Abstract
Background
Breast cancer is the most common cancer in the UK. GPs are encouraged to refer all women whose symptoms may represent cancer, rather than selecting those at highest risk.
Aim
To identify and quantify features of breast cancer in primary care.
Design and setting
A UK case–control study using the Clinical Practice Research Database (CPRD).
Method
Possible features of breast cancer were identified in the year before diagnosis, and odds ratios calculated using conditional logistic regression. Positive predictive values (PPVs) were estimated for consulting women.
Results
A total of 3994 women aged ≥40 years with breast cancer between 2000 and 2009, and 16 873 age-, sex-, and practice-matched controls were studied. Median age at diagnosis was 63 years (interquartile range 55–74 years). Four features were significantly associated with breast cancer: breast lump (odds ratio [OR] 110; 95% confidence interval [CI] = I88 to150), breast pain (OR = 4.2; 95% CI = 3.0 to 6.0), nipple retraction (OR = 26; 95% CI = 10 to 64), nipple discharge (OR = 19; 95% CI = 8.6 to 41): all P-values <0.01. In the year before diagnosis, 1762 (44%) of cases had a breast lump compared with 132 (0.8%) controls. The PPV of breast cancer with a breast lump was 4.8% in women aged 40–49 years, rising to 48% in women aged >70 years. PPVs were lower in women who also reported breast pain.
Conclusion
Generally, the figures support current referral practice. However, the low likelihood of cancer for all the non-lump symptoms means that the current guidance recommends investigation for possible cancer at a more liberal risk threshold than for other cancers. Although supported by patients, this may not meet current NHS criteria for cost–benefit.
Keywords: breast cancer, diagnosis, primary health care
INTRODUCTION
Breast cancer is the most common cancer in UK women, with annually just under 50 000 new diagnoses and 11 700 deaths.1 The lifetime risk of a woman being diagnosed with breast cancer is now 1 in 8. There are two main routes to diagnosis: by screening, or with symptoms. The national screening programme in the UK invites women for 3-yearly mammography from the age of 50 to 70, with extension to 47–73 years underway. This screening programme identifies approximately one-third of total breast cancers.2 The remainder will, in the main, present symptomatically, generally to primary care. Almost all women with breast problems presenting to primary care are recommended for referral to specialist care for cancer to be considered: the exceptions being women <30 years old with a breast lump and suspicious features, or women <50 years old with nipple discharge suggestive of duct ectasia. Women with breast pain are recommended for non-urgent referral if initial treatment is unsuccessful.3 In England, this guidance has largely superseded the 2005 Referral guidelines for suspected cancer.4 There is very little primary care evidence on the features of breast cancer, so the above guidance was largely based on consensus and extrapolation from secondary care evidence. The number of urgent referrals from primary care to a specialist breast unit rose by 42% between 1999 and 2005, while the proportion of women transpiring to have cancer in this urgent group has decreased each year.5
Around 3% of female primary care consultations relate to a breast symptom.6 Textbooks list several symptoms of breast cancer, such as breast lumps,7 nipple bleeding, unilateral eczema (Paget’s disease), and skin changes, such as peau d’orange.4 Symptoms can be present for over a year, and women may delay presentation of their symptoms to their doctor.8,9 Only three relevant, but dated, studies have been published from primary care.6,10,11 The first, from a health maintenance organisation in the US, reported that 16% of the female population aged 40–69 years had consulted about a breast problem in the preceding decade.10 Cancer was detected in 4.5% of these episodes. Pain was the most common breast symptom, with cancer in 1.8%; a likelihood ratio (LR) of 10 was estimated. For breast lumps, 8% were malignant, with an LR of 65. Too few episodes of skin or nipple change were recorded to allow calculation of either of these metrics. A Dutch study identified breast symptoms reported to primary care from 1985 to 2003.6 Breast complaints were most frequent in the 25 to 44-year-old age group, declining steadily as age increased. This matters, as the incidence of breast cancer rises with age. Pain was the most common symptom (LR 10), followed by lump (LR 15), and then nipple complaints (LR 3). Cancer was found in 1%, 8%, and 2% of these women respectively. Finally, a UK study estimated that each GP saw approximately one patient with a breast symptom each week.11
How this fits in
Women are encouraged to be breast aware in order to present symptoms suggestive of cancer to their GP with the minimum of delay. Many women who are referred for further investigation will not have breast cancer. This study confirms the importance of several features including breast lump and pain, nipple discharge, and retraction as significant risk markers. It is the first UK primary care study to estimate the chance of having breast cancer in a patient reporting a symptom suggestive of breast cancer to their GP. The results quantify the risk of breast cancer in women currently referred to secondary care for further investigation and should assist the National Institute for Health and Care Excellence (NICE) in its ongoing update of GP referral guidance.
