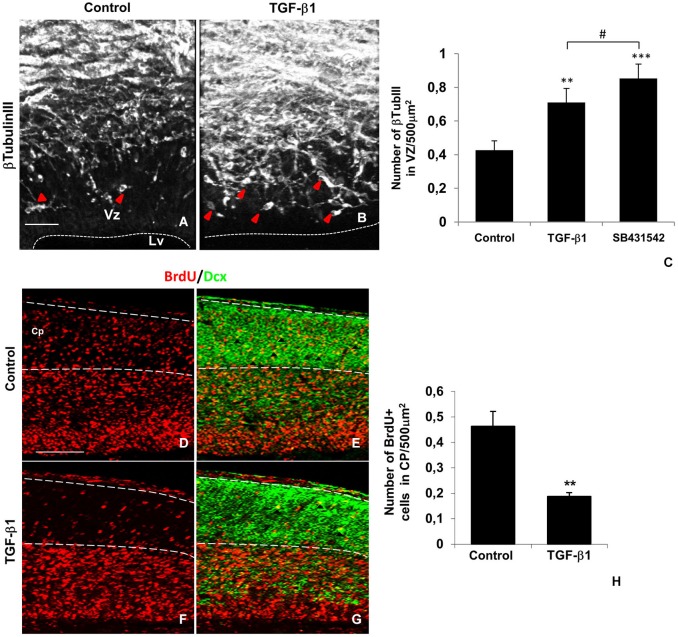
TGF-β1 also affected neuronal generation and placement in CP of the Lc. Interestingly, numerous βTubulinIII+ cells were present in the VZ of TGF-β1-injected brains, counting for an 66% increment (Figures 4A–C), thus suggesting enhanced neurogenesis in this RG cell bodies enriched layer. Pharmacological inhibition of TGF-β1 signaling pathway by SB431542 injection yielded a greater enhancement of βTubulinIII+ cells numbers in VZ, compared to control condition. In order to access if this increment was due to generation of new neurons, we have labeled the cells for BrdU and Doublecortin, which label recent generated neurons from RG cells that migrated through cortical wall and reached their final destination in the CP (Pramparo et al., 2010). We observed a 55% decrease in the number of BrdU+cells in the Lc CP of TGF-β1 injected brains (Figures 4D–H), thus demonstrating that both neuronal migration and positioning are modulated by TGF-β1 in vivo.
Figure 4.
TGF-β1 affects neurogenesis and neuronal positioning in the lateral cortex. TGF-β1 injections (injection at E14 and analysis at E16) increased neurogenesis at the lateral cortex Vz, as shown by the presence of βTubulinIII+ cells (white, arrows) in this layer (A–C). TGF-β1 decreased the number of BrdU+ cells (red) at the cortical plate (Cp) of the lateral cortex (D,F,H). Note that this layer is enriched in Doublecortin+ neurons (green) that were generated at Vz and migrated to the Cp (E,G). *P < 0.05, #P = 0.063. Scales: 50 μm (A,D). Vz: ventricularzone, Lv: lateral ventricle.
TGF-β1 controls the expression of FoxG1 in different cortical areas
Differences between the distinct regions of the brain are mainly generated during developmental controlled axis patterning-related morphogen distribution. Cerebral cortex arealizationor patterning is controlled by the expression of a great repertoire of transcription factors that define neural stem cells and progenitors generation, self-renewal and phenotypes. Those factors, such as FoxG1, are modulated by diverse morphogenetic proteins distinctly distributed in different patterning centers (Takahashi and Liu, 2006; O’Leary and Sahara, 2008).
Quantitative analyses by real time RT-PCR of DMc and Lc tissues revealed that TGF-β1 distinctly modulated the levels of FoxG1 mRNA transcription factors in these regions. Whereas TGF-β1 reduced the expression level of FoxG1 in DMc by 80%, it had no effect in Lc (Figure 5). These results suggest that TGF-β1 controls the expression of a transcription factor related to cortical arealization in vivo.
Figure 5.
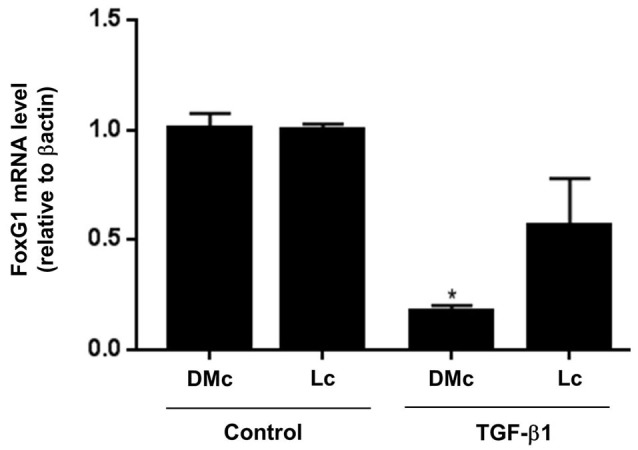
TGF-β1 controls FoxG1 expression in the cerebral cortex. Cerebral cortex tissue from TGF-β1 injected brains (injection at E14 and analysis at E16) were dissected to separate DMc and Lc areas and evaluated for FoxG1 mRNA expression by real time RT-PCR. TGF-β1 injections greatly decreased FoxG1 gene mRNA levels only in DMc area compared to control brains. *P < 0.01.
