After cardiovascular causes, infectious diseases are the next most common cause of death for dialysis patients. The Japanese Society for Dialysis Therapy reported an increased standardized mortality of 7.5-fold [95% confidence limits (CI) 7.3–7.6] for infectious diseases between 2008 and 2009 compared with the general Japanese population. The increased mortality rates for dialysis patients were greatest for sepsis, followed in descending order by peritonitis, influenza, tuberculosis and pneumonia [1]. Patients with chronic kidney disease are more susceptible to some infections, as the azotaemic state alters innate immunity, with reports of reduced monocyte Toll-like receptor 4 expression [2], reduced B-lymphocyte cell populations [3] and impaired polymorphonuclear chemotaxis and phagocytosis [4]. It has also been proposed that changes in the gastrointestinal microbiota, and increased intestinal permeability to endotoxin, lead to a persistent activation of the innate immune system, resulting in the induction of immune-regulatory mediators which then suppress both innate and adaptive immunity [5]. Additionally, immune responses may also be impaired by poor nutritional status, malnutrition and vitamin D deficiency [6].
For many years, it has been recognized that haemodialysis and peritoneal dialysis (PD) patients have a reduced response to vaccination, in terms of developing seroprotective antibody levels, to a number of the commonly available vaccines including hepatitis B, pneumococcus, influenza A H1N1 and tetanus toxoid [7]. The risk of mortality with chest infections in the chronic dialysis patient has been reported to be 14- to 16-fold higher than that for the general population, with >50% of lower respiratory tract infections caused by Streptococcus pneumoniae [8]. Lower respiratory tract infections have additionally been reported to increase the relative risk for cardiovascular events by 3.02 (95% CI: 2.87–3.02) in dialysis patients with pneumonia [9]. Most haemodialysis patients gain fluid between dialysis sessions [10], and this increase in extracellular volume includes lung water. As such, lung water content is increased even in healthy haemodialysis outpatients without any respiratory symptoms prior to dialysis [11]. Whereas it was readily established that the mouth and upper airway are extensively colonized by bacteria, it is only relatively recently that it has been recognized that the lung is also normally colonized. The average person inhales 8000 L of air each day, containing 104–106 bacterial cells per cubic metre. The internal surface area of the lung is some 30 times that of the skin and the lung microbiome is determined by the balance between microbial immigration, elimination and the relative growth rates of different bacteria [12]. The proportion of resident to transient microbes remains to be determined in healthy subjects, and it remains to be established how chronic kidney disease and dialysis affect the lung microbiome. Surfactant in the distal alveoli has bacteriostatic activity against some bacterial species, and it is unknown whether this bacteriostatic activity is impaired in dialysis patients. Similarly, other changes in innate and adaptive immunity will affect the risk for pulmonary infections. The lung microbiome is altered by hypoxia [12], and increased water lung content in the dialysis patient will increase hypoxia in dependent areas of the lung. As such these changes may help explain the increased risk of pulmonary infections in dialysis patients. This risk for pulmonary infections appears to be much greater for haemodialysis patients, and although this may be related to greater changes in lung water content during the dialysis week compared with PD patients, haemodialysis patients additionally often travel together to and from dialysis centres and wait together at the start and end of dialysis sessions increasing the risk of respiratory pathogen transmission, whereas PD is a home-based therapy. Inflammatory changes in the lung have been shown to lead to changes in other organs, the so-called organ ‘cross talk’, and the combination of pulmonary inflammation and increased lung water may account for the increased cardiovascular events reported [9]. To reduce the risk of pulmonary infections, most economically developed countries recommend vaccination programmes for dialysis patients, not only annual influenza vaccinations, but also pneumococcal and haemophilus influenzae vaccines.
In addition to changes in the immune system, dialysis techniques also potentially introduce additional risk factors for infection. Haemodialysis patients who dialyze using central venous access catheters are at the highest risk of access-related infection, followed by arteriovenous grafts, then arteriovenous fistulae [13], and more recently it has been recognized that needling practices may also affect the risk for infection, with greater risk of fistula-associated infection with buttonhole cannulation [14]. It is now acknowledged that the greatest risk for mortality is when patients transition from non-dialysis chronic kidney disease to dialysis, with mortality rates often greater than those of patients opting for non-dialysis conservative care [15]. This excess mortality is linked to unplanned starts with central venous catheter access [16]. As such many renal units have introduced evidence-based clinical care bundles to reduce the rates of haemodialysis access-related infections. Peritoneal dialysis patients are also at increased risk of access-related exit-site infections and peritonitis, and the International Society for Peritoneal Dialysis similarly has recommended a series of interventions designed to reduce infection rates [17].
