Abstract
Introduction
We evaluated ocular outcomes in a 14-day head-down tilt (HDT) bed rest (BR) study designed to simulate the effects of microgravity on the human body.
Methods
Healthy subjects were selected using NASA standard screening procedures. Standardized NASA BR conditions were implemented (e.g., strict sleep-wake cycle, standardized diet, 24-hour-a-day BR, continuous video monitoring). Subjects maintained a 6° HDT position for 14 consecutive days. Weekly ophthalmological examinations were performed in the sitting (pre/post-BR) and HDT (in-bed phase) positions. Equivalency tests with optimal-alpha techniques evaluated pre/post-BR differences in best-corrected visual acuity (BCVA), spherical equivalent, intraocular pressure (IOP), Spectral-domain OCT retinal nerve fiber layer thickness (RNFLT), optic disc and macular parameters.
Results
16 subjects (12 men and 4 women) were enrolled. Nearly all ocular outcomes were within our predefined clinically relevant thresholds following HDTBR, except near BCVA (pre/post-BR mean difference: −0.06 logMAR), spherical equivalent (−0.30 D), Tonopen XL IOP (+3.03 mmHg) and Spectralis OCT average (+1.14 μm), temporal-inferior (+1.58 μm) and nasal-inferior RNFLT (+3.48 μm). Modified Amsler grid, red dot test, confrontational visual field and color vision were within normal limits throughout. No changes were detected on stereoscopic color fundus photography.
Discussion
A few functional and structural changes were detected after 14-day HDTBR, notably an improved BCVA possibly due to learning effect and RNFL thickening without signs of optic disc edema. In general, 6° HDTBR determined a small non-progressive IOP elevation, which returned to baseline levels post-BR. Further studies with different BR duration and/or tilt angle are warranted to investigate microgravity-induced ophthalmological changes.
Keywords: microgravity, intraocular pressure, retinal nerve fiber layer, optimal alpha
INTRODUCTION
Outer space is a hostile environment for the human body, typically affecting the cardiovascular, musculoskeletal, metabolic and immunological systems.[20] Unfortunately, the visual system is no exception. Ocular abnormalities were recently described in several astronauts returning from long-duration spaceflights. Changes included signs of optic disc edema, posterior globe flattening with hyperopic shifts, choroidal folds and cotton wool spots, resulting in some cases in significant visual impairment.[10, 13, 30] It has been hypothesized that, in predisposed individuals, increased intracranial pressure (ICP) secondary to microgravity-induced cephalad fluid shift may be responsible for these findings. However, a better understanding of the pathophysiological mechanisms leading to the above changes is necessary and it may help advance our knowledge of common conditions such as intracranial hypertension and papilledema. In addition, risks to the astronauts’ health must be quantified for safe and successful conduct of future long-duration human explorations, and countermeasures to mitigate such risks must be developed.
Head-down tilt bed rest, which produces cephalad shift of body fluids, has long been used on Earth to simulate the effects of microgravity on the human body and evaluate possible countermeasures.[17] In fact, prolonged head-down bed rest produces similar changes to those occurring in microgravity, such as bone demineralization, muscle loss, reduced metabolic needs, and decreased sensory stimulation.[22] Numerous studies have attempted to characterize the effects of body posture and bed rest on ocular outcomes, such as intraocular pressure (IOP).[15, 23] For example, it appears to be well established that IOP tends to increase in a supine or reclined position, possibly due to posture-induced choroidal engorgement and expansion, and to increased episcleral venous pressure.[30] However, little is known with regard to the effects of head-down bed rest on ocular structures and visual function. Because head-down tilt bed rest is designed to simulate the effects of microgravity on the human body, it is hypothesized that microgravity-induced ophthalmological changes observed in long-duration spaceflight might occur in head-down bed rest subjects. Therefore, the purpose of the present investigation was to evaluate ocular outcomes before, during and after bed rest in a 14-day head-down tilt bed rest study.
METHODS
This was an integrated, multidisciplinary bed rest study conducted at the NASA Flight Analogs Research Unit (FARU), located at The University of Texas Medical Branch (UTMB) at Galveston, Texas. The study protocol was approved in advance by the NASA Johnson Space Center and the UTMB Institutional Review Boards, and all methods adhered to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act (HIPAA). Each subject provided written informed consent before participating.
