Abstract
Glutamate-rich protein is a Plasmodium falciparum (Pf) antigen found in all stages of the parasite and has been reported to induce clinical immunity. The R0 and R2 regions have been found to exhibit a high degree of conservation, therefore serving as a good vaccine design material. We assayed the genetic diversity of Pf glurp genes in the R0 and R2 regions, as well as evaluated the role of seasonality on allelic frequency. A total of 402 genomic DNA samples, extracted from filter paper blood samples, were screened by nested polymerase chain reaction (PCR) analysis of Pf glurp R0 and R2 regions, in addition to fragment analysis of the polymorphic regions to identify allelic diversity of the parasite population. We found an extensive heterogeneity in the R2 region in general, and this heterogeneity is seasonally dependent, indicative of region plasticity. The R0 region displayed genetic conservation, as expected. We conclude that positive genotyping results with glurp R0 region should be seen as indicative of an active Pf infection, requiring adequate treatment. In addition, we advocate extending the possibility that an R0 region genotypic positivity could serve as diagnostic tool, thereby reducing cases of untreated or poorly treated infection, contributory to recrudescence or treatment failure.
Keywords: malaria, glutamate-rich proteins, antigenic diversity, resistance, seasonality
Introduction
Plasmodium falciparum (Pf) remains the most infectious human parasitic agent, afflicting millions globally while imposing significant hardships on individuals and nations.1,2 Ongoing control efforts are targeted toward integrated approaches, including the design of vaccines with potential to reduce disease burden, especially for non-immune travelers to endemic locations and secondarily local control programs. Multiple vaccine design efforts utilizing malaria proteins are underway, including those utilizing parasite surface proteins from developmental stages of the parasite life cycle in humans such as circumsporozoite surface proteins (CSP), merozoite surface proteins (MSPs 1–5), glutamate-rich protein (GLURP), apical membrane antigen 1 (AMA1), and erythrocyte binding antigen 175 (EBA-175).3,4 Such efforts, however, are hampered by extensive antigenic diversity of these proteins, with the degree of diversity directly related to geography and transmission intensities in such locations.5–7
In many endemic countries, the biggest problem is self-or inadequate treatment, thereby building drug pressure, and the added challenge of resistance to available chemotherapeutic agents. Polymerase chain reaction (PCR) genotyping methods, utilizing nested allelic type-specific amplification of surface proteins, have become mainstays in this field. These tools have facilitated the definition of allelic variability in an individual or within a population to deconvolute recurrent or recrudescent infection, as well as to clarify effectiveness of prescribed therapy or drug resistance.8–11 It is an important investigative tool to delineate effectiveness of therapeutic agents during clinical trials or efficacy studies.
To assist vaccine design efforts and overcome the challenge imposed by antigenic diversity, Pf proteins that can induce clinical immunity, common in the parasite life cycle, and can be expressed on merozoites released into peripheral circulation would be significantly important, of which the glurp gene is an example. It is composed of an N-terminal nonrepetitive region (R0 region) and a C-terminal repetitive (R2 region) region. The R0 region has been shown in previous studies to be highly conserved and elicits an antibody response that is very stable over time,12–16 with the added potential of possessing antimalarial parasitic activity.17–19 In fact, one such study showed that glurp-specific immunoglobulin G antibodies in an endemic region significantly contribute to clearance of drug-resistant parasites, thereby enhancing the efficacy of antimalarial therapy.20 These observations, in various field studies, have been corroborated by in vitro assays, further validating the results and the conclusion that glurp R0 region is conserved while the R2 region is heterogeneous.
This conclusion, however, is challenged by recent reports showing that the Pf glurp gene displays some antigenic diversity, with differing allelic variants depending on geography and transmission intensity.7,21,22 If the former is the case, can the R0 region serve as a confirmatory tool in unknown cases of infection or as a marker of recurrent or recrudescent infection in endemic domains? Second, is this conservation stable by seasonality? Third, how widespread is the allelic heterogeneity of the R2 region viz-à-viz geography and seasonality, considering the diversity of other malaria genes in sub-Saharan Africa? We present evidence of genetic diversity in the R0 and R2 regions of Pf glurp gene from Nigeria, as well as allelic frequency by season, and show its utilization as a diagnostic tool for malaria infection.