In other cancers, GPs are expected to select patients for investigation, aided by national guidance.4 The current policy of indiscriminate referral of all breast problems is resource-intensive, and does not use the clinical skills in primary care. It may be advantageous to allow GPs to exercise their traditional ‘gatekeeping’ function in breast cancer referrals. If the risk of cancer is negligible, GPs thereby deliver patient care in the most appropriate setting.12 However, to do so requires primary care research identifying and quantifying the risk of breast cancer in symptomatic women. This study sought to do that.
METHOD
Data sources
This was a case–control study using data from the Clinical Practice Research Database (CPRD) in the UK. The CPRD maintains an anonymised copy of participating practices’ medical records. These contain full information on the patient, including all consultations, recorded symptoms, investigations, and diagnoses. There are stringent checks on validation and data quality.13,14 Similar methods have been used previously for several cancer diagnostic studies.15–17
Identification of cases and controls
A list of 56 breast tumour diagnostic codes (available from the authors) was collated from the CPRD master code library. All women aged ≥40 years with one of these codes diagnosed between 1 January 2000 and 31 December 2009 were identified. This age threshold was used for all cancers in the host programme of research, as positive predictive values (PPVs) were expected to be very low below this age. For each case, five controls, matched to the case by year of birth, sex, and practice, were randomly selected using a computer-generated sequence. Cases and controls with less than one year of data meeting CPRD quality standards before the first diagnostic code were excluded. These stages were performed by CPRD staff. The date of the first cancer code in the records was taken to be the date of diagnosis; this was labelled the index date (index_C; see below). The matched controls were assigned the index date of their case.
The following exclusion criteria were applied: males with breast cancer; ill-defined medcodes giving multiple sites of cancer; skin cancer of the breast; cases with a mastectomy, chemotherapy, or radiotherapy medcode more than 90 days before the index date (as this strongly suggested the index date was wrong); controls diagnosed with breast cancer (or having a mastectomy) before the index date; and women with no consultations in the year before the index date.
Adjustment of index dates
As there can be delay in recording a cancer on GPs’ records, the index dates were advanced where there was strong evidence for the true date of diagnosis being before index_C. The maximum allowed here was 90 days, as this was a reasonable time for administrative reasons to have delayed addition of the breast cancer code to the records. This figure has been used previously in cancer diagnostic studies.18 For patients with a medcode relating to either a relevant surgical procedure (such as mastectomy or lumpectomy) or a non-surgical procedure (chemotherapy or radiotherapy) during the 90-day period before index_C, the index date was advanced to the earliest of these dates within the 90-day period, which was called index_T (index date for treatment). A second stage examined mammography codes. It was assumed that any mammogram code (apart from ‘normal mammogram’) in the 90 days before the new index date was the catalyst for the diagnosis, and so advanced the index date to it, calling it index_M. It was considered that symptom reporting after a positive mammogram would have been influenced by the mammogram, so should be omitted from study.
Selection of possible features of breast cancer
All symptoms, signs, or abnormal investigations previously recorded in the breast cancer literature and cancer charity websites were studied (full list available from authors). For simplicity, these are called ‘features’ from now on. The CPRD stores clinical information on just over 100 000 medcodes, each describing a facet of primary care, such as a symptom. There are several codes for each symptom, differing usually in a qualifier such as duration or severity, so generally containing more information than a specific Read code. All the codes pertaining to individual symptoms were collated into single symptom libraries. All codes for fractures were also identified, as a test for any recording bias between cases and controls (making the assumption that the fracture rate would be approximately equal). Occurrences of symptoms in the year before the index date were identified.
Features were only retained in the study if they occurred in ≥1% of the cases or controls (this was invariably cases). A list of plausible laboratory abnormalities were also assembled a priori using the literature and the authors' clinical knowledge. All abnormal laboratory results in the year before the index date were also identified, using the local laboratory’s normal range, which is supplied with the data. Women without a test were considered to be equivalent to those with a normal result. Some abnormal tests were grouped together, for example, abnormal liver function was defined as the presence of any liver enzyme above the normal range. The variable ‘raised inflammatory markers’ was defined as a raised erythrocyte sedimentation rate, C-reactive protein, or plasma viscosity. These simplifications were necessary because different localities in the UK contributing to the CPRD have different tests available.
