Abstract
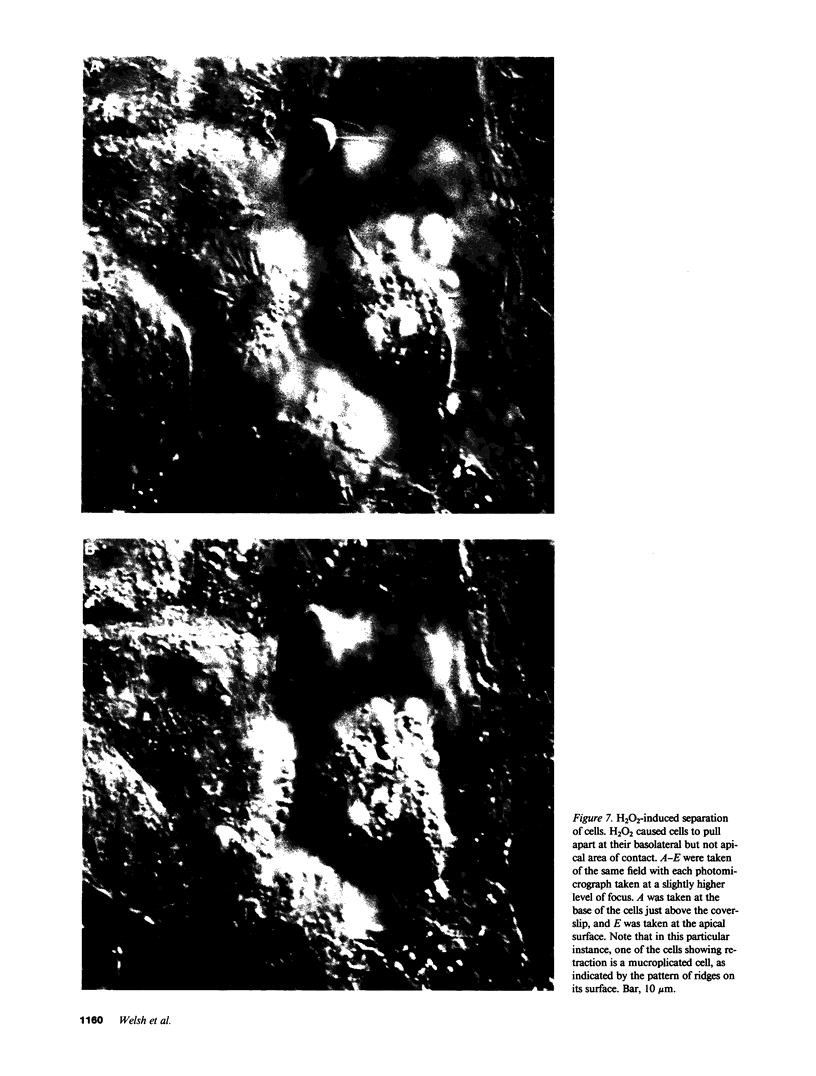
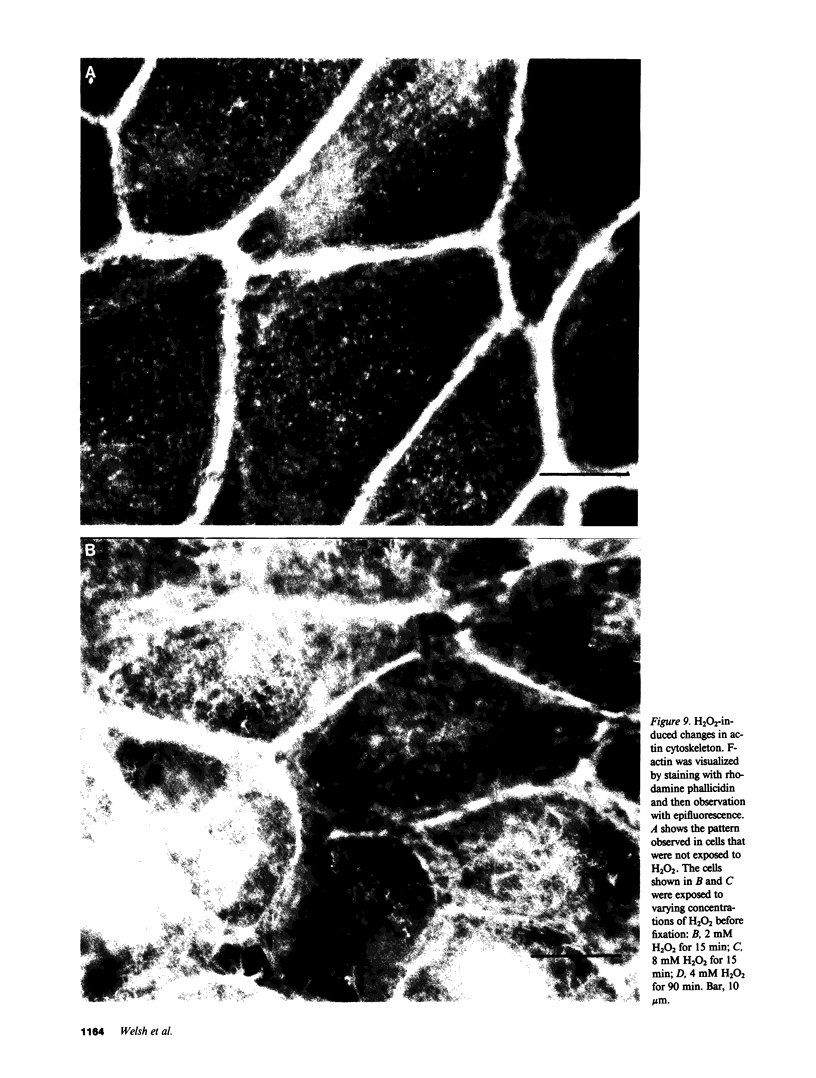
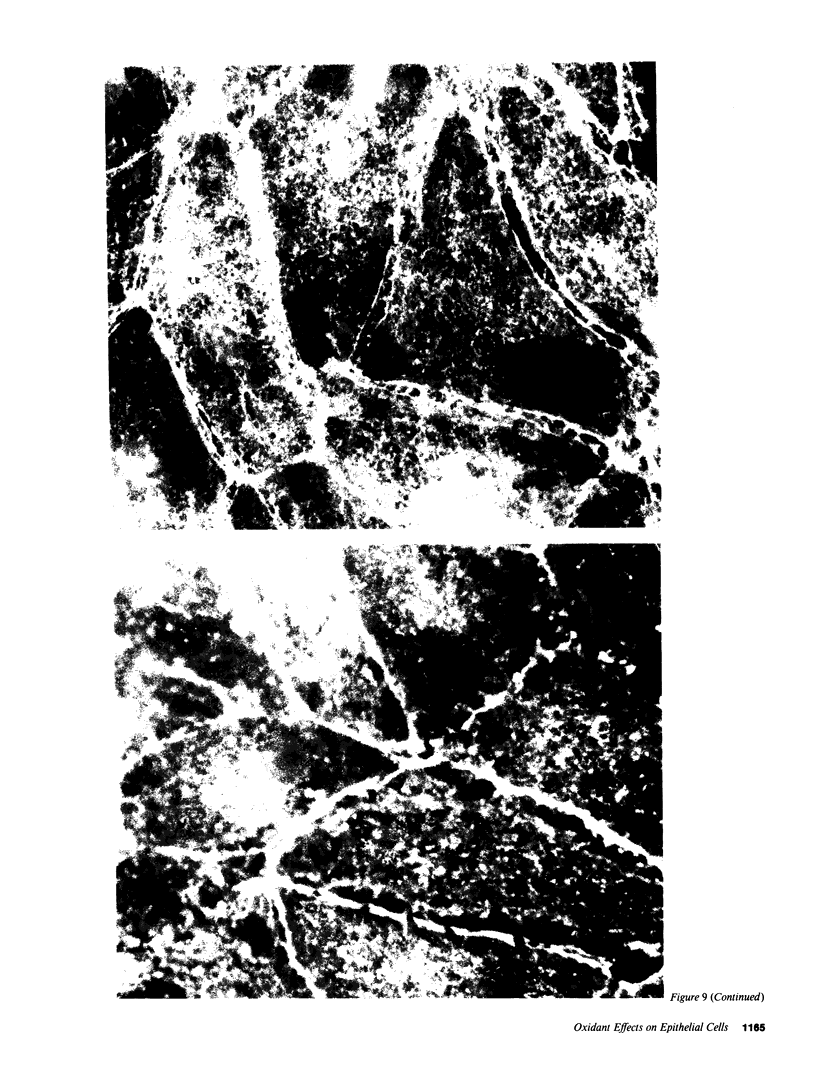
Inflammation of epithelia is an important step in the pathophysiology of a wide variety of diseases. Because reactive oxygen metabolites are important effector molecules of acute inflammation, we examined the effect of oxidants on the barrier function of a cultured epithelium, Madin Darby Canine Kidney cells, by measuring the transepithelial electrical conductance, Gt, of monolayers grown on permeable supports. We found that H2O2, added directly or generated with glucose oxidase, increased Gt. Similar effects were observed with addition of xanthine and xanthine oxidase, a system which enzymatically generates superoxide radical O2-. The oxidant-induced increase in Gt was reversible if the exposure to oxidants was not prolonged (less than 20 min), and if the concentration of H2O2 was less than 5 X 10(-3) M. The increase in Gt suggested that oxidants increase the permeability of the paracellular pathway, a suggestion supported by an oxidant-induced increase in the permeability to 14C-mannitol, which primarily crosses epithelia via the extracellular route. In addition to functional changes in the epithelial monolayer, oxidants changed the cell morphology; after H2O2 exposure, the cells tended to pull apart, most prominently at their basolateral surfaces. These changes were heterogeneous with most areas showing no changes. Some of the morphologic changes could be reversed if the exposure to H2O2 was limited. We also observed a disruption of the normal pattern of the actin-cytoskeleton, particularly in the area of cell to cell junctions, as demonstrated by fluorescent staining of f-actin