Materials and Methods
Subjects and genomic DNA samples
A total of 402 individuals, recruited from the Awoyaya Medical Center, Lagos, Nigeria, during the rainy (July 2012) and dry (December 2013) seasons, served as subjects for this study. These individuals presented at the hospital with complaints of feeling unwell or persistent fever for the last three days, and were referred to the laboratory for diagnostic sample collection and further analyses. Discarded EDTA-anticoagulated blood samples, collected during this process, were spotted onto Whatman filter papers (GE Healthcare Life Sciences), and well-characterized genomic DNA samples were extracted from the dried blood spots with the Qiagen Parasite Blood Mini Kit (Qiagen Inc), with some changes in the manufacturer’s instruction, as described previously.23 Final elution volume was 100 μL, and DNA samples were stored at −20°C until further analysis. As the study involved existing pathological specimens, with the relevant information recorded in such a manner that subjects could not be identified, the research was exempt from the requirement for ethical approval under exemption 4 of the US Department of Health and Human Services regulations §46.101 (b).
Multiplex PCR assay
A multiplex PCR assay for differential diagnosis was carried out, utilizing the 18S rRNA gene, with primers, protocols, and reaction setup following established methods,24 as described previously and amended.11
Genotyping for diversity of glutamate-rich protein (glurp) R0 and R2 regions
We assayed for the genetic diversity of the glutamate-rich protein R0 and R2 regions with respective nested PCR assay, utilizing previously published protocol.14 Briefly, the R0 region was amplified with the primer pairs, DA153 (ATGAGAAACCTTTTCCATAT) and PF55 (TGCTTCATGCTCGCTTTTTTCCGAT) for the primary PCR and PF61 (TACAAGTGAGAATAGAAATAAAC) and PF62 (CACAGTTTCTTCATGTTCGACAGT) for the secondary step PCR. Similar protocol but a different primer pair was utilized for the glurp R2 region with primers PF3 (ACATGCAAGTGTTGATCCTGAAG) and PF2 (ATATTACTATATCCTTTGCTATTCC) as well as PF5 (TGAATTTGAAGATGTTCACACTGAAC) and PF4 (TGTAGGTACCACGGGTTCTTGTGG) serving for first and second round amplifications, respectively. PCR was performed on an Eppendorf Mastercycler Gradient machine (Harlow Scientific) in a total volume of 25 μL and amplified using the Lucigen EconoTaq PLUS GREEN 2X Master Mix PCR system (Lucigen Corporation). Reactions were carried out with the same program-initial denaturation at 95°C for 5 minutes; followed by 35 cycles of 94°C for 1 minute, 48°C for 1 minute, and 72°C for 2 minutes; and a final cycle of 72°C for 10 minutes. In all, 10 μL of PCR products were loaded on a 2% ethidium bromide-stained agarose gel (SeaKem Agarose) and band size determined, as described previously.11 Representative gel images are as shown below.
Results
Out of 402 samples collected, 19 (4.7%) were successfully amplified for Pf glurp gene indicating the presence of active malaria infection (Table 1). Analyzing for glurp regions, we found 18 (94.7%) glurp-positive samples were amplified for the R0 and R2 regions, respectively. Surprisingly, more glurp-positive samples were amplified during the dry season (73.7%) than during the rainy season (26.3%). Per region breakdown, each was amplified similarly during both seasons—100% for R0 and R2 regions during the dry season and 80.0% for the rainy season, respectively.
Table 1.