Analysis
This followed the methods used in several previous studies, with non-parametric methods used for consultation numbers, as the data was not normally distributed.19 The main analytical method was conditional logistic regression. Analysis was carried out in two stages. First, univariable analysis was performed. Any feature with P<0.1 was retained for the multivariable analysis. The final model was derived from multivariable conditional regression analyses using a threshold P<0.05 for retention. Four clinically plausible interactions (available from authors) were tested in the final multivariable model. Likelihood ratio testing was used to compare models.
Sensitivity analyses removed cases with mammography in the 90 days before index or with in situ tumours, in case they had a different symptom profile. Analyses were performed using Stata (version 11).
Calculation of positive predictive values
Positive predictive values (PPVs) were estimated for features shown to be independently associated with breast cancer in the multivariable analysis using Bayes’ theorem, whereby the posterior odds = the prior odds × the likelihood ratio.20 The latter is a univariable value, not the adjusted value from the multivariable analysis. The prior odds were calculated from the age-specific national incidence rate of breast cancer for 2008.21 This was repeated for pairs of symptoms and for second attendances with the same symptom. As 89% of controls consulted their GP in primary care during the study period, compared with 98% of cases, the posterior odds were divided by 0.91 (=0.89/0.98) to give predictive values for the consulting population. We repeated estimation of likelihood ratios and PPVs stratified into 10-year age bands.
Power calculation
As the number of cases in the CPRD were fixed, a power calculation was performed rather than a sample size calculation. Three thousand cases and 10 000 controls (the initial estimates from the CPRD) provided 97% power (5% two-sided alpha) to detect a change in a rare variable from 2% in cases to 1% of controls. For a more common variable, the study had >95% power to detect a change in prevalence of 20% in cases to 17% in controls.
RESULTS
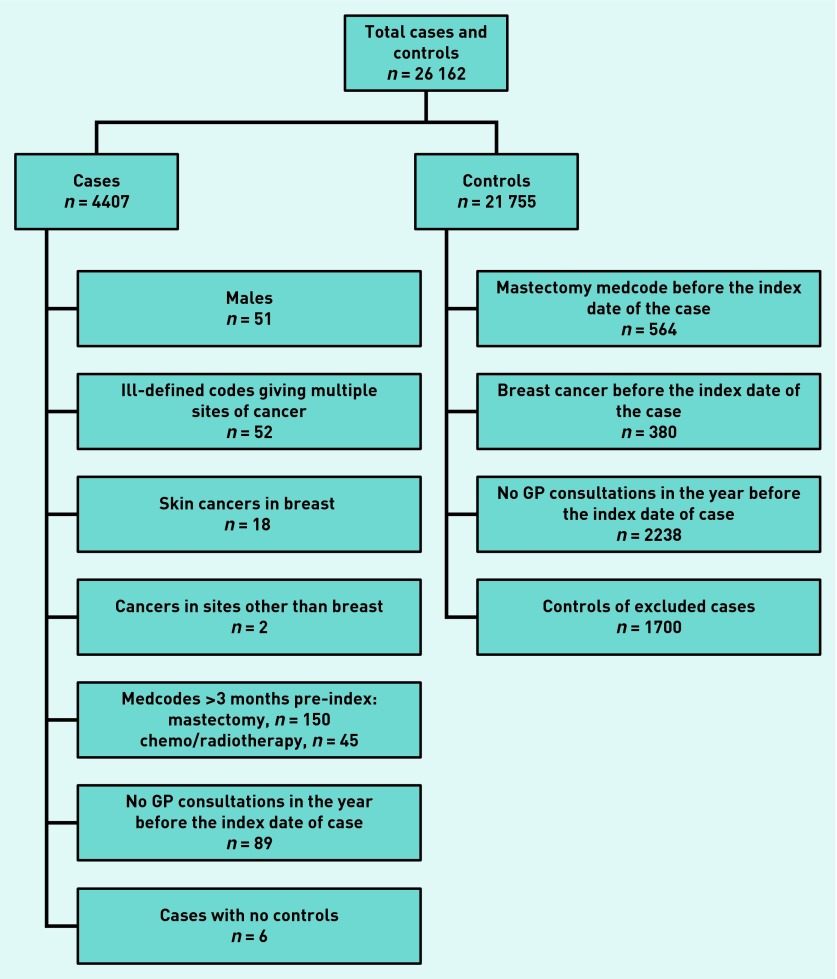
The CPRD provided a total of 26 162 women (4407 cases and 21 755 controls). Application of the exclusion criteria (Figure 1) resulted in 20 867 eligible women (3994 cases and 16 873 controls). The median age at diagnosis was 63 years (interquartile range 55–74 years). The frequency of consultations is given in Table 1.
Figure 1.
Application of exclusion criteria for cases and matched controls.
Table 1.