In this issue of the journal, Van Diepen et al. reviewed the case notes of 452 incident dialysis patients starting dialysis between 1997 and 2007 and followed while being treated by their primary dialysis modality until 2009. Data on all infectious complications were retrospectively collected and the rates and types of infection were examined, according to dialysis modality. Their study concluded that after taking into account as many confounding factors as possible, infection rates were higher for haemodialysis patients over the first 6 months, but overall PD patients had a higher infection risk, which was mainly attributable to dialysis-related technique infections. Whether this increased risk for infections not requiring hospitalization could simply be accounted for by PD catheter exit infections is unclear. However, their results suggested an increased risk for non-dialysis technique-related infections in haemodialysis patients, such as sepsis and pneumonia. Other studies have also tried to answer the question as to whether the choice of dialysis modality confers a higher risk for infection and results have been mixed, with some reporting increased infection risk for haemodialysis and others for PD. The major difference between Van Diepen et al.'s study and earlier reports is their exhaustive effort to adjust for possible confounding factors, using additional statistical analysis, which adds extra validity to their conclusions. The Khan comorbidity score, which has been previously validated in dialysis patients, was significantly higher in those patients initiating haemodialysis compared with PD. During their retrospective case note reviews, data on infections, which occurred in both outpatient and hospital setting, were collated. After adjustment for comorbidity [18], PD patients were found to have had more infections. However, this group had a greater number of less severe infections as the association between infection risk and modality was weaker when only infection-related hospitalizations were considered. The authors detailed how they defined infection during their case note review, but the study would have been strengthened if they had used a defined standard set of agreed criteria, such as the Centre for Disease Control/National Healthcare Safety Network surveillance definitions [19]. Patients were followed for an average of nearly 2 years on their first dialysis modality, and the risk for infection was higher in the haemodialysis group for the first 6 months, when patients were more likely to be dialysing using central venous catheters. Data on vascular access type was not available at the start of the study, with only a snapshot of vascular access available at 3 months [16]. To really help understand why infection risk fell during the first 3 months in their haemodialysis cohort, changes in the proportion of patients dialyzing using central venous catheters and arteriovenous fistulae are crucial.
To reduce infection in haemodialysis patients dialysing in the Royal Free London network, we implemented a clinical care bundle approach and introduced a number of evidence-based interventions. From 2010 to 2012, arteriovenous fistula dialysis access rates increased from 64 to 78%. We introduced a rolling 3 monthly Staphylococcus aureus nasal screening programme, with all positive patients decolonized using a 5-day course of nasal mupirocin and chlorhexidine body and hair washing. Patients dialysing using a central venous catheter had their exit-site examination recorded at each dialysis session and antibiotics promptly started if there was evidence of local infection. Chlorhexidine impregnated dressings were used at the exit site for the first 6 months and catheters locked with 46% citrate solution [20]. High-risk patients [i.e. those with a previous S. aureus (SA) bacteraemia or those who are consistently positive for SA on nasal carriage] had mupirocin additionally applied at the exit site post-dialysis. An active surveillance programme has been running since the start of 2010, with antibiotic start data, catheter infection rates and S. aureus bacteraemia (SAB) rates calculated and fedback to our individual dialysis centres and at the dialysis group level every 6 months. From 2010 to 2012, catheter access infection rates have fallen from 0.99/1000 catheter days in the first 6 months of 2010 to 0.42 in the latter 6 months of 2012 (R2 linear test of trend = 0.86). The reduction in both catheter-related SAB rates and SAB rates overall were less marked over this time period (Figure 1). This may well have been due to the rolling out of a buttonhole fistula needling policy as the first-line approach for needling arteriovenous fistulae in 2010, following which we then observed a rise in SAB in this set of patients [14]. Despite the introduction of a strict fistula disinfection and needle tracking policy, the infection rates did not fall and buttonhole needling of fistulae was subsequently withdrawn at the end of 2012.