Subjects
Subject qualification criteria and bed rest conditions are detailed elsewhere.[17] In brief, to qualify for participation, subjects passed extensive physical and psychological exams. A standardized diet (based on the NASA space flight nutritional requirements) was provided throughout the study. To ensure nutritional intake, subjects were required to consume all food served to them.[8] In addition, subjects adhered to a strict sleep/wake schedule and did not nap during the day, with lights turned on at 6:00 am and turned out at 10:00 pm. Subject health was monitored by the attending physician and nursing staff 24-hour a day for the entire duration of the study.
To become acclimated to the FARU, participants spent 13 days in the pre-bed rest phase, in which they were ambulatory and underwent baseline assessment of their nutritional, cardiovascular, neurological and fitness status. The in-bed phase consisted of 14 consecutive days of 6° head-down tilt bed rest. All daily activities, including showering and toileting were performed in this position.[17] Finally, subjects were ambulatory and remained at the FARU for 7 more days post-bed rest to collect post study measures and receive rehabilitation to compensate for the deconditioning that occurs after prolonged bed rest.
Procedure
Subjects were evaluated on a weekly basis for 5 consecutive weeks. Specifically, pre- and post-bed rest ocular examinations were performed in the sitting position at the UTMB University Eye Center. These tests occurred 10 and 3 days prior to starting bed rest (BR −10 and BR −3, respectively) and on the 3rd day post bed rest (BR +2). In-bed ocular examinations were performed in the 6° head-down tilt position at the FARU on the 3rd and 10th day of bed rest (BR 3 and BR 10, respectively). A schematic representation of the ocular testing protocol is presented in Table I.
Table I.
OCULAR TESTING PROTOCOL.
| Pre-BR | BR | Post-BR | |||
|---|---|---|---|---|---|
| BR −10 | BR −3 | BR 3 | BR 10 | BR +2 | |
| BCVA (Distance and Near) | • | • | • | • | • |
| Cycloplegic Refraction | |||||
| Modified Amsler Grid | |||||
| Red Dot Test | |||||
| Color Vision (HRR Plates) | |||||
| Confrontational Visual Field | |||||
| IOP (Handheld) | |||||
|
| |||||
| IOP (Goldmann) | • | • | • | ||
|
| |||||
| SD-OCT | • | • | |||
| Color Fundus Photography | |||||
BCVA, best-corrected visual acuity; BR, bed rest; HRR, Hardy-Rand-Rittler; IOP, intraocular pressure; SD-OCT, Spectral-domain optical coherence tomography
Distance and near best-corrected visual acuity (BCVA) was assessed monocularly by one examiner using wall-mounted and handheld Early Treatment Diabetic Retinopathy Study (ETDRS) charts (Precision Vision, La Salle, IL), respectively. A standardized protocol was followed: after manifest refraction, subjects were required to consecutively identify the optotypes of each chart line from the left to the right, starting from the top line. There were no termination criteria; rather, the number of optotypes correctly identified was recorded and converted to logMAR units for further evaluation.
Goldmann applanation tonometry (GAT) was performed by two operators in a masked, standardized fashion: the tonometer dial was covered to eliminate potential bias from knowing prior IOP values. The dial was reset and IOP recorded by the operator not taking the measurement. In addition, for reproducible measurements, the operator had to achieve contact between the inner edges of the fluorescein bands at the systolic peak of the cardiac cycle. To account for inter-operator differences, in each eye the mean of the two operators’ IOP was considered for further evaluation.
Handheld tonometry was performed using iCare (Icare Finland Oy, Espoo, Finland). In each eye, the mean of four consecutive measurements obtained on the same visit was considered. In-bed IOP was measured with subjects maintaining a 6° head-down body tilt, assuming a right and left lateral decubitus for assessment of the left and the right eye IOP, respectively. To account for IOP changes possibly induced by transitioning from the supine to the lateral decubitus position,[15] in each eye a minimum 5-minute interval was given prior to starting the measurements. For the first 5 subjects enrolled, iCare was not available at the time of the first ocular examination. Therefore, at BR −10 and for the entire duration of their study, handheld tonometry was performed in 4 subjects using Tonopen XL (Reichert Inc., Depew, NY), while in 1 subject it was measured using Tonopen AVIA (Reichert Inc., Depew, NY). All the different tonometers used in this study were not considered interchangeable for the purpose of the analysis.