Consultations in the year before diagnosis
| Cases Median (IQR) (n = 3994) | Controls Median (IQR) (n = 16 873) | Significance (rank sum test) | |
|---|---|---|---|
| Consultations in the year before index | 9 (5–15) | 7 (4–13) | <0.0001 |
| Consultations in the 6 months before index | 9 (4–16) | 6 (2–12) | <0.0001 |
IQR = interquartile range.
Clinical features
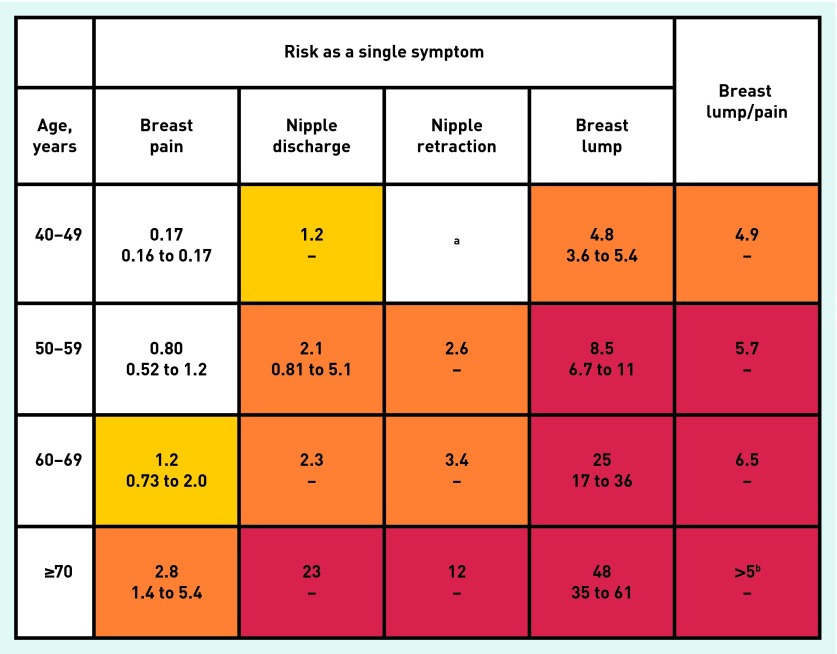
Twenty-three symptoms and 22 abnormal test results were studied initially. These are shown in Appendix 1, along with the stages at which they were omitted. The frequency, univariable likelihood ratio, and multivariable odds ratio for features associated with breast cancer are shown in Table 2. Fractures were similar in 70 cases (1.8%) and 274 controls (1.6%), P = 0.53. A total of 1887 (47%) of the cases had at least one of the four features from Table 2 recorded in their notes, compared with 275 (1.6%) controls. A total of 1762 (44%) of cases had a record of a breast lump compared with just 132 (0.8%) controls. Antagonistic interactions were identified for a woman reporting a breast lump and breast pain (interaction OR = 0.13, P = 0.002) and a breast lump and nipple discharge (interaction OR = 0.02, P = 0.001). Figure 2 shows the PPVs for breast cancer by age group for women reporting symptoms to their GP. PPVs were calculated only for breast lump combined with breast pain as the numbers were insufficient for reliable estimates of other symptom combinations.
Table 2.
Clinical features of breast cancer (all ages)
| Symptom in the preceding year | Cases, n (%) n = 3994 | Controls, n (%) n = 16 873 | Likelihood ratio (95% CI) | Odds ratio in multivariable analysis (95% CI) |
|---|---|---|---|---|
| Breast lump | 1762 (44.1) | 132 (0.8) | 56 (47 to 67) | 110 (88 to 150) |
| Breast pain | 95 (2.4) | 127 (0.8) | 3.2 (2.4 to 4.1) | 4.2 (3.0 to 6.0) |
| Nipple retraction | 40 (1.0) | 9 (0.1) | 19 (9.1 to 39) | 26 (10 to 64) |
| Nipple discharge | 41 (1.0) | 15 (0.1) | 12 (6.4 to 21) | 19 (8.6 to 41) |
Note: All associations in the model have P < 0.001.
Figure 1.
Positive predictive value (PPV) for breast cancer for women reporting features of the cancer to primary care (aged ≥40 years). 1) The top figure in each cell is the PPV when the feature is present. The two smaller figures represent the 95% CIs for the PPV. These have not been calculated when any cell in the 2×2 table was < 5 (invariably this was because too few controls had the feature). 2) The yellow shading is for symptoms with a PPV > 1.0%; the orange shading is when the PPV is >2.0%; and the red shading is for PPVs >5.0%. 3) The breast lump/pain column is the PPV when a woman has reported both a breast lump and breast pain at least once each during the year before the index date. aNipple retraction was only reported by 4 cases and 0 controls aged 40–49 years. Thus no PPV can be calculated, although it is likely to be high. bBreast lump and breast pain were reported by 8 cases and 0 controls. Again, no PPV can be calculated, but given the relatively large number of cases with this combination, it has been estimated as >5%.
