Abstract
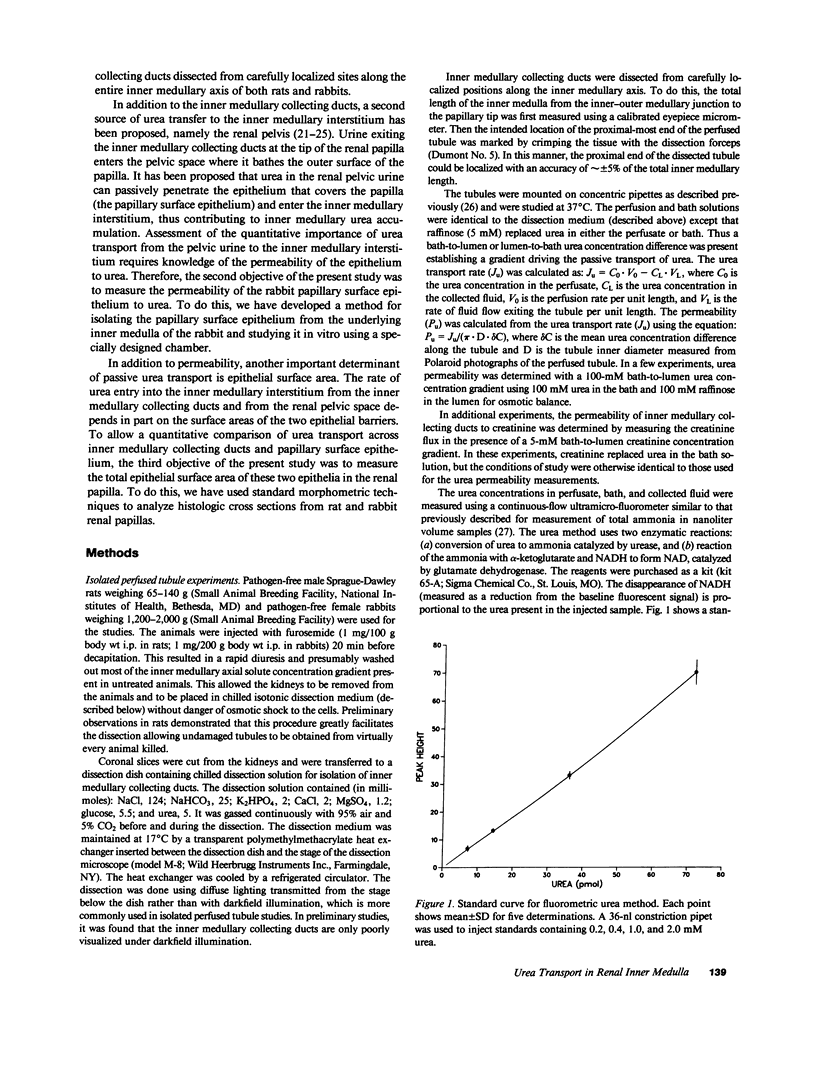
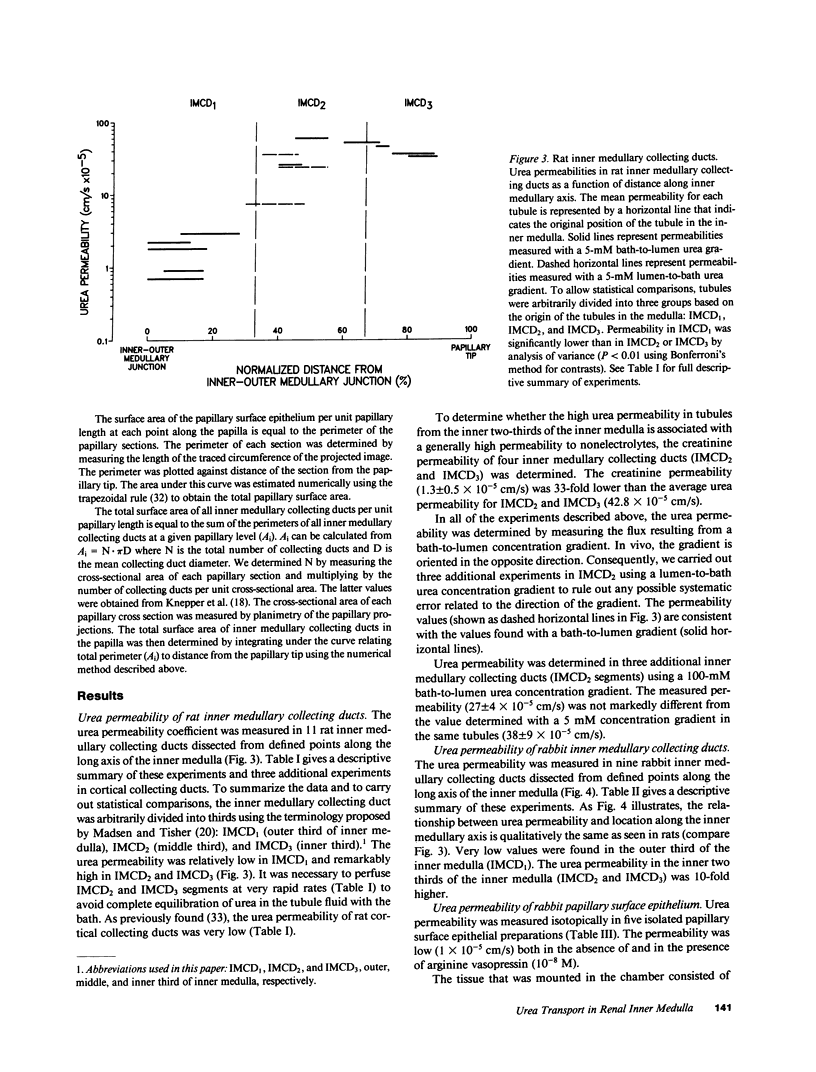
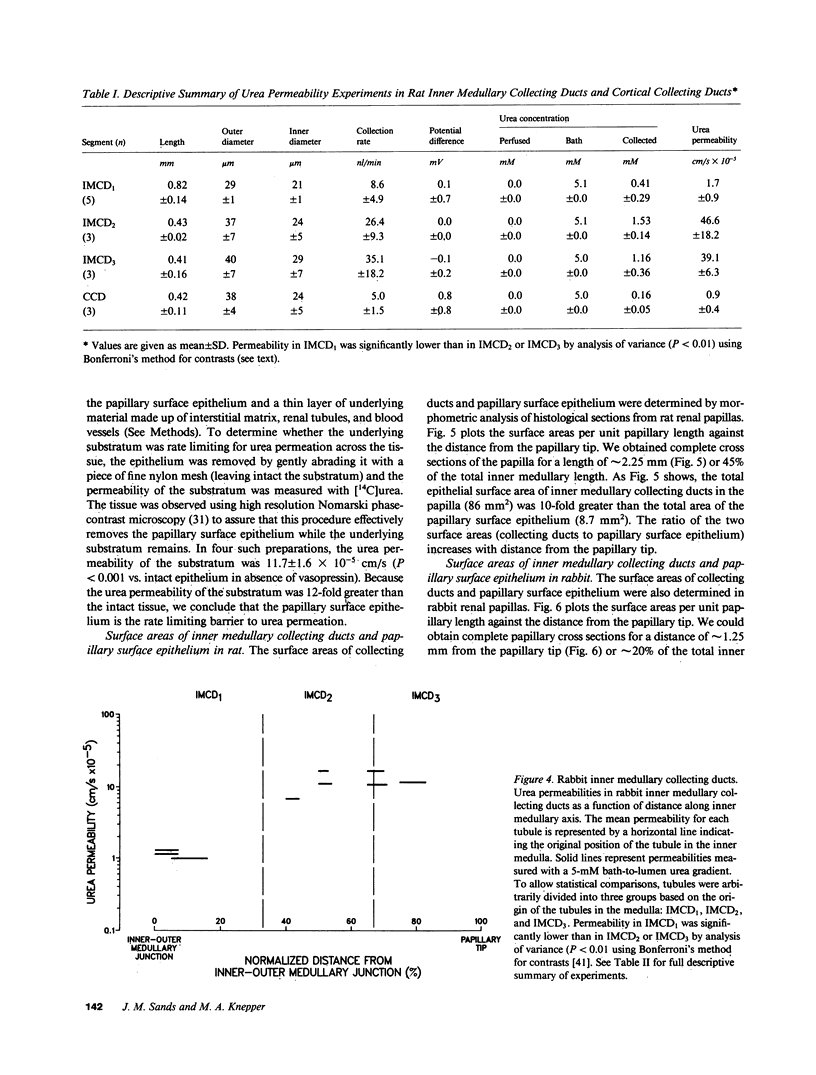
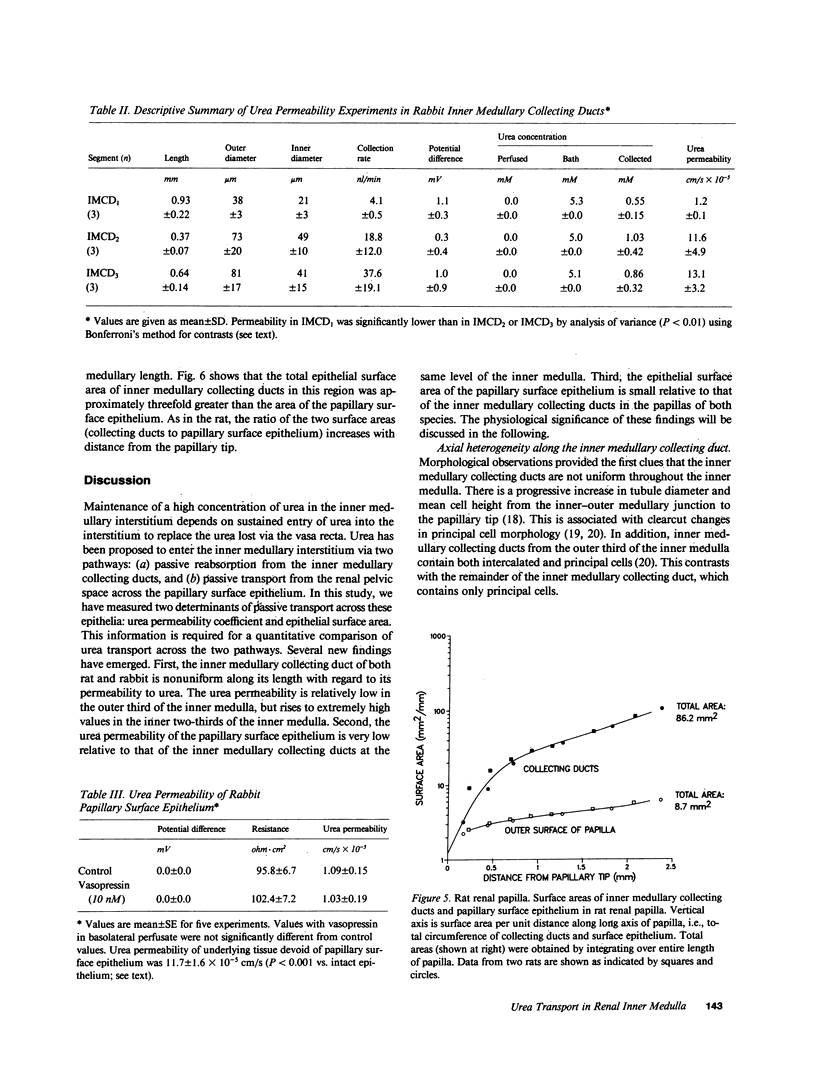
To compare passive urea transport across the inner medullary collecting ducts (IMCDs) and the papillary surface epithelium (PSE) of the kidney, two determinants of passive transport were measured, namely permeability coefficient and surface area. Urea permeability was measured in isolated perfused IMCDs dissected from carefully localized sites along the inner medullas of rats and rabbits. Mean permeability coefficients (X 10(-5) cm/s) in rat IMCDs were: outer third of inner medulla (IMCD1), 1.6 +/- 0.5; middle third (IMCD2), 46.6 +/- 10.5; and inner third (IMCD3), 39.1 +/- 3.6. Mean permeability coefficients in rabbit IMCDs were: IMCD1, 1.2 +/- 0.1; IMCD2, 11.6 +/- 2.8; and IMCD3, 13.1 +/- 1.8. The rabbit PSE was dissected free from the underlying renal inner medulla and was mounted in a specially designed chamber to measure its permeability to urea. The mean value was 1 X 10(-5) cm/s both in the absence and presence of vasopressin (10 nM). Morphometry of renal papillary cross sections revealed that the total surface area of IMCDs exceeds the total area of the PSE by 10-fold in the rat and threefold in the rabbit. We conclude: the IMCD displays axial heterogeneity with respect to urea permeability, with a high permeability only in its distal two-thirds; and because the urea permeability and surface area of the PSE are relatively small, passive transport across it is unlikely to be a major source of urea to the inner medullary interstitium.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERLINER R. W., LEVINSKY N. G., DAVIDSON D. G., EDEN M. Dilution and concentration of the urine and the action of antidiuretic hormone. Am J Med. 1958 May;24(5):730–744. doi: 10.1016/0002-9343(58)90377-2. [DOI] [PubMed] [Google Scholar]