Characteristics of glurp-positive genotyping analysis.
| glurp GENE AMPLIFIED | FREQUENCY (%) | R0 REGION AMPLIFIED | FREQUENCY (%) | R2 REGION AMPLIFIED | FREQUENCY (%) | |
|---|---|---|---|---|---|---|
| Total | 19/402 | 4.7 | 18/19 | 94.7 | 18/19 | 94.7 |
| Rainy season | 5/19 | 26.3 | 4/5 | 80.0 | 4/5 | 80.0 |
| Dry season | 14/19 | 73.7 | 14/14 | 100.0 | 14/14 | 100.0 |
Genetic diversity and allelic frequency
The degree of genetic diversity observed in the glurp-positive samples was dependent on the region analyzed. For the R0 region, all positive samples had a single genotype of sizes 380 and 1301 base pairs (Fig. 1). This observation is potentially indicative of gene conservation across regions and seasons, and possible evidence of a single Pf strain.
Figure 1.
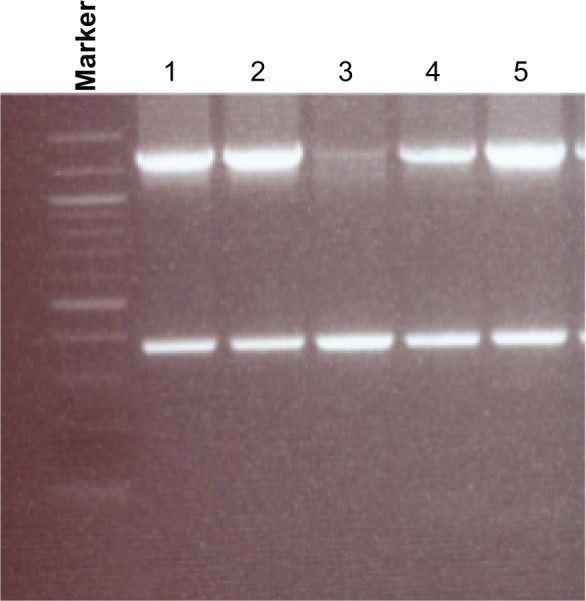
Agarose gel electrophoresis analysis showing genetic diversity detected in the Plasmodium falciparum glurp gene R0 region. All glurp-positive samples had one genotype, with band sizes of 380 and 1301 bp. Marker: 100 bp ladder, where the 500 bp band stains most intensely (New England Biolabs). Lanes 1–5: individual samples amplified for the glurp R0 region.
However, the R2 region displayed widespread and extensive genetic diversity. We found a total of 18 different genotypes among glurp-positive samples (Fig. 2), with allelic variants ranging from 660 to 1090 base pairs, the majority (55.5%) occurring at more than 10% (Fig. 3). Two genotypes (981–1000 bp and 1021–1040 bp) had the highest frequencies (16.7%) in our study, followed by bin sizes 841–860 bp and 881–900 bp (11.1%). There was more diversity in the glurp gene during the dry season than during the rainy season. Fewer genotypes—4 out of 18 (22.2%)—were observed during the rainy season with the majority of variants found during the dry season (77.8%). Seasonally, the highest frequencies of allelic variants (841–860 bp, 881–900 bp, and 981–1000 bp) were observed during the rainy season (25%), while the variant with the highest frequency during the dry season was 981–1000 bp (21.5%). Seasonally, majority of the genotypes (64.3%) encountered during the dry season occurred at less than 10%. In all, 18 individuals had multiple alleles of R0 and R2 regions amplified.
Figure 2.