Discussion
In this study, we provide evidence for the role of TGF-β1 as a modulator of RG-astrocyte differentiation in vivo. Our data is pioneer in two aspects: (1) by demonstration of TGF-β1 action in radial-glial-astrocyte differentiation in vivo; (2) by showing distinct effects of TGF-β1 in different subpopulations of RG cells. First, we demonstrated that RG cells express the TGF-β receptor and activate the Smad pathway in response do TGF-β1. Then, we demonstrated that TGF-β1 disrupts RG cells polarized morphology and promotes premature astrocytogenesis and neuronal displacement in specific areas of the cerebral cortex. Our findings show that RG cells are potential targets for TGF-β signaling pathway and suggest that these effects are region dependent. Our data not only contribute to the understanding of the mechanism underlying fate decision and specific phenotype acquisition in the cerebral cortex, but support the hypothesis of the existence of distinct RG subpopulations with different potentials in the cerebral cortex.
RG cells as potential targets of TGF-β1 in vivo: impact on RG polarity and astrocytic differentiation
Evidence suggests that VZ cells are direct targets of different TGF-β family members (Miller, 2003; Mecha et al., 2008), however, the cellular pattern of expression of TGF-β1 signaling pathway members in the developing CNS has not been well characterized. Here, we have shown TGFRII expression in the developing telencephalon, specifically in the VZ/SVZ of the cerebral cortex. Additionally, we precisely identified its distribution in RG soma and fibers, an issue only previously suggested by other authors (Miller, 2003). Moreover, the levels of TGFRII and one of its downstream effectors, phosphorylated Smad2, seems to be negatively modulated through development. These results are corroborated by previous data that showed TGF-β1 and Smad2/3 proteins expression in different CNS regions including cerebral cortex VZ, neurons and progenitor layers in vivo (Miller, 2003; Sousa Vde et al., 2004; Mecha et al., 2008; Powrozek and Miller, 2009). In addition, our data is in accordance with previous reports that demonstrated that TGF-β signaling members are expressed in higher levels in early moments of the telencephalon development, and that are determining for the generation of different cell types of the CNS and other regions (Luukko et al., 2001).
RG cell polarity and radial processes extension are essential characteristics that are directly related to RG maintenance of its progenitor potential and scaffold property for neuronal migration (Rakic, 1971). RG differentiation into astrocytes involves disruption of its polarity and gradual acquisition of immature astrocyte morphology (Voigt, 1989; Hartfuss et al., 2001). Here, we have shown that TGF-β1 induces specific disorganization of nestin positive RG fibers and displacement of their cell nucleus labeled for pH3. Moreover, we observed the appearance of BLBP positive cells bearing an intermediate morphology between RG and astrocytes throughout the cortical wall.
Several mechanisms have been proposed to control RG cell polarity and correct positioning of migrating neurons such as modulation of cytoskeleton molecules (Yokota et al., 2007, 2009, 2010; Weimer et al., 2009) and ECM signal transduction (Haubst et al., 2006; Voss et al., 2008). Here we observed that disruption of RG polarity induced by TGF-β1 is followed by impaired organization of the basal membrane that covers pial surface of the telencephalon, where RG cells attach their pial process endfeet (Götz and Huttner, 2005). Laminin labeling revealed an ectopic distribution pattern of this protein in pial region of the cerebral cortex, associated with deficiencies in CP formation and displaced cell bodies. Our data is supported by previous results that TGF-β1 is a potent regulator of the synthesis of laminin, fibronectin, the adhesion protein nCAM and integrins (Brionne et al., 2003; Siegenthaler and Miller, 2004; Gomes et al., 2005). Further, similar phenotypes were found in mutant mice for C3G protein, a guanine nucleotide exchange factor for small GTPases of the Ras family, and also in laminin γ1III4 mutant (Haubst et al., 2006; Voss et al., 2008). In these mice, it is observed a robust loss of radial cell polarity, disruption of basal membrane and neuronal migration and CP deficits. Thus, although we cannot fully rule out additional mechanisms, our data strongly suggested an association between TGF-β1-control of laminin organization and maintenance of RG polarity.
TGF-β1 promotes premature gliogenesis in dorsomedial area of the cerebral cortex: implications for RG heterogeneity
In rodents, by the end of gestation, RG-astrocyte differentiation, is characterized within several molecular mechanisms by replacement of RG markers, such as BLBP and nestin, by astrocytic markers such as GFAP, the glutamate transporter GLAST and the calcium binding protein S100β (Dahl, 1981; Pixley and de Vellis, 1984).The correct timing of RG-astrocyte transformation is a crucial step to ensure correct number of neurons and cerebral cortex lamination. Here, we report that activation of TGF-β1 pathwayled to a premature appearance of GFAP+ cells in different regions of the embryonic telencephalon, mainly, in the cingulate cortex, neuroepithelium related to the third ventricle, and also at the pial region of the preoptic area. Although it has been reported the expression of TGF-β isoforms and also its different roles in these regions (Bouret et al., 2004; Dobolyi and Palkovits, 2008; Srivastava et al., 2014), the role of TGF-β1 in dorsomedial area of the cerebral cortex, cingulate cortex, specifically on astrocyte differentiation, is poorly known.