- Bargman J., Leonard S. L., McNeely E., Robertson C., Jamison R. L. Examination of transepithelial exchange of water and solute in the rat renal pelvis. J Clin Invest. 1984 Nov;74(5):1860–1870. doi: 10.1172/JCI111605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre J. V., Karnovsky M. J., Lechene C. P. Renal papillary epithelial morphology in antidiuresis and water diuresis. Am J Physiol. 1978 Jul;235(1):F69–F76. doi: 10.1152/ajprenal.1978.235.1.F69. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Roman R. J., Lechene C. Effect of urea concentration of pelvic fluid on renal concentrating ability. Am J Physiol. 1980 Dec;239(6):F609–F618. doi: 10.1152/ajprenal.1980.239.6.F609. [DOI] [PubMed] [Google Scholar]
- Burg M. B., Green N. Function of the thick ascending limb of Henle's loop. Am J Physiol. 1973 Mar;224(3):659–668. doi: 10.1152/ajplegacy.1973.224.3.659. [DOI] [PubMed] [Google Scholar]
- Burg M. B. Perfusion of isolated renal tubules. Yale J Biol Med. 1972 Jun-Aug;45(3-4):321–326. [PMC free article] [PubMed] [Google Scholar]
- Chandhoke P. S., Saidel G. M., Knepper M. A. Role of inner medullary collecting duct NaCl transport in urinary concentration. Am J Physiol. 1985 Nov;249(5 Pt 2):F688–F697. doi: 10.1152/ajprenal.1985.249.5.F688. [DOI] [PubMed] [Google Scholar]
- Good D. W., Vurek G. G. Picomole quantitation of ammonia by flow-through fluorometry. Anal Biochem. 1983 Apr 1;130(1):199–202. doi: 10.1016/0003-2697(83)90670-x. [DOI] [PubMed] [Google Scholar]
- Gunther R. A., Rabinowitz L. Urea and renal concentrating ability in the rabbit. Kidney Int. 1980 Feb;17(2):205–222. doi: 10.1038/ki.1980.24. [DOI] [PubMed] [Google Scholar]