The two sensitivity analyses (removing cases and their corresponding controls from the final multivariable model where (1) cases had a mammogram in the 90 days before their index date or (2) where the case’s initial diagnosis of breast cancer was for carcinoma in situ) made little material change to the results. Of the 3994 cases, 967 (24%) had an abnormal mammogram at any time before their diagnosis, but only 236 (5.9%) of these were in the 90 days immediately before diagnosis.
DISCUSSION
Summary
This is the first study into the risk of clinical features of breast cancer in primary care during the mammographic era. As expected, most of the symptoms reported from secondary care studies were also strongly associated with breast cancer in primary care. The risk of breast cancer in a woman presenting with breast pain in particular was relatively low, though higher in women presenting with a breast lump, nipple retraction, or nipple discharge; all of these risks increasing with age. The strong antagonistic interaction between breast lump and pain suggests that painful lumps are less predictive of cancer than painless ones.
Strengths and limitations
This study is large, and uses primary care data. This is crucial, because selection of women for investigation is performed by primary care, so primary care data must be used to study the selection process. The CPRD is the largest and most established of the longitudinal patient databases from primary care. It has been used in nearly 1000 research papers and its validity has been well documented.13,14,22 The patient population in the database is also broadly representative of the UK population. Additionally, laboratory results are transmitted directly to the database, allowing us to use the local normal range to identify abnormal results, and avoiding transcription errors (though no laboratory result proved to be significant).
It was not possible to check the accuracy of diagnosis in the cases by histology, or determine the staging. However, most cases had multiple records of breast cancer. It is unlikely that such a serious disease would be recorded incorrectly with any frequency. Our dataset omitted women <40 years old, so we cannot know what the risk for the 30–39 year age group is — though they are currently recommended for referral. However, the chief limitation is that accurate recording of symptoms by GPs were relied upon. Most symptoms are recorded in the main field of the records, but some are in an inaccessible part of the CPRD — the so-called ‘free-text’ area. Encouragingly, a recent study of ovarian cancer identified relatively little hidden data in these fields.23 When calculating likelihood ratios and PPVs, under-recording is only important if the proportion of under-recording was differentially higher in either cases or controls. Furthermore, concern that cases had more opportunity for having symptoms recorded — by virtue of their slightly higher number of consultations — is lessened by the study's fracture analysis, where there was no difference between cases and controls.
The size of the CPRD database allowed for the study of multiple symptoms in the same woman and the combination of pain and lump. Furthermore, symptom recording was prospective, in that the doctor did not know if it would transpire that the woman would have breast cancer at the time of symptom recording. The decision to use national statistics to estimate the prior odds of cancer was deliberate. They are highly accurate, and sidestep any possible sampling errors from the alternative, which is estimating the incidence of breast cancer in the CPRD itself.
Comparison with existing literature
Women with breast cancer attended their doctors a median of nine times in the year before diagnosis, compared with seven times for controls; this excess is less extreme than seen in other cancers in similar cancer studies.15,16,24 The findings also mirror data from the National Cancer Patient Experience Survey (which included male breast cancer), where only 7.4% of breast cancer patients reported consulting their GP at least three times before diagnosis.25 Both findings probably reflect current recommendations for GPs, to refer most women presenting with symptoms of breast cancer to secondary care without further investigation.
Most other primary care research is dated, and so of limited value, particularly in the mammography era. The current study's results are broadly comparable with the reports from the US and the Netherlands: both of these studies estimated a PPV from lump of 8%, whereas this study ranged from 4.8–48% depending on age. Similarly, pain had PPVs of 1% and 1.8% previously — in this study the figure was 0.17–2.8%. Likelihood ratios were similar for lump, higher for nipple symptoms, and lower for pain. No material difference was identified in our sensitivity analysis excluding women with a recorded screening mammography; this should be interpreted with caution, as we do not know how complete recording of mammographic screening is.
Implications for practice
The findings strongly support referral for investigation when a woman has a breast lump. Furthermore, patients value discussing cancer risks,26 and it may be helpful to offer referred women rough guidance on the likelihood of their lump transpiring to be cancer. Even at the lowest age band, 40–49 years, women had a risk of 4.8%. Most patients would request cancer investigation at levels well below this.26 Breast pain accompanying a lump generally reduced the risk, though not enough to avert referral. This is an area where more research may be able to define a subset of women in whom referral could be avoided — our categories of lump and pain are broad, and low-risk subgroups may be identifiable. Database research has its limitations in that qualifiers for symptoms are often omitted, such as duration, severity, and symptoms deemed of lesser importance, so future work on painful breast lumps will have to use other methods. As few women had both pain and lump recorded, this is one of the less robust findings — though no previous primary care study has reported the combination.
