Valved conduits are frequently used in congenital heart surgery to establish continuity between the right ventricle and the pulmonary arteries. The Contegra bovine jugular vein (Medtronic Inc, Minneapolis, MN) is a conduit that incorporates a tri-leaflet valve and affords off-the-shelf availability, good handling characteristics, and excellent hemodynamics. However, complications related to the use of this device have been reported, with conduit failure occurring mainly as a consequence of stenosis, conduit thrombosis, and valve regurgitation. We present a case of aneurysmal conduit failure of a 14-mm Contegra conduit used to reconstruct the right ventricular outflow tract.
Because the Contegra bovine jugular vein conduit (Medtronic Inc, Minneapolis, MN) was introduced for right ventricular outflow tract reconstruction in 1999, aneurysmal dilatation of the conduit has been very rarely reported [1, 2]. When identified, it is restricted to the proximal portion of the conduit, and it represents a false aneurysm or pseudoaneurysm resulting from partial dehiscence of the conduit from the right ventricle [3]. We report a case of uniform aneurysmal dilatation (without pseudoaneurysm formation) of a Contegra conduit (Medtronic Inc) with preserved competency of the valve.
A 2.5-year-old baby girl was born weighing 1.5 kg at 34 weeks gestation. A diagnosis of Vacterl syndrome, double outlet right ventricle, and pulmonary atresia with borderline left ventricle was made. She had anatomic repair surgery at 12 days of age with the interposition of an 8-mm pulmonary homograft between the right ventricle (RV) and the pulmonary artery (PA) and ventricular septal defect closure was made with a Dacron patch (C. R. Bard Inc, Murray Hill, NJ). The atrial septal defect was left open. She did well initially, but she was diagnosed with mitral stenosis at age 1 year. The mitral valve was balloon dilated, but recurrence of mitral stenosis by catheterization (mean gradient, 12 mm Hg; left atrial pressure, 28; left ventricular end-diastolic pressure, 18 mm Hg) and PA hypertension (60/46, mean 25 mm Hg) led to mitral valvuloplasty consisting of surgical resection of a supramitral ring, resection of secondary chords of the anterior leaflet, and conduit change 4 months later. The RV–PA homograft was replaced at that time with a 14-mm Contegra RV–PA conduit. She recovered uneventfully, but she also had both mitral stenosis (mean gradient, 5 mm Hg) and pulmonary hypertension (60/20 mean, 35 mm Hg) develop 13 months later. Imaging by echocardiography, magnetic resonance imaging, and angiography demonstrated severe aneurysmal dilatation of the 14-mm Contegra conduit to 26 to 28 mm (Fig 1, Fig 2). No distal stenosis was identified, and no conduit regurgitation was found. There was a similar uniform dilatation of the valve leaflets, and the valve remained competent as assessed by preoperative catheterization, echocardiography, and magnetic resonance imaging. Dynamic compression of a malpositioned right coronary artery by the dilated conduit was suspected by angiography and magnetic resonance imaging (Fig 3).
Fig 1.
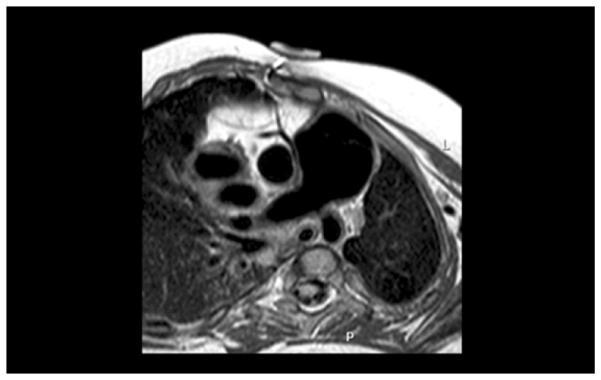
Angiographic picture demonstrating aneurysmal dilation of the Contegra conduit (Medtronic, Inc., Minneapolis, MN). There was no evidence of distal conduit stenosis by angiogram, echocardiogram, or magnetic resonance imaging, and there was no measurable gradient at catheterization.
Fig 2.
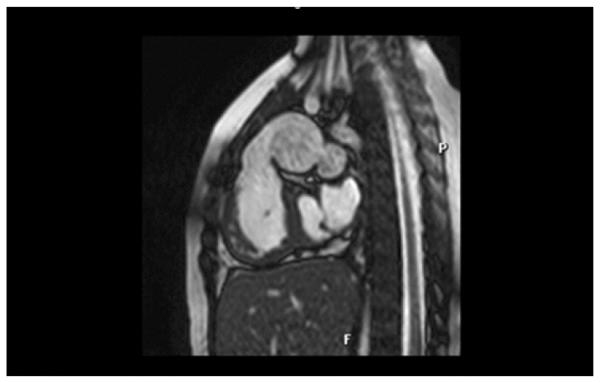
Magnetic resonance imaging frame showing dilatation of the Contegra conduit (Medtronic, Inc., Minneapolis, MN).