Equipment
Cirrus HD-OCT
(Carl Zeiss Meditec, Dublin, CA; version 5.0.0.326) is a commercially available Spectral-domain OCT (SD-OCT) characterized by an acquisition rate of 27 000 axial scans/sec and an axial resolution in tissue of 5 μm.
The Optic Disc Cube 200×200 scan protocol was used to evaluate optic disc and retinal nerve fiber layer (RNFL) thickness changes over time. A cube of data over a 6×6 mm2 area centered on the optic disc is generated by acquiring 200 horizontal scan lines (B-scans), each composed of 200 axial scans (A-scans). For optic disc assessment, built-in automated software identifies and outlines the optic disc and cup margins to obtain neuroretinal rim area, optic disc area, average and vertical cup-to-disc ratio, and cup volume. For RNFL thickness analysis, built-in automated algorithm identifies the center of the optic disc and places a scan circle of 3.46 mm diameter around it. After automated RNFL segmentation, the system samples from the data cube 256 A-scans along the path of the scan circle to obtain average and sectorial (4 quadrants and 12 clock-hours) RNFL thickness. Measurements are statistically compared to the normative database, and color-coded classification results are displayed on the printout to evaluate. Cirrus HD-OCT macular scans were obtained using the Macular Cube 512×128 protocol. A cube of data over a 6×6 mm2 area centered on the macula is generated by acquiring 128 B-scans, each composed of 512 A-scans. After automated fovea identification, the software places an ETDRS calculation grid centered on the fovea. The grid is composed of three concentric circles (diameters of 1, 3, and 6 mm) defining the central, inner and outer regions, and four perpendicular radial lines dividing both the inner and outer regions into four quadrants (temporal, superior, nasal and inferior, respectively). Average and sectorial retinal thickness, and average scan cube volume were considered for further evaluation. Post-bed rest changes in retinal thickness were evaluated using the Macular Change Analysis option, which automatically realigns a follow-up scan to the corresponding baseline, thus ensuring placement of the ETDRS grid in the same location over time.
Cirrus HD-OCT scans with signal strength < 6 were excluded, as per manufacturer’s recommendation. Additional scan exclusion criteria were the presence of retinal or RNFL segmentation artifacts, motion artifacts, media opacities, floaters or missing data on the scan circle or within the ETDRS grid area.
Spectralis OCT
(Heidelberg Engineering, GmbH, Heidelberg, Germany; version 5.1.3.0) is a commercially available SD-OCT that acquires 40 000 A-scans/sec with an axial resolution of 3.9 μm and a transverse resolution of 14 μm.
The Volume Scan acquisition protocol (512 A-scans × 19 B-scans) in High Speed mode was used to analyze a 20°×15° area centered on the macula and the optic disc, respectively. After scan acquisition, the software automatically places an ETDRS grid in the center of the fundus image for evaluation of the retinal thickness and volume. Because the diameter of the external circle exceeded the 20°×15° scan area, only the retinal thickness and volume from the central and inner sectors of the ETDRS grid were considered for further evaluation.
The Circle Scan protocol in High Speed mode was used to obtain average and sectorial (temporal, temporal-superior, nasal-superior, nasal, nasal-inferior and temporal inferior) RNFL thickness measurements.
Post-bed rest Spectralis OCT scans were obtained using the AutoRescan™ feature. This feature uses a retinal map created by a real-time eye tracking system that combines confocal scanning laser ophthalmoscopy and SD-OCT scans to compensate for eye movements, allowing for automatic placement of the follow-up scans in the same location as baseline.
All Spectralis OCT funduscopic images were evenly illuminated with signal-to-noise ratio >15 dB, the minimum quality score as per manufacturer’s recommendation.