- Helman S. I., Miller D. A. In vitro techniques for avoiding edge damage in studies of frog skin. Science. 1971 Jul 9;173(3992):146–148. doi: 10.1126/science.173.3992.146. [DOI] [PubMed] [Google Scholar]
- Imbert M., de Rouffignac C. Role of sodium and urea in the renal concentrating mechanism in Psammomys obesus. Pflugers Arch. 1976 Jan 30;361(2):107–114. doi: 10.1007/BF00583453. [DOI] [PubMed] [Google Scholar]
- JARAUSCH K. H., ULLRICH K. J. Untersuchungen zum Problem der Harnkonzentrierung und Harnverdünnung; Uber die Verteilung von Elektrolyten (Na, K, Ca, Mg, Cl, anorganischem Phosphat), Harnstoff, Aminosäuren und exogenem Kreatinin in Rinde und Mark der Hundeniere bei verschiedenen Diuresezuständen. Pflugers Arch. 1956;262(6):537–550. doi: 10.1007/BF00362116. [DOI] [PubMed] [Google Scholar]
- KLUMPER J. D., ULLRICH K. J., HILGER H. H. Das Verhalten des Harnstoffs in den Sammelrohren der Säugetierniere. Pflugers Arch. 1958;267(3):238–243. doi: 10.1007/BF00362427. [DOI] [PubMed] [Google Scholar]
- Knepper M. A., Danielson R. A., Saidel G. M., Post R. S. Quantitative analysis of renal medullary anatomy in rats and rabbits. Kidney Int. 1977 Nov;12(5):313–323. doi: 10.1038/ki.1977.118. [DOI] [PubMed] [Google Scholar]
- Knepper M. A., Good D. W., Burg M. B. Ammonia and bicarbonate transport by rat cortical collecting ducts perfused in vitro. Am J Physiol. 1985 Dec;249(6 Pt 2):F870–F877. doi: 10.1152/ajprenal.1985.249.6.F870. [DOI] [PubMed] [Google Scholar]
- Knepper M. A. Urea transport in isolated thick ascending limbs and collecting ducts from rats. Am J Physiol. 1983 Nov;245(5 Pt 1):F634–F639. doi: 10.1152/ajprenal.1983.245.5.F634. [DOI] [PubMed] [Google Scholar]