Here the reported event was region-dependent since in DMc area the appearance of GFAP+ cells and disruption of RG processes were more robust than in Lc area. This observation might be related to 2 alternatives: (1) distinct responsiveness of different brain regions to TGF-β1; (2) heterogeneity of radial glial cells. The first possibility is supported by our previous report that GFAP gene promoter from different brain regions distinctly responds to TGF-β1 (Sousa Vde et al., 2004). It is also possible that TGF-β1 might exert its actions controlling size of a brain area (Falk et al., 2008) by acting into the different subpopulations of RG cells and other progenitors previously described to contribute to cell diversity in CNS (Pinto and Götz, 2007; Stancik et al., 2010), and that this event accounts for diversity in the responsiveness to TGF-β1. Whether this is due to different levels of TGF-β receptor or intracellular signaling molecules, or even, by cell autonomous defined potentials, remains to be determined.
Several molecules have been described to guarantee the maintenance of RG self-renewal, BLBP expression and morphology characteristics, such as the proteins of Neuregulin family and its receptor ErbBs, and Notch1 (Gaiano and Fishell, 2002; Patten et al., 2003; Schmid et al., 2003; Yoon et al., 2004; Anthony et al., 2005; Ghashghaei et al., 2006, 2007). Thus, alterations of ErB2 and Notch1 expression in RG cells could lead to a premature astrocyte differentiation under TGF-β1 influence. This hypothesis is supported by reports that interaction between TGF-β1 signaling pathway proteins and radializing factors such as Notch intracellular cleaved domain (NICD) and ErbB4 is necessary to regulate the expression of target genes in neural precursors (Blokzijl et al., 2003) and the correct time of gliogenesis (Sardi et al., 2006). The exact mechanisms by which TGF-β1 pathway controls RG-astrocyte differentiation in the dorsomedial area of the cerebral cortex will require further investigation.
We reported here that activation of TGF-β1 signaling pathway in the cerebral cortex down regulates the expression of FoxG1 in DMcarea.FoxG1 is a member of the forkhead family of transcription factors, expressed by cells with high proliferation rates; it controls neurogenesis, by maintaining the undifferentiated state of neural progenitors (Dou et al., 2000; Siegenthaler and Miller, 2008). In addition, FoxG1 is mainly expressed in lateral areas of the mice cerebral cortex (Miller, 2003).Mutant mice models for FoxG1 functions share several similarities with many of the phenotypes described here, including reduction of cortical thickness and layers of the dorsal area. For example, mutant mice for FoxG1, present reduction of dorsal area, and pronounced increase of BMPs, a member of TGF-β family, expression in the telencephalon (Takahashi and Liu, 2006). Further, FoxG1 was described as a potent inhibitor of TGF-β signaling due to its association with Smad proteins (Dou et al., 2000; Siegenthaler and Miller, 2008). Although TGF-β1 affects more robustly DMc area, we also observe the effect of this factor in Lc, such as mild RG fibers morphology and neurogenesis induction, it is possible that other transcription factors responsible for arealization of the cortex might mediate TGF-β1 actions in Lc (O’Leary and Sahara, 2008).
Thus, it is possible that TGF-β1 controls the balance between gliogenesis and neurogenesis by modulating the expression and activation of different transcription factors in vivo. Since FoxG1 is a lateral transcription factor, a gliogenic inhibitor, and negatively regulates Smads signaling, it is possible that FoxG1 is a mediator of TGF-β1 signaling in DMc.
Besides the role of TGF-β1 in the modulation of transcription factors at transcriptional level, it is possible that the lateral morphogen gradients might exert an inhibitory action on medial ones. It correlates with our observation that endogenous TGF-β signaling pathway might not be active or engaged in promotion of astrocytogenesis at this developmental stage, since pharmacological inhibition of endogenous TGF-β signaling by SB431542 did not affect RG morphological phenotype, as well as GFAP + cells numbers. Although we have shown that TGF-β1 is a potent inductor of astrocyte differentiation (Stipursky and Gomes, 2007; Stipursky et al., 2012a), this data confirm that RG cells are mainly committed in promoting neurogenesis at this stage (Noctor et al., 2001).
TGF-β1 affects neurogenesis and neuronal positioning in the cortical plate
Injection of TGF-β1 decreased the number of BrdU+ cells in the developing CP of the lateral area of the cortex. This effect might be the consequence of neurogenesis and/or migration deficits. The last hypothesis is more likely, since increased number of βTubulinIII+ cells was observed in the VZ, and although in the present work we cannot completely guarantee the identity of the pH3+ cells in the SVZ, it is possible that these cells could also contribute to neurogenic effect promoted by TGF-β1.