FIGURE 1:
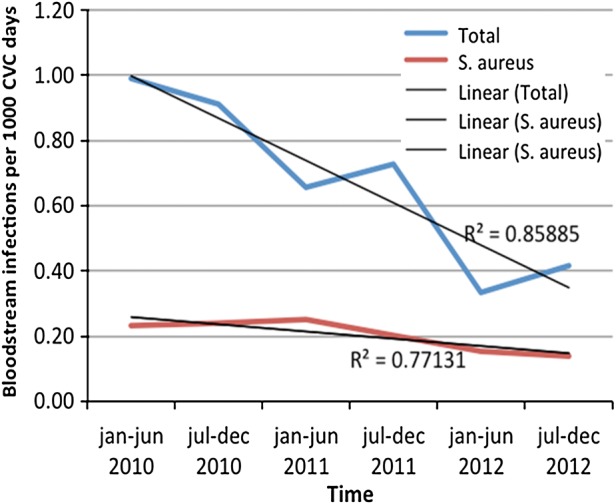
The effect of changes in clinical practice with introduction of active nasal and exit site screening and for haemodialysis patients dialysing with central venous access catheters in combination with a preventative combination bundle approach on all and SA blood stream infections 2010–2012.
In Van Diepen et al.'s study, PD patients predominantly suffered from dialysis technique-related infections [21]. It has been documented for some time that PD peritonitis remains the commonest cause of patients transferring from PD to haemodialysis [22]. Typically most cases are caused by Gram-positive organisms, which often migrate from the skin and colonize the PD catheter [23]. Infection can also follow a failure to follow sterile precautions when performing PD exchanges, external contamination, gastrointestinal bacterial translocation, haematogenous spread and occasionally following gynaecological and rectal instrumentation. In addition, fungal infections may occur, particularly after preceding broad-spectrum antibiotic prescription [24]. At the time of their study, surveillance of nasal S. aureus and eradication therapy was not part of their routine clinical practice, and similarly neither were PD exit-site antibiotics routinely prescribed [17], and as such the number of exit site and tunnel infections reported may have been somewhat higher than would be expected today. Although the International Society for Peritoneal Dialysis Clinical Guidelines recommend the use of prophylactic exit site antibiotics, or nasal antibiotics or both [17], and this may reduce the incidence of exit-site infections, the effect on reducing peritonitis rates is somewhat variable in clinical practice [25], with some centres which have a low background rate of PD peritonitis reporting minimal or no beneficial effect, whereas centres with higher background peritonitis rates reporting a reduction [26].
The dialysis infection literature would benefit from an equally thorough but more up-to-date review of the question as to whether one dialysis modality confers a greater infection risk. This information could then be used to counsel patients when they have to choose a dialysis modality. As with other surveillance data on infection, it could be used to target infection prevention interventions. Evidence-based guidelines for the prevention of infection in dialysis patients are now readily available [17, 27]. A review of infection rates is needed in from those centres where these infection prevention bundles have been introduced, as clinical practice varies widely. For example, catheter insertion may be undertaken in surgical theatres, radiological intervention suites or ward procedure rooms with variation in skin cleaning preparations, use of prophylactic antibiotics including antibiotic choice, dosage and duration of prophylaxis, SA eradication therapy and pre-insertion topical exit-site care. Central venous dialysis catheter choice may also affect the risk for infection, not only in terms of whether tunnelled and cuffed, but also the effect of differing designs; dual lumen versus two single-lumen catheters, biomaterials, catheter surface smoothness, size and composition of catheter cuffs and more recently coating with heparin, antiseptics, antibiotics, silver and bismuth. Thereafter, catheter care varies between centres in terms of whether aseptic precautions are used for catheter connection and disconnection, topical exit-site care and the use of catheter locks. As each of these components of the clinical care bundle designed to reduce infection risk has an economic cost, it is important for both the patient and also for healthcare economics to ascertain which components were most successful in reducing the risk of infection, and equally which were least effective.