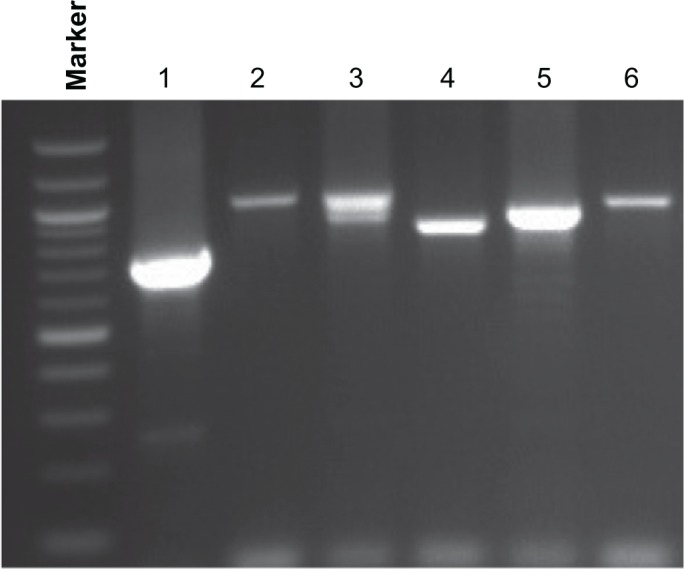
Agarose gel electrophoresis analysis showing genetic diversity detected with the Plasmodium falciparum glurp gene R2 region. PCR products were categorized into molecular weight groups differing by 20 bp. The glurp alleles ranged in size from 660 to 1,090 bp. Marker: 100 bp ladder, where the 500 bp band stains most intensely (New England Biolabs). Lanes 1–6: individual samples amplified for the glurp R2 region.
Figure 3.
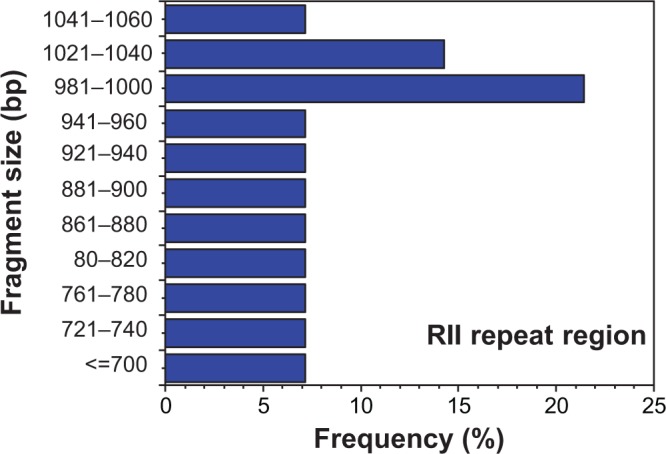
Allelic frequencies of Plasmodium falciparum glurp gene (R2 region) detected by polymerase chain reaction. PCR products were categorized into molecular weight groups differing by 20 bp. Two genotypes (981–1000 bp and 1021–1040 bp) were the most common (16.7%) in our study, followed by bin sizes 841–860 bp and 881–900 bp (11.1%).
Discussion
There is a serious imperative for the elucidation of the population genetic profile of Pf glurp gene, owing to its basis as a candidate for malaria vaccine design. Despite documented reports of its stability over time and the capacity to induce clinical immunity, other reports of heterogeneity, especially in the R2 region, would be of huge concern if the expected outcome is a vaccine capable to restrain the current trend for malaria infection. Nigeria, like many countries in West Africa, is a malaria-endemic country with many individuals with asymptomatic or sub-clinical infections,11 and as such taking advantage of the glurp gene R0 region conservation as a diagnostic tool would be a positive development. In other words, genotyping results with glurp R0 region positivity should count as an active infection, and adequate antimalarial therapy is administered. In fact, the observation that even less variation is found in similar geographical locations when field isolates are examined14 extends the possibility of this region as a diagnostic tool. The stability of the R0 region in our study confirms previous findings elsewhere14,25 and could potentially serve as another tool in the battle against malaria. The two glurp R0 genotypes found in our study confirm the possibility of multiple infections, which is not unexpected in an endemic region.