Risks with the two nipple symptoms, retraction and discharge, were much lower, and for women below 60 they were in the range of 1–3%. Furthermore, nipple complaints were surprisingly rare, although the prevalence was approximately twice that reported in a recent primary care study of breast complaints, with 210 (0.1%) women of 84 285 reporting a nipple problem in that study.6 The breast pain findings also support current recommendations. The risk of cancer is low, but not zero, so non-urgent referral appears appropriate (the focus may not necessarily be on excluding cancer, but perhaps on treatment).
Few cancer referral recommendations in National Institute for Health and Care Excellence (NICE) guidance relate to risks below 5%, so, arguably, the current recommendations for referral with almost all breast symptoms is offering a rapid service valued by patients,26 though at the cost of high proportions of the non-lump referrals transpiring to be benign. No health economic assessment of providing such a comprehensive service has been published, and this is a clear research direction that warrants attention. It is recognised that false-positive mammograms can induce considerable anxiety in women. It is certainly plausible that referral for investigation may also do this, despite no cancer being found. Thus analysis of the overall benefits/harms of the current high-referral strategy for breast disease needs to include clinical as well as economic costs.
This large study of breast cancer symptoms has been able to estimate risks of cancer for the main symptoms previously reported. The PPVs can help GPs and women, even when referral is actioned. Most women will be revealed not to have cancer — and GPs can share the PPVs with the woman. Generally, the figures support current referral practice, though the low likelihood of cancer for all the non-lump symptoms means that the current guidance suggests investigation for possible cancer at a more liberal risk threshold than for most other cancers. This is supported by patients, but may not meet current NHS criteria for cost–benefit.
Appendix 1.
Symptoms and abnormal test results
| Cases, n n = 3994 | Cases, % |
Controls, n n = 16 873 |
Controls, % | Exclusion reason | |
|---|---|---|---|---|---|
| Symptoms | |||||
| Fatigue (1st occurrence) | 150 | 3.8 | 605 | 3.6 | UV failure |
| Fatigue (2nd occurrence) | 13 | 0.3 | 75 | 0.4 | <1% frequency |
| Chest pain (1st occurrence) | 173 | 4.3 | 685 | 4.1 | UV failure |
| Chest pain (2nd occurrence) | 43 | 1.1 | 146 | 0.9 | UV failure |
| Cough (1st occurrence) | 396 | 9.9 | 1733 | 10.3 | UV failure |
| Cough (2nd occurrence) | 113 | 2.8 | 469 | 2.8 | UV failure |
| Cough (3rd occurrence) | 36 | 0.9 | 174 | 1.0 | UV failure |
| Loss of appetite (1st occurrence) | 9 | 0.2 | 36 | 0.2 | <1% frequency |
| Weight loss (1st occurrence) | 33 | 0.8 | 101 | 0.6 | <1% frequency |
| Breast lump (1st occurrence) | 1762 | 44.1 | 132 | 0.8 | |
| Breast lump (2nd occurrence) | 322 | 8.1 | 36 | 0.2 | |
| Breast lump (3rd occurrence) | 47 | 1.2 | 2 | 0.0 | |
| Breast fibroadenoma | 5 | 0.1 | 4 | 0.0 | <1% frequency |
| Breast pain (1st occurrence) | 95 | 2.4 | 127 | 0.8 | |
| Breast pain (2nd occurrence) | 13 | 0.3 | 14 | 0.1 | <1% frequency |
| Breast nodularity | 8 | 0.2 | 2 | 0.0 | <1% frequency |
| Nipple bleed | 0 | 0.0 | 0 | 0.0 | <1% frequency |
| Nipple retraction | 37 | 0.9 | 8 | 0.0 | |
| Nipple discharge | 37 | 0.9 | 14 | 0.1 | |
| Paget’s nipple/eczema | 5 | 0.1 | 4 | 0.0 | <1% frequency |
| Nipple pain | 16 | 0.4 | 3 | 0.0 | <1% frequency |
| Breast skin changes | 3 | 0.1 | 0 | 0.0 | < 1% frequency |
| Breast cyst | 9 | 0.2 | 23 | 0.1 | < 1% frequency |
| Breast infection | 7 | 0.2 | 4 | 0.0 | < 1% frequency |
| Breast (other) | 3 | 0.1 | 0 | 0.0 | < 1% frequency |
| Axillary lymphadenopathy | 3 | 0.1 | 0 | 0.0 | < 1% frequency |
| Cervical lymphadenopathy | 0 | 0.0 | 11 | 0.1 | < 1% frequency |
| Fever/sweating | 12 | 0.3 | 63 | 0.4 | < 1% frequency |
| Hair loss | 8 | 0.2 | 29 | 0.2 | < 1% frequency |
|
| |||||
| Tests | |||||