- Kokko J. P., Rector F. C., Jr Countercurrent multiplication system without active transport in inner medulla. Kidney Int. 1972 Oct;2(4):214–223. doi: 10.1038/ki.1972.97. [DOI] [PubMed] [Google Scholar]
- Lacy E. R., Schmidt-Nielsen B. Anatomy of the renal pelvis in the hamster. Am J Anat. 1979 Mar;154(3):291–320. doi: 10.1002/aja.1001540302. [DOI] [PubMed] [Google Scholar]
- Lacy E. R. The mammalian renal pelvis: physiological implications from morphometric analyses. Anat Embryol (Berl) 1980;160(2):131–144. doi: 10.1007/BF00301856. [DOI] [PubMed] [Google Scholar]
- Lory P., Gilg A., Horster M. Renal countercurrent system: role of collecting duct convergence and pelvic urea predicted from a mathematical model. J Math Biol. 1983;16(3):281–304. doi: 10.1007/BF00276508. [DOI] [PubMed] [Google Scholar]
- MURDAUGH H. V., Jr, SCHMIDT-NIELSEN B., DOYLE E. M., O'DELL R. Renal tubular regulation of urea excretion in man. J Appl Physiol. 1958 Sep;13(2):263–268. doi: 10.1152/jappl.1958.13.2.263. [DOI] [PubMed] [Google Scholar]
- Marsh D. J. Solute and water flows in thin limbs of Henle's loop in the hamster kidney. Am J Physiol. 1970 Mar;218(3):824–831. doi: 10.1152/ajplegacy.1970.218.3.824. [DOI] [PubMed] [Google Scholar]
- Morgan T., Berliner R. W. Permeability of the loop of Henle, vasa recta, and collecting duct to water, urea, and sodium. Am J Physiol. 1968 Jul;215(1):108–115. doi: 10.1152/ajplegacy.1968.215.1.108. [DOI] [PubMed] [Google Scholar]
- Pennell J. P., Sanjana V., Frey N. R., Jamison R. L. The effect of urea infusion on the urinary concentrating mechanism in protein-depleted rats. J Clin Invest. 1975 Feb;55(2):399–409. doi: 10.1172/JCI107944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer E. W. Comparative anatomical observations of the mammalian renal pelvis and medulla. J Anat. 1968 Jan;102(Pt 2):321–331. [PMC free article] [PubMed] [Google Scholar]