Statistical Analysis
All statistical analyses were conducted using Stata version 13.0 (StataCorp LP, College Station, TX) and IBM SPSS Statistics version 20.0.0 (IBM Corporation, Armonk, NY). Our experimental design is an equivalency study, in which repeated observations (pre- and post-bed rest, right and left eye) of ocular outcomes were compared with the null hypothesis that the pre/post-bed rest differences exceeded previously specified clinically relevant thresholds. Rejecting the null hypothesis in this case suggests that the pre/post-bed rest differences are within clinically acceptable limits, while failure to reject the null hypothesis suggests that the observed pre/post-bed rest differences are clinically relevant. Equivalency study designs are superior at detecting similarity over traditional statistics designed to detect differences because of the reversal of what the null and the alternative hypotheses represent.[24, 34] In fact, a non-significant result from a traditional comparative statistic test would only indicate that the null hypothesis cannot be rejected, without proving that pre/post-bed rest observations are equivalent. As our intent was to evaluate equivalency between the pre/post bed rest conditions, a control group of ambulatory or supine subjects was not necessary for the purpose of this study.
The clinically relevant thresholds for the equivalency analysis were determined by our senior authors (GV and GT) based on their clinical and research expertise and they are reported along with the results. However, we also report the 95% Confidence Interval (CI) of the differences for alternative interpretations. In equivalency studies, attention must be given to the 95% CI for a correct interpretation of the results. For example, if the 95% CI of the pre/post-bed rest delta exceeds the clinically relevant interval (±clinically relevant threshold) the null hypothesis is not rejected, suggesting that the observed pre/post-bed rest difference is clinically relevant. An explanation of the pre-defined clinical significant thresholds for the ocular parameters follows here. Because the study involved young and healthy individuals, ages 28–54 years, a 0.1 logMAR (i.e., 1 ETDRS chart line) change in BCVA was considered as clinically relevant. A 0.5 D threshold in cycloplegic refraction was adopted in this microgravity-analog study, consistent with the hyperopic shift ≥ 0.5 D documented in the astronauts who experienced clinically relevant vision changes after long-duration spaceflights.[13] Weekly ocular examinations were performed at approximately the same time in the morning. Therefore, the effects of circadian IOP variations were not considered in determining IOP thresholds. Rather, the thresholds adopted correspond to the test-retest variability of the tonometers, as reported in the literature.[5] A 5% change from baseline in Spectralis OCT peripapillary retinal thickness and volume was considered as clinically relevant, based on the findings of a previous bed-rest investigation.[31] Given the excellent reproducibility of macular measurements using the Volume Scan acquisition protocol,[36] a 2.5% change from baseline was used as the threshold for Spectralis OCT macular thickness and volume. For the remaining Spectralis OCT measures [11, 37] and for the Cirrus HD-OCT macular [4, 6, 36], optic disc [26] and RNFL parameters [4, 12, 33], it was determined that post-bed rest changes from baseline exceeding the instrument’s test-retest variability be used as clinically relevant thresholds.
Consistent with typical equivalency trial methods, we employed the two-one-sided t-test approach to evaluate pre/post-bed rest differences.[28] Given our experimental design and our clinical and scientific interests to minimize both Type I (rejecting the null when equivalence is due to chance) and Type II errors (failing to reject the null and concluding non-equivalency when the differences are due to chance), we employed optimal alpha techniques for our statistical tests of equivalency, setting equal weights to both types of errors.[19]
RESULTS
Sixteen healthy subjects (12 men and 4 women) participated in this study. Subjects’ age averaged 37.8 ± 8.8 years. Height and weight averaged 174.9 ± 8.3 cm and 76.4 ± 10.4 Kg, respectively. Table II summarizes the baseline ophthalmic characteristics of the study participants. Table III shows the results of the pre/post-bed rest equivalency tests, and the observed mean pre/post-bed rest delta with 95% CIs per each outcome. Nearly all of the ocular outcomes were within our predefined thresholds following head-down bed rest. For example, the 95% CI of the pre/post-bed rest delta for iCare IOP ranged from −1.69 to 0.83 mmHg, which was within the ±2 mmHg threshold. Measures (mean [95% CI] of the pre/post-bed rest delta) that exceeded our clinically relevant thresholds include near BCVA (−0.06 [−0.10 to −0.02] logMAR), Retinomax K-plus 3 spherical equivalent (−0.30 [−0.56 to −0.04] D), Tonopen XL IOP (+3.03 [−5.24 to 11.31] mmHg), and Spectralis OCT average (+1.14 [0.69 to 1.59] μm), temporal-inferior (+1.58 [0.78 to 2.38] μm) and nasal-inferior RNFL thickness (+3.48 [2.20 to 4.76] μm). In each subject, measures of modified Amsler grid test, red dot test, confrontational visual field and color vision were within normal limits in both eyes at all time points. In addition, no changes from baseline were detected on stereoscopic color fundus photography.