The role of TGF-β1 in neurogenesis is controversial; whereas it has been shown as inductor of neurogenesis in the cerebral cortex during embryonic stage and in the adult hippocampus (Vogel et al., 2010; Stipursky et al., 2012a; He et al., 2014); others have reported its action as negative modulator of neurogenesis in the adult SVZ (Roussa et al., 2004; Wachs et al., 2006; Siegenthaler and Miller, 2008). Although TGF-β1 has been shown to induce radial neuronal migration in the cerebral cortex, its effect in RG cell has not been previously addressed (Siegenthaler and Miller, 2004). Here we suggest that although TGF-β1 promotes neuronal generation from RG cells and as we previously demonstrated in vitro (Stipursky et al., 2012a), the morphological alterations triggered in radial processes in the lateral area of the cortex, even in a less extension that in DMc area, counteracts its effect and prevent neuronal migration and the accuracy in the establishment of these new generated neurons in the CP.
It is interesting that pharmacological inhibition of TGF-β1 signaling pathway by injection of SB431542 yielded an even greater increase of βTubulinIII+ cells in VZ, when compared with TGF-β1 injected brains. Although apparently contradictory, this result might indicate that endogenous TGF-β signaling pathway might be committed to control neuron generation in cerebral cortex during the neurogenic stage of the CNS development (Vogel et al., 2010). Further, it is possible that different levels of TGF-β signaling activation might be critical to elicit positive or negative responses to this factor. Accordingly, it has been demonstrated that opposite actions of TGF-β1 in neuronal migration is concentration dependent (Siegenthaler and Miller, 2004).
Together our results points to a new feature of TGF-β1 action in patterning the developing telencephalon. By acting in different RG populations, TGF-β1 promotes the generation of astrocytes and/or neurons in a regional dependent manner. Deficits in pathways that operate in RG physiology might generate dysfunctional cells, disorders in neuronal migration and premature astrocytogenesis, leading to diverse types of lamination defects in the developing cortex, such as observed in Lisencephaly and the congenital abnormality cortical dysplasia. Identification and characterization of the mechanisms underlying RG maintenance and differentiation might contribute to generation of therapeutic approaches to cell restocking in CNS parenchyma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Eva S. Anton (Associate Professor, Neuroscience Center, University of North Carolina at Chapel Hill) for significant comments and scientific support, and Marcelo Meloni and Adiel Batista do Nascimento for technical assistance. Thisworkwassupportedbygrantsfrom: Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (Flávia Carvalho Alcantara Gomes); Conselho Nacional para o Desenvolvimento Científico e Tecnológico (Daniel Francis; Flávia Carvalho Alcantara Gomes; Joice Stipursky; Lays Souza; Rômulo Sperduto Dezonne); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior(Carolina A. Moraes; Ana Paula Bérgamo de Araújo).
Glossary
Abbreviations
- BLBP
brain lipid binding protein
- BMP
bone morphogenetic protein
- BrdU
bromodeoxiuridine
- BSA
bovine serum albumin
- CNS
central nervous system
- CP
cortical plate
- DAPI
4_,6-diamidino-2-phenylindole dihydrochloride
- DMc
dorsomedial cortex
- DMEM/F12
Dulbecco’s modified Eagle’s medium supplemented with nutrient mixture F-12
- E14
embryonic day fourteen
- E18
embryonic day eighteen
- P0
postnatal day 0
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FGFb
basic fibroblast growth factor
- GFAP
glial fibrillary acidic protein
- IDM
intermediate differentiating morphology
- Lc
lateral cortex
- LV
lateral ventricle
- MAPK
mitogen-activated protein kinase
- PBS
phosphate-bufferedsaline
- PI3K
phosphatidylinositol-3 kinase
- RG
radial glia
- SMAD
homologue protein for SMA protein from C. elegans and mothers against decapentaplegic (MAD) from Drosophila
- SVZ
subventricular zone
- TBS-T
Tris-bufferedsaline- Tween20
- Tc
total cortex
- TGFRII
transforming growth factor beta type II receptor
- TGF-β1
transforming growth factor beta 1
- VZ
ventricular zone.