The evidence for multiple genotypes of R2 region found in our study indicates the heterogeneity and diversity of this region. Despite the significant reports on the diversity of other malaria surface proteins, reports on glurp gene diversity are very few and limited. This present observation probably reflects the degree of ongoing diversification of this gene, potentially ruling out its worthiness of consideration as an antimalarial agent. Previous reports have concluded that the Pf glurp gene in the R2 region is very heterogeneous with extensive diversity within and between regions and is related to transmission intensity.14,26,27
The difference between the glurp R0 and R2 region diversity in our study as it relates to seasonality is a very significant observation. In many endemic countries, majority of individuals with active malaria infections are encountered during the rainy season, wholly because of the preponderance of mosquito and environmental conditions that facilitate infection. Our current observation confirms previous reports that residents of malaria-endemic regions assume malaria infection associated with fever, during the rainy season and self-treat than during the dry season. The fact that a handful of glurp-positive cases were recorded during the rainy season indicates these are potentially recrudescent cases arising from treatment failures, and as such sought medical attention. A recent study from Tanzania28 clearly exemplifies the challenge faced in many sub-Saharan African countries on the presumption that malaria infection is responsible for the majority of febrile illness.29–34 According to Crump et al, there was a 60.7% frequency of presumptive malaria diagnosis, but true malaria infection was 1.6%, determined after laboratory examination of blood samples. If the percentage of presumptive diagnosis made by medical personnel is this high, we can extrapolate that the population would probably be higher, potentially leading to self-treatment. Unfortunately, many other infections34 that probably were the cause of febrile illness that requires urgent and immediate attention are left untreated with serious and potentially tragic consequences.
We conclude that PCR-positivity of this gene could potentially serve as evidence of failed or inadequate treatment and assist in proper treatment when patients present at the hospital. In a newly published report evaluating parasite subpopulations and genetic diversity of the msp1, msp2 and glurp genes during and following artesunate treatment, it was reported that most cases of positive malaria but failed treatment were recrudescent rather than recurrent or new infections.22 The major challenge, though, is the major concern that such self-treatments could contribute to or advance the development of resistance to currently available antimalarial medications.
Acknowledgments
We are grateful to Oluwatosin Olaogun, Yinka Olafisoye, and Opeyemi Oloyede for sample collection, and Kristen Navarro for technical assistance.
Glossary
List of Abbreviations
- Pf
Plasmodium falciparum
- glurp
glutamate-rich protein
- PCR-RFLP
polymerase chain reaction-restriction fragment length polymorphism
Footnotes
Author Contributions
Conceived and designed the experiment, and optimized protocols: BNT. Carried out DNA extraction and genotyping assays: BNT and KCD. Drafted the manuscript: BNT. All authors read and approved the final version of the manuscript.
ACADEMIC EDITOR: Raúl Rivas, Editor in Chief
FUNDING: Financial support was provided by the College of Health Sciences and Technology, Rochester Institute of Technology. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis. 2004;4:327–336. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . World Malaria Report 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- 3.Moran M, Guzman J, Ropars A, Jorgensen M, McDonald A, et al. The Malaria Product Pipeline: Planning for the Future. Sydney, Australia: The George Institute for International Health; 2007. [Google Scholar]