- Roch-Ramel F., Chomety F., Peters G. Urea concentrations in tubular fluid and in renal tissue of nondiuretic rats. Am J Physiol. 1968 Aug;215(2):429–438. doi: 10.1152/ajplegacy.1968.215.2.429. [DOI] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Permeability of medullary nephron segments to urea and water: Effect of vasopressin. Kidney Int. 1974 Dec;6(6):379–387. doi: 10.1038/ki.1974.123. [DOI] [PubMed] [Google Scholar]
- Rocha A. S., Kudo L. H. Water, urea, sodium, chloride, and potassium transport in the in vitro isolated perfused papillary collecting duct. Kidney Int. 1982 Nov;22(5):485–491. doi: 10.1038/ki.1982.201. [DOI] [PubMed] [Google Scholar]
- Sands J. M., Knepper M. A., Spring K. R. Na-K-Cl cotransport in apical membrane of rabbit renal papillary surface epithelium. Am J Physiol. 1986 Sep;251(3 Pt 2):F475–F484. doi: 10.1152/ajprenal.1986.251.3.F475. [DOI] [PubMed] [Google Scholar]
- Schütz W., Schnermann J. Pelvic urine composition as a determinant of inner medullary solute concentration and urine osmolarity. Pflugers Arch. 1972;334(2):154–166. doi: 10.1007/BF00586788. [DOI] [PubMed] [Google Scholar]
- Stephenson J. L. Concentration of urine in a central core model of the renal counterflow system. Kidney Int. 1972 Aug;2(2):85–94. doi: 10.1038/ki.1972.75. [DOI] [PubMed] [Google Scholar]
- TRUNIGER B., SCHMIDT-NIELSEN B. INTRARENAL DISTRIBUTION OF UREA AND RELATED COMPOUNDS: EFFECTS OF NITROGEN INTAKE. Am J Physiol. 1964 Nov;207:971–978. doi: 10.1152/ajplegacy.1964.207.5.971. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Schmidt-Nielsen B. Urea transport in the collecting duct of rats on normal and low protein diet. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;295(2):147–156. doi: 10.1007/BF00362746. [DOI] [PubMed] [Google Scholar]