References
- Altschul S. F., Madden T. L., Schaffer A. A., Zhang J., Zhang Z., Miller W., et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony T. E., Klein C., Fishell G., Heintz N. (2004). Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron 41, 881–890. 10.1016/s0896-6273(04)00140-0 [DOI] [PubMed] [Google Scholar]
- Anthony T. E., Mason H. A., Gridley T., Fishell G., Heintz N. (2005). Brain lipid-binding protein is a direct target of Notch signaling in radial glial cells. Genes Dev. 19, 1028–1033. 10.1101/gad.1302105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton E. S., Marchionni M. A., Lee K. F., Rakic P. (1997). Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development 124, 3501–3510. [DOI] [PubMed] [Google Scholar]
- Barnabé-Heider F., Wasylnka J. A., Fernandes K. J., Porsche C., Sendtner M., Kaplan D. R., et al. (2005). Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron 48, 253–265. 10.1016/j.neuron.2005.08.037 [DOI] [PubMed] [Google Scholar]
- Bentivoglio M., Mazzarello P. (1999). The history of radial glia. Brain Res. Bull. 49, 305–315. 10.1016/s0361-9230(99)00065-9 [DOI] [PubMed] [Google Scholar]
- Blokzijl A., Dahlqvist C., Reissmann E., Falk A., Moliner A., Lendahl U., et al. (2003). Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J. Cell Biol. 163, 723–728. 10.1083/jcb.200305112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S., De Seranno S., Beauvillain J. C., Prevot V. (2004). Transforming growth factor beta1 may directly influence gonadotropin-releasing hormone gene expression in the rat hypothalamus. Endocrinology 145, 1794–1801. 10.1210/en.2003-1468 [DOI] [PubMed] [Google Scholar]
- Brionne T. C., Tesseur I., Masliah E., Wyss-Coray T. (2003). Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron 40, 1133–1145. 10.1016/s0896-6273(03)00766-9 [DOI] [PubMed] [Google Scholar]
- Costa M. R., Götz M., Berninger B. (2010). What determines neurogenic competence in glia? Brain Res. Rev. 63, 47–59. 10.1016/j.brainresrev.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Culican S. M., Baumrind N. L., Yamamoto M., Pearlman A. L. (1990). Cortical radial glia: identification in tissue culture and evidence for their transformation to astrocytes. J. Neurosci. 10, 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D. (1981). The vimentin-GFA protein transition in rat neuroglia cytoskeleton occurs at the time of myelination. J. Neurosci. Res. 6, 741–748. 10.1002/jnr.490060608 [DOI] [PubMed] [Google Scholar]
- Dezonne R. S., Stipursky J., Araujo A. P., Nones J., Pavão M. S., Porcionatto M., et al. (2013). Thyroid hormone treated astrocytes induce maturation of cerebral cortical neurons through modulation of proteoglycan levels. Front. Cell. Neurosci. 7:125. 10.3389/fncel.2013.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz L. P., Almeida J. C., Tortelli V., Vargas Lopes C., Setti-Perdigão P., Stipursky J., et al. (2012). Astrocyte-induced synaptogenesis is mediated by transforming growth factor beta signaling through modulation of D-serine levels in cerebral cortex neurons. J. Biol. Chem. 287, 41432–41445. 10.1074/jbc.m112.380824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz L. P., Tortelli V., Garcia M. N., Araújo A. P., Melo H. M., Seixas da Silva G. S., et al. (2014). Astrocyte transforming growth factor beta 1 promotes inhibitory synapse formation via CaM kinase II signaling. Glia 62, 1917–1931. 10.1002/glia.22713 [DOI] [PubMed] [Google Scholar]
- Dobolyi A., Palkovits M. (2008). Expression of latent transforming growth factor beta binding proteins in the rat brain. J. Comp. Neurol. 507, 1393–1408. 10.1002/cne.21621 [DOI] [PubMed] [Google Scholar]
- Dou C., Lee J., Liu B., Liu F., Massague J., Xuan S., et al. (2000). BF-1 interferes with transforming growth factor beta signaling by associating with Smad partners. Mol. Cell. Biol. 20, 6201–6211. 10.1128/mcb.20.17.6201-6211.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espósito M. S., Piatti V. C., Laplagne D. A., Morgenstern N. A., Ferrari C. C., Pitossi F. J., et al. (2005). Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J. Neurosci. 25, 10074–10086. 10.1523/jneurosci.3114-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk S., Wurdak H., Ittner L. M., Ille F., Sumara G., Schmid M. T., et al. (2008). Brain area-specific effect of TGF-beta signaling on Wnt-dependent neural stem cell expansion. Cell Stem Cell 2, 472–483. 10.1016/j.stem.2008.03.006 [DOI] [PubMed] [Google Scholar]
- Gaiano N., Fishell G. (2002). The role of notch in promoting glial and neural stem cell fates. Annu. Rev. Neurosci. 25, 471–490. 10.1146/annurev.neuro.25.030702.130823 [DOI] [PubMed] [Google Scholar]