Although the introduction of clinical care infection prevention bundles has reduced the incidence of catheter-associated bacteraemias in haemodialysis patients, similar care pathways have not substantially reduced the risk of peritonitis in European PD patients [26, 28]. Peritonitis rates have historically been lower in Hong Kong and Japan compared with Northern Europe, North America and Australia. Although there are differences in climate, the bacterial causes of peritonitis are similar, with Gram-positive skin commensals, followed by SA predominating [17], suggesting that the skin microbiome is not substantially different. Dietary intake may differ, and changes in the diet, for example, soy protein-rich diets and those rich in plant-derived polysaccharides or resistant starches are known to alter the gastrointestinal microbiota [5]. Increased gut permeability and bacterial translocation are the most likely cause for the majority of episodes of gut microbiome-derived Gram-negative and Gram-negative peritonitis. Both elderly haemodialysis and PD patients are at increased risk of diverticular disease due to the combination of fluid restriction and dietary modifications designed to reduce phosphate and potassium intake. Changes in the gut microbiome alter innate and adaptive immunity, leading to effects at distant sites. The combination of these effects may account for the increased risk for developing peritonitis with bacteria originating from the skin microbiome. On the other hand, the introduction of neutral pH and low glucose degradation product PD solutions has not made any significant impact on reducing peritonitis rates, suggesting that these changes in peritoneal dialysate composition do not have any major effect on the gut microbiome, and intestinal permeability.
Infection in dialysis patients remains an important healthcare problem. Studies have reported that although hospitalizations rates for dialysis patients have recently fallen overall, those for infection-related admissions have not [29]. Prevention should be the priority as infections are now harder to treat with multi-drug resistant bacteria on the rise and few newer antibiotics in the pipeline [30]. As such, centres should ensure that patients are actively vaccinated against respiratory pathogens to reduce the risk of pulmonary infections and also aim to limit fluid overload, in particular interdialytic fluid gains in haemodialysis patients. To reduce access-associated infections, centres should aim to increase the number of patients starting haemodialysis with arteriovenous fistulae, so limiting the use of central venous access catheters. Preventative infection clinical care bundles have reduced catheter-associated bacteraemias, but have not been as successful in preventing peritonitis in PD patients, and as such more research is required to reduce the risk of peritonitis in this group of dialysis patients.
ACKNOWLEDGEMENTS
This study was supported by Royal Free Hospital.
CONFLICTS OF INTEREST STATEMENT
The authors have no conflicts of interest.
REFERENCES
- 1.Wakasugi M, Kawamura K, Yamamoto S, et al. High mortality rate of infectious diseases in dialysis patients: a comparison with the general population in Japan. Ther Apher Dial. 2012;16:226–231. doi: 10.1111/j.1744-9987.2012.01062.x. [DOI] [PubMed] [Google Scholar]
- 2.Koc M, Toprak A, Arikan H, et al. Toll-like receptor expression in monocytes in patients with chronic kidney disease and haemodialysis: relation with inflammation. Nephrol Dial Transplant. 2011;26:955–963. doi: 10.1093/ndt/gfq500. [DOI] [PubMed] [Google Scholar]
- 3.Pahl MV, Gollapudi S, Sepassi L, et al. Effect of end-stage renal disease on B-lymphocyte subpopulations, IL-7, BAFF and BAFF receptor expression. Nephrol Dial Transplant. 2010;25:205–212. doi: 10.1093/ndt/gfp397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen G, Haag-Weber M, Horl W. Immune dysfunction in uraemia. Kidney Int Suppl. 1997;62:S79–S82. [PubMed] [Google Scholar]
- 5.Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut and abnormal immunity in kidney disease. Kidney Int. 2013;83:1010–1016. doi: 10.1038/ki.2012.440. [DOI] [PubMed] [Google Scholar]
- 6.Stubbs JR, Idiculla A, Slusser J, et al. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21:353–361. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soni R, Horowitz B, Unruh M. Immunisation in end-stage renal disease: opportunity to improve outcomes. Semin Dial. 2013;26:416–426. doi: 10.1111/sdi.12101. [DOI] [PubMed] [Google Scholar]
- 8.Sarnak MJ, Jaber BL. Pulmonary infectious mortality among patients with end-stage renal disease. Chest. 2001;120:1883–1887. doi: 10.1378/chest.120.6.1883. [DOI] [PubMed] [Google Scholar]