Fig 3.
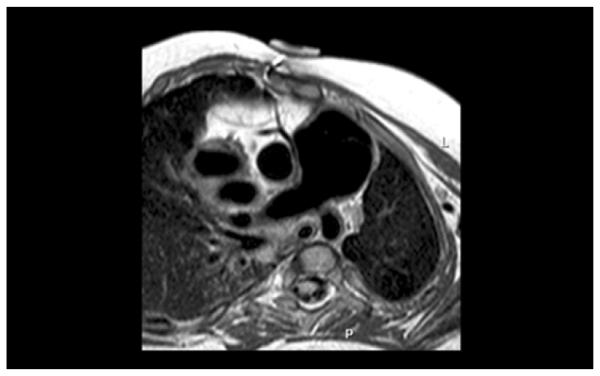
Magnetic resonance image depicting the relationship between the dilatated Contegra conduit (Medtronic, Minneapolis, MN) and the right coronary artery.
The patient was taken to the operating room for mitral valvuloplasty and conduit change. On inspection, the conduit was uniformly dilated from the proximal ventricular anastomosis to the distal anastomosis. At this time, the posterior leaflet of the mitral valve was enlarged with a pericardial patch and a recurrent supramitral ring was resected, and the anterior leaflet was thinned out. In addition, both commissures were scored. With respect to the conduit, no distal stenosis was identified. The conduit was replaced with a 20-mm aortic homograft. Gram stain and bacterial and fungal cultures were negative. Six months after surgery the patient is alive with no stenosis or regurgitation across the conduit and with a mean mitral gradient of 6 mm Hg.
Comment
Aneurysmal dilatation of the Contegra conduit (Medtronic Inc) has been rarely reported [1, 2, 3]. When identified, it is usually confined to the proximal anastomosis (pseudoaneurysm) and is commonly associated with distal anastomotic stenosis [4]. In our patient, true aneurysmal dilatation of the 14-mm unsupported Contegra device was observed in the setting of elevated pulmonary artery pressure, but without evidence for distal conduit or branch pulmonary artery stenosis. The mechanism(s) of failure are unknown, but mechanical stress and/or immunologic factors could be involved [5]. However, this generalized dilatation of the conduit is rare and may represent a new means of failure related to the Contegra graft. Close follow-up on this device in patients is warranted.
Acknowledgments
Funding:
This work was supported by the National Institutes of Health under award number: T32 HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Delmo-Walter EM, Alexi-Meskishvili V, Abdul-Khaliq H, Meyer R, Hetzer R. Aneurysmal dilatation of the Contegra bovine jugular vein conduit after reconstruction of the right ventricular outflow tract. Ann Thorac Surg. 2007;83:682–2. doi: 10.1016/j.athoracsur.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 2.Boudjemline Y, Bonnet D, Agnoletti G, Vouhe P. Aneurysm of the right ventricular outflow following bovine valved venous conduit insertion. Eur J Cardiothotac Surg. 2003;23:122–124. doi: 10.1016/s1010-7940(02)00660-7. [DOI] [PubMed] [Google Scholar]
- 3.Tiete AR, Sachweh JS, Roemer U, Kozlik-Feldmann R, Reichart B, Daebritz SH. Right ventricular outflow tract reconstruction with the Contegra bovine jugular vein conduit: a word of caution. Ann Thorac Surg. 2004;77:2151–2156. doi: 10.1016/j.athoracsur.2003.12.068. [DOI] [PubMed] [Google Scholar]
- 4.Kadner A, Dave H, Stallmach T, Turina M, Petre R. Formation of a stenotic fibrotic membrane at the distal anastomosis of bovine jugular vein grafts (Contegra) after right ventricular outflow tract reconstruction. J Thorac Cardiovasc Surg. 2004;127:285–286. doi: 10.1016/j.jtcvs.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Wojtalik M, Mrowczynski W, Zeromski J, Bartkowski R. Does contegra xenograft implantation evoke cellular immunity in children? Interact Cardiovasc Thorac Surg. 2003;2:273–278. doi: 10.1016/S1569-9293(03)00058-6. [DOI] [PubMed] [Google Scholar]