- Ghashghaei H. T., Weber J., Pevny L., Schmid R., Schwab M. H., Lloyd K. C., et al. (2006). The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc. Natl. Acad. Sci. U S A 103, 1930–1935. 10.1073/pnas.0510410103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei H. T., Weimer J. M., Schmid R. S., Yokota Y., Mccarthy K. D., Popko B., et al. (2007). Reinduction of ErbB2 in astrocytes promotes radial glial progenitor identity in adult cerebral cortex. Genes Dev. 21, 3258–3271. 10.1101/gad.1580407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes F. C., Sousa Vde O., Romão L. (2005). Emerging roles for TGF-beta1 in nervous system development. Int. J. Dev. Neurosci. 23, 413–424. 10.1016/j.ijdevneu.2005.04.001 [DOI] [PubMed] [Google Scholar]
- Götz M., Hartfuss E., Malatesta P. (2002). Radial glial cells as neuronal precursors: a new perspective on the correlation of morphology and lineage restriction in the developing cerebral cortex of mice. Brain Res. Bull. 57, 777–788. 10.1016/s0361-9230(01)00777-8 [DOI] [PubMed] [Google Scholar]
- Götz M., Huttner W. B. (2005). The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777–788. 10.1038/nrm1739 [DOI] [PubMed] [Google Scholar]
- Hartfuss E., Galli R., Heins N., Gotz M. (2001). Characterization of CNS precursor subtypes and radial glia. Dev. Biol. 229, 15–30. 10.1006/dbio.2000.9962 [DOI] [PubMed] [Google Scholar]
- Hatten M. E. (1999). Central nervous system neuronal migration. Annu. Rev. Neurosci. 22, 511–539. 10.1146/annurev.neuro.22.1.511 [DOI] [PubMed] [Google Scholar]
- Haubst N., Georges-Labouesse E., De Arcangelis A., Mayer U., Gotz M. (2006). Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development 133, 3245–3254. 10.1242/dev.02486 [DOI] [PubMed] [Google Scholar]
- He F., Ge W., Martinowich K., Becker-Catania S., Coskun V., Zhu W., et al. (2005). A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat. Neurosci. 8, 616–625. 10.1038/nn1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Zhang H., Yung A., Villeda S. A., Jaeger P. A., Olayiwola O., et al. (2014). ALK5-dependent TGF-beta signaling is a major determinant of late-stage adult neurogenesis. Nat. Neurosci. 17, 943–952. 10.1038/nn.3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner R. F., Hodge R. D., Daza R. A., Englund C. (2006). Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum and adult hippocampus. Neurosci. Res. 55, 223–233. 10.1016/j.neures.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Hunter K. E., Hatten M. E. (1995). Radial glial cell transformation to astrocytes is bidirectional: regulation by a diffusible factor in embryonic forebrain. Proc. Natl. Acad. Sci. U S A 92, 2061–2065. 10.1073/pnas.92.6.2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javelaud D., Mauviel A. (2005). Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene 24, 5742–5750. 10.1038/sj.onc.1208928 [DOI] [PubMed] [Google Scholar]
- Kriegstein A., Alvarez-Buylla A. (2009). The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149–184. 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A. R., Götz M. (2003). Radial glia diversity: a matter of cell fate. Glia 43, 37–43. 10.1002/glia.10250 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Luukko K., Ylikorkala A., Mäkelä T. P. (2001). Developmentally regulated expression of Smad3, Smad4, Smad6 and Smad7 involved in TGF-beta signaling. Mech. Dev. 101, 209–212. 10.1016/s0925-4773(00)00556-6 [DOI] [PubMed] [Google Scholar]
- Malatesta P., Hack M. A., Hartfuss E., Kettenmann H., Klinkert W., Kirchhoff F., et al. (2003). Neuronal or glial progeny: regional differences in radial glia fate. Neuron 37, 751–764. 10.1016/s0896-6273(03)00116-8 [DOI] [PubMed] [Google Scholar]
- Massagué J. (1998). TGF-beta signal transduction. Annu. Rev. Biochem. 67, 753–791. 10.1146/annurev.biochem.67.1.753 [DOI] [PubMed] [Google Scholar]
- Massagué J., Gomis R. R. (2006). The logic of TGFbeta signaling. FEBS Lett. 580, 2811–2820. 10.1016/j.febslet.2006.04.033 [DOI] [PubMed] [Google Scholar]
- Mecha M., Rabadán M. A., Peña-Melián A., Valencia M., Mondéjar T., Blanco M. J. (2008). Expression of TGF-betas in the embryonic nervous system: analysis of interbalance between isoforms. Dev. Dyn. 237, 1709–1717. 10.1002/dvdy.21558 [DOI] [PubMed] [Google Scholar]
- Mi H., Haeberle H., Barres B. A. (2001). Induction of astrocyte differentiation by endothelial cells. J. Neurosci. 21, 1538–1547. 10.1046/j.1432-0436.2001.680405.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. W. (2003). Expression of transforming growth factor-beta in developing rat cerebral cortex: effects of prenatal exposure to ethanol. J. Comp. Neurol. 460, 410–424. 10.1002/cne.10658 [DOI] [PubMed] [Google Scholar]
- Miyata T., Kawaguchi A., Okano H., Ogawa M. (2001). Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31, 727–741. 10.1016/s0896-6273(01)00420-2 [DOI] [PubMed] [Google Scholar]
- Munoz-Garcia D., Ludwin S. K. (1986). Gliogenesis in organotypic tissue culture of the spinal cord of the embryonic mouse. I. Immunocytochemical and ultrastructural studies. J. Neurocytol. 15, 273–290. 10.1007/bf01611431 [DOI] [PubMed] [Google Scholar]