- 9.Dinits-Pensy M, Forrest GN, Cross AS, et al. The use of vaccines in adult patients with renal disease. Am J Kidney Dis. 2005;46:997–1011. doi: 10.1053/j.ajkd.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Papakrivopoulou E, Booth J, Pinney J, et al. Comparison of volume status in asymptomatic haemodialysis and peritoneal dialysis outpatients. Nephron Extra. 2012;2:48–54. doi: 10.1159/000337338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoccali C, Puntorieri E, Mallamaci F. Lung congestion as a hidden threat in end-stage kidney disease: a call to action. Nephrol Dial Transplant. 2013;28:2657–2660. doi: 10.1093/ndt/gft425. [DOI] [PubMed] [Google Scholar]
- 12.Dickson RP, Martinez FJ, Huffnagle GB. Infections in chronic lung diseases 1. Lancet. 2014;384:691–702. doi: 10.1016/S0140-6736(14)61136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleven RM, Edwards JR, Andrus ML, et al. Dialysis Surveillance Report: National Healthcare Safety Network (NHSN)-data summary for 2006. Semin Dial. 2008;21:24–28. doi: 10.1111/j.1525-139X.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- 14.Kandil H, Collier S, Yewetu E, et al. Arteriovenous fistula survival with buttonhole (constant site) cannulation for hemodialysis access. ASAIO J. 2014;60:95–98. doi: 10.1097/MAT.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 15.Carson RC, Juszczak M, Davenport A, et al. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol. 2009;4:1611–1619. doi: 10.2215/CJN.00510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ocak G, Halbesma N, le Cessie S, et al. Haemodialysis catheters increase mortality as compared to arteriovenous accesses especially in elderly patients. Nephrol Dial Transplant. 2011;26:2611–2617. doi: 10.1093/ndt/gfq775. [DOI] [PubMed] [Google Scholar]
- 17.Piraino B, Bernardini J, Brown E, et al. ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int. 2011;31:614–630. doi: 10.3747/pdi.2011.00057. [DOI] [PubMed] [Google Scholar]
- 18.Van Manen JG, Korevaar JC, Dekker FW, et al. Adjustment for comorbidity in studies on health status in ESRD patients: which comorbidity index to use? J Am Soc Nephrol. 2003;14:478–485. doi: 10.1097/01.asn.0000043902.30577.c9. [DOI] [PubMed] [Google Scholar]
- 19.Horan TCM, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Willicombe MK, Vernon K, Davenport A. Embolic complications from central venous hemodialysis catheters used with hypertonic citrate locking solution. Am J Kidney Dis. 2010;55:348–351. doi: 10.1053/j.ajkd.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 21.Van Diepen AT. The association between dialysis modality and the risk for dialysis technique and non-dialysis technique related infections. Nephrol Dial Transplant. 2014;29:2244–2250. doi: 10.1093/ndt/gfu285. [DOI] [PubMed] [Google Scholar]
- 22.Davenport A. Peritonitis remains the major clinical complication of peritoneal dialysis: the London, UK, peritonitis audit 2002–2003. Perit Dial Int. 2009;29:297–302. [PubMed] [Google Scholar]
- 23.Davenport A, Dealler SF. The epidermo-peritoneal potential in patients treated with continuous ambulatory peritoneal dialysis. Int J Artif Organs. 1993;16:71–74. [PubMed] [Google Scholar]
- 24.Davenport A, Wellsted Pan Thames Renal Audit Peritoneal Dialysis Group. Does antifungal prophylaxis with daily oral fluconazole reduce the risk of fungal peritonitis in peritoneal dialysis patients? The Pan Thames Renal Audit. Blood Purif. 2011;32:181–5. doi: 10.1159/000328735. [DOI] [PubMed] [Google Scholar]
- 25.van Diepen AT, Jassal SV. A qualitative systematic review of the literature supporting a causal relationship between exit-site infection and subsequent peritonitis in patients with end-stage renal disease treated with peritoneal dialysis. Perit Dial Int. 2013;33:604–610. doi: 10.3747/pdi.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davenport A Pan Thames Renal Audit Peritoneal Dialysis Group. Do topical antibiotics reduce exit site infection rates and peritonitis episodes in peritoneal dialysis patients? The Pan Thames Renal Audit. J Nephrol. 2012;25:819–824. doi: 10.5301/jn.5000071. [DOI] [PubMed] [Google Scholar]
- 27.O'Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 Suppl 1):S1–34. doi: 10.1016/j.ajic.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Brown MC, Simpson K, Kerssens JJ, et al. Peritoneal dialysis-associated peritonitis rates and outcomes in a national cohort are not improving in the post-millennium (2000–2007) Perit Dial Int. 2011;31:639–650. doi: 10.3747/pdi.2010.00185. [DOI] [PubMed] [Google Scholar]
- 29.Lafrance JP, Rahme E, Iqbal S, et al. Trends in infection-related hospital admissions and impact of length of time on dialysis among patients on long-term dialysis: a retrospective cohort study. CMAJ Open. 2014;2:E109–E114. doi: 10.9778/cmajo.20120027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]