| Low calcium | 33 | 0.8 | 89 | 0.5 | < 1% frequency |
| High calcium | 24 | 0.6 | 73 | 0.4 | < 1% frequency |
| High cholesterol | 650 | 16.3 | 2699 | 16.0 | UV failure |
| High creatinine | 172 | 4.3 | 685 | 4.1 | UV failure |
| Low gamma | 3 | 0.1 | 29 | 0.2 | < 1% frequency |
| High gamma | 48 | 1.2 | 160 | 0.9 | UV failure |
| Low glucose | 8 | 0.2 | 33 | 0.2 | < 1% frequency |
| High glucose | 257 | 6.4 | 1022 | 6.1 | UV failure |
| Low haemoglobin | 167 | 4.2 | 671 | 4.0 | UV failure |
| High haemoglobin | 26 | 0.7 | 85 | 0.5 | <1% frequency |
| High inflammatory markers (IM) | 168 | 4.2 | 639 | 3.8 | UV failure |
| High liver function tests (LFT) | 311 | 7.8 | 1223 | 7.2 | UV failure |
| Low mean corpuscular volume (MCV) | 56 | 1.4 | 218 | 1.3 | UV failure |
| High MCV | 67 | 1.7 | 297 | 1.8 | UV failure |
| Low platelets | 33 | 0.8 | 123 | 0.7 | <1% frequency |
| High platelets | 91 | 2.3 | 369 | 2.2 | UV failure |
| Low sodium | 121 | 3.0 | 435 | 2.6 | UV failure |
| High sodium | 24 | 0.6 | 116 | 0.7 | <1% frequency |
| High thyroid-stimulating hormone (TSH) | 103 | 2.6 | 413 | 2.4 | UV failure |
| High urate | 7 | 0.2 | 23 | 0.1 | <1% frequency |
| Low white cell count (WCC) | 34 | 0.9 | 195 | 1.2 | UV failure |
| High WCC | 98 | 2.5 | 371 | 2.2 | UV failure |
Notes: 1) Frequency failures — frequency <1% in each of cases and controls. Nipple retraction and nipple discharge were just below 1% in univariate models but included in the multivariate model. 2) UV failure — P >0.1 in the univariable model.
Funding
The Policy Research Unit in Cancer Awareness, Screening and Early Diagnosis receives funding for a research programme from the Department of Health Policy Research Programme. It is a collaboration between researchers from seven institutions (Queen Mary University of London, University College London, King’s College London, London School of Hygiene and Tropical Medicine, Hull York Medical School, Durham University, and Peninsula Medical School). The views expressed are those of the authors and not necessarily those of the NHS or the Department of Health.
Ethical approval
Ethical approval was granted by the Independent Scientific Advisory Committee (ISAC) in November 2009, reference number 09_111.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
William Hamilton is the clinical lead for the upcoming NICE revision of cancer referral guidelines. There are no other relevant competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Cancer Research UK. CancerStats — incidence 2009 — UK. London: Cancer Research UK; 2012. http://publications.cancerresearchuk.org/downloads/Product/CS_CS_INCIDENCE.pdf (accessed 14 Oct 2014) [Google Scholar]
- 2.Health and Social Care Information Centre. Breast screening programme, England, 2011–12. Leeds: HSCIC; 2013. https://catalogue.ic.nhs.uk/publications/screening/breast/bres-scre-prog-eng-2011-12/bres-scre-prog-eng-2011-12-rep.pdf (accessed 14 Oct 2014) [Google Scholar]
- 3.Willett A, Michell MJ, Lee MJR. Best practice diagnostic guidelines for patients presenting with breast symptoms. COI; 2010. http://www.associationofbreastsurgery.org.uk/media/4585/best_practice_diagnostic_guidelines_for_patients_presenting_with_breast_symptoms.pdf (accessed 14 Oct 2014) [Google Scholar]
- 4.National Institute for Health and Care Excellence. Referral guidelines for suspected cancer. London: NICE; 2005. NICE clinical guideline 27 (CG27) https://www.nice.org.uk/guidance/cg27/resources/guidance-referral-guidelines-for-suspected-cancer-pdf (accessed 14 Oct 2014) [Google Scholar]
- 5.Potter S, Govindarajulu S, Shere M, et al. Referral patterns, cancer diagnoses, and waiting times after introduction of two week wait rule for breast cancer: prospective cohort study. BMJ. 2007;335(7614):288–291. doi: 10.1136/bmj.39258.688553.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberl MM, Phillips RL, Jr, Lamberts H, et al. Characterizing breast symptoms in family practice. Ann Fam Med. 2008;6(6):528–533. doi: 10.1370/afm.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anonymous. The palpable breast lump: information and recommendations to assist decision-making when a breast lump is detected. The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Canadian Association of Radiation Oncologists. CMAJ. 1998;158(Suppl 3):S3–S8. [PubMed] [Google Scholar]