- Nakashima K., Yanagisawa M., Arakawa H., Kimura N., Hisatsune T., Kawabata M., et al. (1999). Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 284, 479–482. 10.1126/science.284.5413.479 [DOI] [PubMed] [Google Scholar]
- Nishino J., Yamashita K., Hashiguchi H., Fujii H., Shimazaki T., Hamada H. (2004). Meteorin: a secreted protein that regulates glial cell differentiation and promotes axonal extension. EMBO J. 23, 1998–2008. 10.1038/sj.emboj.7600202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor S. C., Flint A. C., Weissman T. A., Dammerman R. S., Kriegstein A. R. (2001). Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714–720. 10.1038/35055553 [DOI] [PubMed] [Google Scholar]
- Noctor S. C., Flint A. C., Weissman T. A., Wong W. S., Clinton B. K., Kriegstein A. R. (2002). Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J. Neurosci. 22, 3161–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary D. D., Sahara S. (2008). Genetic regulation of arealization of the neocortex. Curr. Opin. Neurobiol. 18, 90–100. 10.1016/j.conb.2008.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten B. A., Peyrin J. M., Weinmaster G., Corfas G. (2003). Sequential signaling through Notch1 and erbB receptors mediates radial glia differentiation. J. Neurosci. 23, 6132–6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L., Götz M. (2007). Radial glial cell heterogeneity–the source of diverse progeny in the CNS. Prog. Neurobiol. 83, 2–23. 10.1016/j.pneurobio.2007.02.010 [DOI] [PubMed] [Google Scholar]
- Pixley S. K., de Vellis J. (1984). Transition between immature radial glia and mature astrocytes studied with a monoclonal antibody to vimentin. Brain Res. 317, 201–209. 10.1016/0165-3806(84)90097-x [DOI] [PubMed] [Google Scholar]
- Powrozek T. A., Miller M. W. (2009). Ethanol affects transforming growth factor beta1-initiated signals: cross-talking pathways in the developing rat cerebral wall. J. Neurosci. 29, 9521–9533. 10.1523/jneurosci.2371-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pramparo T., Youn Y. H., Yingling J., Hirotsune S., Wynshaw-Boris A. (2010). Novel embryonic neuronal migration and proliferation defects in Dcx mutant mice are exacerbated by Lis1 reduction. J. Neurosci. 30, 3002–3012. 10.1523/jneurosci.4851-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radakovits R., Barros C. S., Belvindrah R., Patton B., Muller U. (2009). Regulation of radial glial survival by signals from the meninges. J. Neurosci. 29, 7694–7705. 10.1523/jneurosci.5537-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. (1971). Guidance of neurons migrating to the fetal monkey neocortex. Brain Res. 33, 471–476. 10.1016/0006-8993(71)90119-3 [DOI] [PubMed] [Google Scholar]
- Rakic P. (1995). Radial versus tangential migration of neuronal clones in the developing cerebral cortex. Proc. Natl. Acad. Sci. U S A 92, 11323–11327. 10.1073/pnas.92.25.11323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romão L. F., Sousa Vde O., Neto V. M., Gomes F. C. (2008). Glutamate activates GFAP gene promoter from cultured astrocytes through TGF-beta1 pathways. J. Neurochem. 106, 746–756. 10.1111/j.1471-4159.2008.05428.x [DOI] [PubMed] [Google Scholar]
- Roussa E., Farkas L. M., Krieglstein K. (2004). TGF-beta promotes survival on mesencephalic dopaminergic neurons in cooperation with Shh and FGF-8. Neurobiol. Dis. 16, 300–310. 10.1016/j.nbd.2004.03.006 [DOI] [PubMed] [Google Scholar]
- Sardi S. P., Murtie J., Koirala S., Patten B. A., Corfas G. (2006). Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell 127, 185–197. 10.1016/j.cell.2006.07.037 [DOI] [PubMed] [Google Scholar]
- Schmid R. S., Mcgrath B., Berechid B. E., Boyles B., Marchionni M., Sestan N., et al. (2003). Neuregulin 1-erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Proc. Natl. Acad. Sci. U S A 100, 4251–4256. 10.1073/pnas.0630496100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler J. A., Miller M. W. (2004). Transforming growth factor beta1 modulates cell migration in rat cortex: effects of ethanol. Cereb. Cortex 14, 791–802. 10.1093/cercor/bhh039 [DOI] [PubMed] [Google Scholar]
- Siegenthaler J. A., Miller M. W. (2008). Generation of Cajal-Retzius neurons in mouse forebrain is regulated by transforming growth factor beta-Fox signaling pathways. Dev. Biol. 313, 35–46. 10.1016/j.ydbio.2007.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sild M., Ruthazer E. S. (2011). Radial glia: progenitor, pathway and partner. Neuroscientist 17, 288–302. 10.1177/1073858410385870 [DOI] [PubMed] [Google Scholar]
- Sousa Vde O., Romão L., Neto V. M., Gomes F. C. (2004). Glial fibrillary acidic protein gene promoter is differently modulated by transforming growth factor-beta 1 in astrocytes from distinct brain regions. Eur. J. Neurosci. 19, 1721–1730. 10.1111/j.1460-9568.2004.03249.x [DOI] [PubMed] [Google Scholar]