with rhodamine phallicidin. These functional and structural findings indicate that oxidants increase the permeability of the paracellular pathway in a cultured epithelium. The changes can be reversible, and are accompanied by alterations in organization of the cell cytoskeleton. These studies demonstrate the dynamic nature of the interaction between epithelial cells and oxygen metabolites.
Full text
PDF
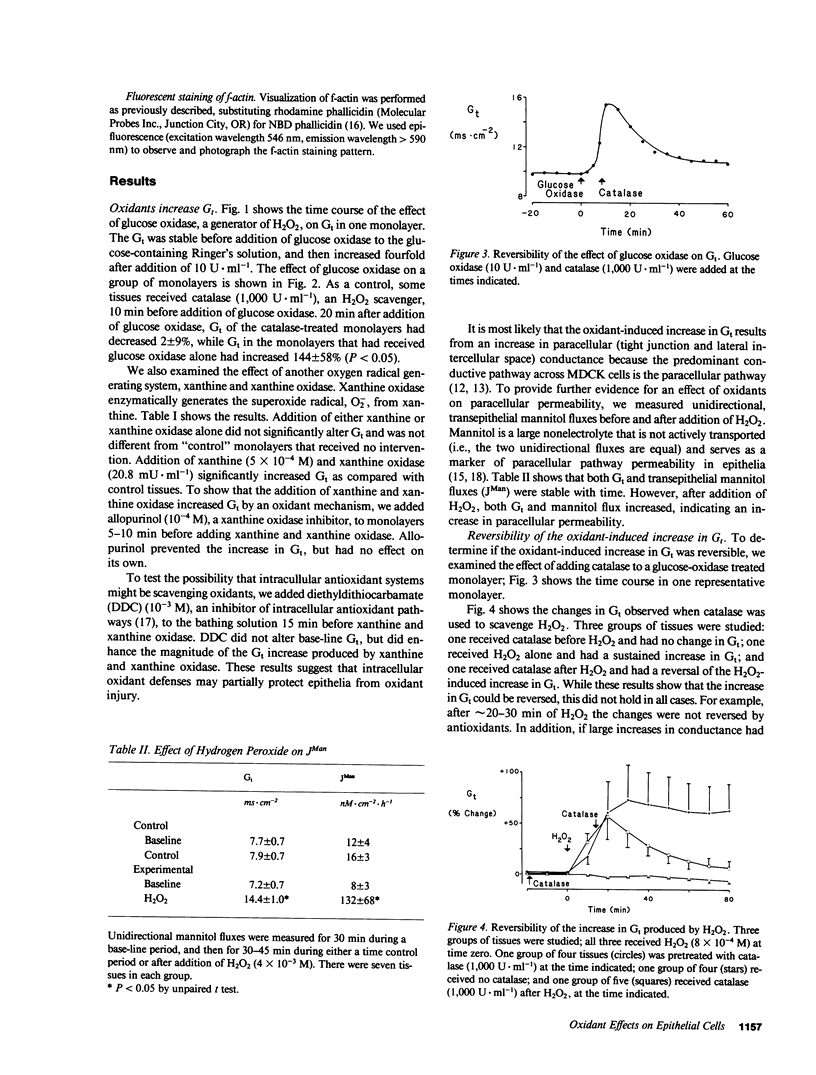








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Gordon J. L. Differential effects of hydrogen peroxide on indices of endothelial cell function. J Exp Med. 1984 Feb 1;159(2):592–603. doi: 10.1084/jem.159.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard G. R., Lucht W. D., Niedermeyer M. E., Snapper J. R., Ogletree M. L., Brigham K. L. Effect of N-acetylcysteine on the pulmonary response to endotoxin in the awake sheep and upon in vitro granulocyte function. J Clin Invest. 1984 Jun;73(6):1772–1784. doi: 10.1172/JCI111386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M., Ehrenfeld J., Meza I., Martínez-Palomo A. Structural and functional membrane polarity in cultured monolayers of MDCK cells. J Membr Biol. 1980;52(2):147–159. doi: 10.1007/BF01869120. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Stefani E., Palomo A. M. Occluding junctions in a cultured transporting epithelium: structural and functional heterogeneity. J Membr Biol. 1980 Mar 31;53(1):19–32. doi: 10.1007/BF01871169. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975 Jun 1;141(6):1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson D. C. Na and Cl transport across the isolated turtle colon: parallel pathways for transmural ion movement. J Membr Biol. 1977 Dec 15;37(3-4):213–233. doi: 10.1007/BF01940933. [DOI] [PubMed] [Google Scholar]