- 4.Barry AE, Schultz L, Buckee CO, Reeder JC. Contrasting population structures of the genes encoding ten leading vaccine-candidate antigens of the human malaria parasite, Plasmodium falciparum. PLoS One. 2009;4:e8497. doi: 10.1371/journal.pone.0008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira MU, Ribeiro WL, Tonon AP, Kawamoto F, Rich SM. Sequence diversity and evolution of the malaria vaccine candidate merozoite surface protein-1 (MSP-1) of Plasmodium falciparum. Gene. 2003;304:65–75. doi: 10.1016/s0378-1119(02)01180-0. [DOI] [PubMed] [Google Scholar]
- 6.Machado RL, Povoa MM, Calvosa VS, et al. Genetic structure of Plasmodium falciparum populations in the Brazilian Amazon region. J Infect Dis. 2004;190:1547–1555. doi: 10.1086/424601. [DOI] [PubMed] [Google Scholar]
- 7.Mwingira F, Nkwengulila G, Schoepflin S, et al. Plasmodium falciparum msp1, msp2 and glurp allele frequency and diversity in sub-Saharan Africa. Malar J. 2011;10:79–88. doi: 10.1186/1475-2875-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magesa SM, Mdira KY, Farnert A, Simionsen PE, Bygbjerg IC, Jakobsen PH. Distinguishing Plasmodium falciparum treatment failures from re-infections by using PCR genotyping in a holoendemic area in Northeastern Tanzania. Am J Trop Med Hyg. 2001;65:477–483. doi: 10.4269/ajtmh.2001.65.477. [DOI] [PubMed] [Google Scholar]
- 9.Mugittu K, Adjuik M, Snounou G, et al. Molecular genotyping to distinguish between recrudescents and new infections in treatment trials of Plasmodium falciparum malaria conducted in Sub-Saharan Africa: adjustment of parasitological outcomes and assessment of genotyping effectiveness. Trop Med Int Health. 2006;9:1350–1359. doi: 10.1111/j.1365-3156.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 10.Mugittu K, Priotto G, Guthmann JP, et al. Molecular genotyping in a malaria treatment trial in Uganda—unexpected high rate of new infections within two weeks after treatment. Trop Med Int Health. 2007;12:219–223. doi: 10.1111/j.1365-3156.2007.01813.x. [DOI] [PubMed] [Google Scholar]
- 11.Thomas BN, Petrella CR, Thakur TJ, Crespo SR, Diallo DA. Genetic polymorphism of Plasmodium falciparum merozoite surface protein-1 and 2 and diversity of drug resistance genes in blood donors from Bamako, Mali. Infect Dis Res Treat. 2012;6:49–57. [Google Scholar]
- 12.Dodoo D, Theisen M, Kurtzhals JA, et al. Naturally acquired antibodies to the glutamate-rich protein are associated with protection against Plasmodium falciparum malaria. J Infect Dis. 2000;181:1202–1205. doi: 10.1086/315341. [DOI] [PubMed] [Google Scholar]
- 13.Oeuvray C, Theisen M, Rogier C, Trape JF, Jepsen S, Druilhe P. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect Immun. 2000;68:2617–2620. doi: 10.1128/iai.68.5.2617-2620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Stricker K, Vuust J, Jepsen S, Oeuvray C, Theisen M. Conservation and heterogeneity of the glutamate-rich protein (GLURP) among field isolates and laboratory lines of Plasmodium falciparum. Mol Biochem Parasitol. 2000;111:123–130. doi: 10.1016/s0166-6851(00)00304-2. [DOI] [PubMed] [Google Scholar]
- 15.Theisen M, Soe S, Oeuvray C, et al. The glutamate-rich protein (GLURP) of Plasmodium falciparum is a target for antibody-dependent monocyte-mediated inhibition of parasite growth in vitro. Infect Immun. 1998;66:11–17. doi: 10.1128/iai.66.1.11-17.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theisen M, Dodoo D, Toure-Balde A, et al. Selection of glutamate-rich protein long synthetic peptides for vaccine development: antigenicity and relationship with clinical protection and immunogenicity. Infect Immun. 2001;69:5223–5239. doi: 10.1128/IAI.69.9.5223-5229.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogh B, Petersen E, Dziegiel M, David K, Hanson A, Borre M, Holm A, Vuust J, Jepsen S. Antibodies to a recombinant glutamate-rich Plasmodium falciparum protein: evidence for protection of individuals living in a holoendemic area of Liberia. Am J Trop Med Hyg. 1992;46:307–13. doi: 10.4269/ajtmh.1992.46.307. [DOI] [PubMed] [Google Scholar]