- Srivastava V. K., Hiney J. K., Dees W. L. (2014). Actions and interactions of alcohol and transforming growth factor beta1 on prepubertal hypothalamic gonadotropin-releasing hormone. Alcohol. Clin. Exp. Res. 38, 1321–1329. 10.1111/acer.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancik E. K., Navarro-Quiroga I., Sellke R., Haydar T. F. (2010). Heterogeneity in ventricular zone neural precursors contributes to neuronal fate diversity in the postnatal neocortex. J. Neurosci. 30, 7028–7036. 10.1523/jneurosci.6131-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipursky J., de Sampaio e Spohr T. C. L., Romão L., Gomes F. C. (2009). “Neuron-astrocyte interaction in cell fate commitment in the central nervous system,” in Perspectives of Stem Cells (Springer; ), 145–170. [Google Scholar]
- Stipursky J., Francis D., Gomes F. C. (2012a). Activation of MAPK/PI3K/SMAD pathways by TGF-beta(1) controls differentiation of radial glia into astrocytes in vitro. Dev. Neurosci. 34, 68–81. 10.1159/000338108 [DOI] [PubMed] [Google Scholar]
- Stipursky J., Gomes F. C. (2007). TGF-beta1/SMAD signaling induces astrocyte fate commitment in vitro: implications for radial glia development. Glia 55, 1023–1033. 10.1002/glia.20522 [DOI] [PubMed] [Google Scholar]
- Stipursky J., Spohr T. C., Sousa V. O., Gomes F. C. (2012b). Neuron-astroglial interactions in cell-fate commitment and maturation in the central nervous system. Neurochem. Res. 37, 2402–2418. 10.1007/s11064-012-0798-x [DOI] [PubMed] [Google Scholar]
- Takahashi H., Liu F. C. (2006). Genetic patterning of the mammalian telencephalon by morphogenetic molecules and transcription factors. Birth Defects Res. C Embryo Today 78, 256–266. 10.1002/bdrc.20077 [DOI] [PubMed] [Google Scholar]
- Takizawa T., Nakashima K., Namihira M., Ochiai W., Uemura A., Yanagisawa M., et al. (2001). DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev. Cell 1, 749–758. 10.1016/s1534-5807(01)00101-0 [DOI] [PubMed] [Google Scholar]
- Uemura A., Takizawa T., Ochiai W., Yanagisawa M., Nakashima K., Taga T. (2002). Cardiotrophin-like cytokine induces astrocyte differentiation of fetal neuroepithelial cells via activation of STAT3. Cytokine 18, 1–7. 10.1006/cyto.2002.1006 [DOI] [PubMed] [Google Scholar]
- Vogel T., Ahrens S., Büttner N., Krieglstein K. (2010). Transforming growth factor beta promotes neuronal cell fate of mouse cortical and hippocampal progenitors in vitro and in vivo: identification of Nedd9 as an essential signaling component. Cereb. Cortex 20, 661–671. 10.1093/cercor/bhp134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt T. (1989). Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J. Comp. Neurol. 289, 74–88. 10.1002/cne.902890106 [DOI] [PubMed] [Google Scholar]
- Voss A. K., Britto J. M., Dixon M. P., Sheikh B. N., Collin C., Tan S. S., et al. (2008). C3G regulates cortical neuron migration, preplate splitting and radial glial cell attachment. Development 135, 2139–2149. 10.1242/dev.016725 [DOI] [PubMed] [Google Scholar]
- Wachs F. P., Winner B., Couillard-Despres S., Schiller T., Aigner R., Winkler J., et al. (2006). Transforming growth factor-beta1 is a negative modulator of adult neurogenesis. J. Neuropathol. Exp. Neurol. 65, 358–370. 10.1097/01.jnen.0000218444.53405.f0 [DOI] [PubMed] [Google Scholar]
- Walantus W., Castaneda D., Elias L., Kriegstein A. (2007). In utero intraventricular injection and electroporation of E15 mouse embryos. J. Vis. Exp. 239. 10.3791/239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer J. M., Yokota Y., Stanco A., Stumpo D. J., Blackshear P. J., Anton E. S. (2009). MARCKS modulates radial progenitor placement, proliferation and organization in the developing cerebral cortex. Development 136, 2965–2975. 10.1242/dev.036616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y., Eom T. Y., Stanco A., Kim W. Y., Rao S., Snider W. D., et al. (2010). Cdc42 and Gsk3 modulate the dynamics of radial glial growth, inter-radial glial interactions and polarity in the developing cerebral cortex. Development 137, 4101–4110. 10.1242/dev.048637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y., Gashghaei H. T., Han C., Watson H., Campbell K. J., Anton E. S. (2007). Radial glial dependent and independent dynamics of interneuronal migration in the developing cerebral cortex. PLoS One 2:e794. 10.1371/journal.pone.0000794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y., Kim W. Y., Chen Y., Wang X., Stanco A., Komuro Y., et al. (2009). The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex. Neuron 61, 42–56. 10.1016/j.neuron.2008.10.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K., Nery S., Rutlin M. L., Radtke F., Fishell G., Gaiano N. (2004). Fibroblast growth factor receptor signaling promotes radial glial identity and interacts with Notch1 signaling in telencephalic progenitors. J. Neurosci. 24, 9497–9506. 10.1523/jneurosci.0993-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]