- 8.Bish A, Ramirez A, Burgess C, Hunter M. Understanding why women delay in seeking help for breast cancer symptoms. J Psychosom Res. 2005;58(4):321–326. doi: 10.1016/j.jpsychores.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Ramirez A, Westcombe A, Burgess C, et al. Factors predicting delayed presentation of symptomatic breast cancer: a systematic review. Lancet. 1999;353(9159):1127–1131. doi: 10.1016/s0140-6736(99)02142-x. [DOI] [PubMed] [Google Scholar]
- 10.Barton MB, Elmore JG, Fletcher SW. Breast symptoms among women enrolled in a health maintenance organization: frequency, evaluation, and outcome. Ann Intern Med. 1999;130(8):651–657. doi: 10.7326/0003-4819-130-8-199904200-00005. [DOI] [PubMed] [Google Scholar]
- 11.Newton P, Hannay DR, Laver R. The presentation and management of female breast symptoms in general practice in Sheffield. Fam Pract. 1999;16(4):360–365. doi: 10.1093/fampra/16.4.360. [DOI] [PubMed] [Google Scholar]
- 12.Department of Health. Cancer reform strategy. London: DoH; 2007. http://www.nhs.uk/NHSEngland/NSF/Documents/Cancer%20Reform%20Strategy.pdf (accessed 14 Oct 2014) [Google Scholar]
- 13.Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract. 2010 doi: 10.3399/bjgp10X483562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrett E, Thomas SL, Schoonen WM, et al. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shephard E, Stapley S, Neal RD, et al. Clinical features of bladder cancer in primary care. Br J Gen Pract. 2012 doi: 10.3399/bjgp12X654560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor A, Stapley S, Hamilton W. Jaundice in primary care: a cohort study of adults aged >45 years using electronic medical records. Fam Pract. 2011;29(4):416–420. doi: 10.1093/fampra/cmr118. [DOI] [PubMed] [Google Scholar]
- 17.Stapley S, Peters TJ, Neal RD, et al. The risk of oesophago-gastric cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer. 2013;108(1):25–31. doi: 10.1038/bjc.2012.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliss-Brookes L, McPhail S, Ives A, et al. Routes to diagnosis for cancer: determining the patient journey using multiple routine data sets. Br J Cancer. 2012 doi: 10.1038/bjc.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton W. The CAPER studies: five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer. 2009;101(Suppl 2):S80–S86. doi: 10.1038/sj.bjc.6605396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knottnerus JA, editor. The evidence base of clinical diagnosis. London: BMJ Books; 2002. [Google Scholar]
- 21.Cancer Research UK. Incidence statistics. London: Cancer Research UK; 2008. http://info.cancerresearchuk.org/cancerstats/incidence/ (accessed 14 Oct 2014) [Google Scholar]
- 22.Fombonne E, Heavey L, Smeeth L, et al. Validation of the diagnosis of autism in general practitioner records. BMC Public Health. 2004;4:5. doi: 10.1186/1471-2458-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tate AR, Martin AGR, Ali A, Cassell JA. Using free text information to explore how and when GPs code a diagnosis of ovarian cancer: an observational study using primary care records of patients with ovarian cancer. BMJ Open. 2011 doi: 10.1136/bmjopen-2010-000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton W, Round A, Sharp D, Peters T. Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer. 2005;93(4):399–405. doi: 10.1038/sj.bjc.6602714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyratzopoulos G, Neal RD, Barbiere JM, et al. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 2012;13(4):353–365. doi: 10.1016/S1470-2045(12)70041-4. [DOI] [PubMed] [Google Scholar]
- 26.Banks J, Hollinghurst S, Bigwood L, et al. Preferences for cancer investigation: a vignette-based study of primary-care attendees. Lancet Oncol. 2014;15(2):232–240. doi: 10.1016/S1470-2045(13)70588-6. [DOI] [PubMed] [Google Scholar]