- 18.Boudin C, Chumpitazi B, Dziegiel M, Peyron F, Picot S, Hogh B, Ambroise-Thomas P. Possible role of specific immunoglobulin M antibodies to Plasmodium falciparum antigens in immunoprotection of humans living in a hyperendemic area, Burkina Faso. J Clin Microbiol. 1993;31:636–41. doi: 10.1128/jcm.31.3.636-641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dziegiel M, Rowe P, Bennett S, Allen SJ, Olerup O, Gottschau A, Borre M, Riley EM. Immunoglobulin M and G antibody responses to Plasmodium falciparum glutamate-rich protein: correlation with clinical immunity in Gambian children. Infect Immun. 1993;61:103–8. doi: 10.1128/iai.61.1.103-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enevold A, Nkya WM, Theisen M, et al. Potential impact of host immunity on malaria treatment outcome in Tanzanian children infected with Plasmodium falciparum. Malar J. 2007;6:153. doi: 10.1186/1475-2875-6-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiwuwa MS, Ribacke U, Moll K, Byarugaba J, Lundblom K, Färnert A, Fred K, Wahlgren M. Genetic diversity of Plasmodium falciparum infections in mild and severe malaria of children from Kampala, Uganda. Parasitol Res. 2013;112:1691–700. doi: 10.1007/s00436-013-3325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gosi P, Lanteri CA, Tyner SD, et al. Evaluation of parasite subpopulations and genetic diversity of the msp1, msp2 and glurp genes during and following artesunate monotherapy treatment of Plasmodium falciparum malaria in Western Cambodia. Malar J. 2013;12:403. doi: 10.1186/1475-2875-12-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thakur TJ, Guindo A, Cullifer LR, et al. Endothelin-1 but not endothelial nitric oxide synthase gene polymorphism is associated with sickle cell disease in Africa. Gene Regul Syst Bio. 2014;8:119–126. doi: 10.4137/GRSB.S14836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kho WG, Chung JY, Sim EJ, et al. A multiplex polymerase chain reaction for a differential diagnosis of Plasmodium falciparum and Plasmodium vivax. Parasitol Int. 2003;52:229–236. doi: 10.1016/s1383-5769(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 25.Escalante AA, Lal AA, Ayala FJ. Genetic polymorphism and natural selection in the malaria parasite Plasmodium falciparum. Genetics. 1998;149:189–202. doi: 10.1093/genetics/149.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul RE, Packer MJ, Walmsley M, et al. Mating patterns in malaria parasite populations of Papua New Guinea. Science. 1995;269:1709–1711. doi: 10.1126/science.7569897. [DOI] [PubMed] [Google Scholar]
- 27.Robert F, Ntoumi F, Angel G, et al. Extensive genetic diversity of Plasmodium falciparum isolates collected from patients with severe malaria in Dakar, Senegal. Trans R Soc Trop Med Hyg. 1996;90:704–711. doi: 10.1016/s0035-9203(96)90446-0. [DOI] [PubMed] [Google Scholar]
- 28.Crump JA, Morrissey AB, Nicholson WL, Massung RF, Stoddard RA, Galloway RL, Ooi EE, Maro VP, Saganda W, Kinabo GD, Muiruri C, Bartlett JA. Etiology of severe non-malaria febrile illness in Northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis. 2013;7:e2324. doi: 10.1371/journal.pntd.0002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koram KA, Molyneux ME. When is “malaria” malaria? The different burdens of malaria infection, malaria disease, and malaria-like illnesses. Am J Trop Med Hyg. 2007;77:1–5. [PubMed] [Google Scholar]
- 30.Nankabirwa J, Zurovac D, Njogu J, et al. Malaria misdiagnosis in Uganda—implications for policy change. Malar J. 2009;8:66. doi: 10.1186/1475-2875-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye Y, Madise N, Ndugwa R, Ochola S, Snow RW. Fever treatment in the absence of malaria transmission in an urban informal settlement in Nairobi, Kenya. Malar J. 2009;8:160. doi: 10.1186/1475-2875-8-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisoffi Z, Buonfrate D. When fever is not malaria. Lancet Glob Health. 2013;1:e11–e12. doi: 10.1016/S2214-109X(13)70013-5. [DOI] [PubMed] [Google Scholar]
- 33.Oladosu OO, Oyibo WA. Overdiagnosis and overtreatment of malaria in children that presented with fever in Lagos, Nigeria. ISRN Infect Dis. 2013;2013:6. [Google Scholar]
- 34.Stoler J, Al Dashti R, Anto F, Fobil JN, Awandare GA. Deconstructing “malaria”: West Africa as the next front for dengue fever surveillance and control. Acta Trop. 2014;134:58–65. doi: 10.1016/j.actatropica.2014.02.017. [DOI] [PubMed] [Google Scholar]