- Del Maestro R. F. An approach to free radicals in medicine and biology. Acta Physiol Scand Suppl. 1980;492:153–168. [PubMed] [Google Scholar]
- Diamond J. M. Tight and leaky junctions of epithelia: a perspective on kisses in the dark. Fed Proc. 1974 Nov;33(11):2220–2224. [PubMed] [Google Scholar]
- Garner W. H., Garner M. H., Spector A. H2O2-induced uncoupling of bovine lens Na+,K+-ATPase. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2044–2048. doi: 10.1073/pnas.80.7.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem. 1977 Oct 10;252(19):6721–6728. [PubMed] [Google Scholar]
- López-Vancell R., Beaty G., Stefani E., Rodríguez-Boulan E. E., Cereijido M. Changes in paracellular and cellular ionic permeabilities of monolayers of MDCK cells infected with influenza or vesicular stomatitis viruses. J Membr Biol. 1984;81(3):171–180. doi: 10.1007/BF01868711. [DOI] [PubMed] [Google Scholar]
- Maridonneau I., Braquet P., Garay R. P. Na+ and K+ transport damage induced by oxygen free radicals in human red cell membranes. J Biol Chem. 1983 Mar 10;258(5):3107–3113. [PubMed] [Google Scholar]
- Martinez-Palomo A., Meza I., Beaty G., Cereijido M. Experimental modulation of occluding junctions in a cultured transporting epithelium. J Cell Biol. 1980 Dec;87(3 Pt 1):736–745. doi: 10.1083/jcb.87.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J. R., Harkin M. M., Johnson K. J., Ward P. A. Suppression by superoxide dismutase of immune-complex--induced pulmonary alveolitis and dermal inflammation. Am J Pathol. 1981 Jan;102(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- Meza I., Ibarra G., Sabanero M., Martínez-Palomo A., Cereijido M. Occluding junctions and cytoskeletal components in a cultured transporting epithelium. J Cell Biol. 1980 Dec;87(3 Pt 1):746–754. doi: 10.1083/jcb.87.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal C. K., Murad F. Properties and oxidative regulation of guanylate cyclase. J Cyclic Nucleotide Res. 1977 Dec;3(6):381–391. [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med. 1976 Nov 2;144(5):1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C., Anderson P., Goodman E., Dunham P., Weissmann G. Phosphatidate and oxidized fatty acids are calcium ionophores. Studies employing arsenazo III in liposomes. J Biol Chem. 1981 Mar 25;256(6):2736–2741. [PubMed] [Google Scholar]
- Shasby D. M., Lind S. E., Shasby S. S., Goldsmith J. C., Hunninghake G. W. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood. 1985 Mar;65(3):605–614. [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Sullivan J. M., Peach M. J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982 Nov;51(5):657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Stefani E., Cereijido M. Electrical properties of cultured epithelioid cells (MDCK). J Membr Biol. 1983;73(2):177–184. doi: 10.1007/BF01870440. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Vanbenthuysen K. M., Shasby D. M., McMurtry I. F., Repine J. E. Oxygen-radical-mediated permeability edema and vasoconstriction in isolated perfused rabbit lungs. Am Rev Respir Dis. 1982 Nov;126(5):802–806. doi: 10.1164/arrd.1982.126.5.802. [DOI] [PubMed] [Google Scholar]
- Thor H., Hartzell P., Orrenius S. Potentiation of oxidative cell injury in hepatocytes which have accumulated Ca2+. J Biol Chem. 1984 May 25;259(10):6612–6615. [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]