Table II.
BASELINE (MEAN [SD], UNLESS OTHERWISE SPECIFIED) OPHTHALMIC CHARACTERISTICS OF THE STUDY PARTICIPANTS (N = 16).
| Distance BCVA, logMAR | −0.16 (0.06) |
| Near BCVA, median (IQR), logMAR | −0.14 (0.11) |
| Spherical equivalent (Reichert RK600), median (IQR), D | −0.02 (1.68) |
| Intraocular pressure (GAT), mmHg | 15.40 (3.08) |
| Cirrus HD-OCT: | |
| Average RNFL thickness, μm | 93.41 (7.35) |
| Rim area, mm2 | 1.37 (0.18) |
| Disc area, median (IQR), mm2 | 1.64 (0.45) |
| Average Cup/Disc, median (IQR) | 0.46 (0.23) |
| Macular thickness, μm | 260.31 (21.00) |
| Macular cube volume, mm3 | 10.18 (0.44) |
| Spectralis OCT: | |
| Average RNFL thickness, μm | 96.94 (8.97) |
| Peripapillary retinal thickness,* μm | 350.89 (28.85) |
| Peripapillary retinal volume,* mm3 | 0.50 (0.03) |
| Macular thickness, μm | 279.94 (20.65) |
| Macular volume, mm3 | 0.22 (0.02) |
BCVA, best-corrected visual acuity; GAT, Goldmann applanation tonometer; IQR, interquartile range; MAR, minimum angle of resolution; RNFL, retinal nerve fiber layer
Mean of the central and inner sectors of the Early Treatment Diabetic Retinopathy Study grid
Table III.
PRE/POST-BED REST EQUIVALENCY TESTS.
| Outcome | Clinical Threshold for Equivalence | Observed Mean Pre/Post Delta | Optimal Alpha 95% CI | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Best-Corrected Visual Acuity, logMAR | ||||
| Distance | 0.10 | −0.01 | −0.05 | 0.03 |
| Near | 0.10 | −0.06 | −0.10 | −0.02 |
|
| ||||
| Spherical Equivalent, D | ||||
| Retinomax K-plus 3 | 0.50 | −0.30 | −0.56 | −0.04 |
| Reichert RK 600 | 0.50 | −0.16 | −0.36 | 0.03 |
|
| ||||
| Intraocular pressure, mmHg | ||||
| iCare | 2.00 | −0.43 | −1.69 | 0.83 |
| Tonopen AVIA | 4.00 | −2.50 | N/A | N/A |
| Tonopen XL | 4.00 | 3.03 | −5.24 | 11.31 |
| Goldmann | 2.00 | −0.32 | −0.97 | 0.33 |
|
| ||||
| Cirrus HD-OCT | ||||
| Optic Disc Cube 200×200 Protocol | ||||
| Average RNFLT, μm | 4.00 | 0.43 | −0.69 | 1.69 |
| Rim Area, mm2 | 3.26 | 0.01 | −0.11 | 0.12 |
| Disc Area, mm2 | 3.93 | −0.01 | −0.12 | 0.11 |
| Average Cup/Disc | 0.67 | −0.01 | −0.05 | 0.03 |
| Vertical Cup/Disc | 1.41 | −0.02 | −0.06 | 0.03 |
| Cup Volume, mm3 | 0.38 | −0.00 | −0.02 | 0.01 |
| Quadrants RNFLT, μm | ||||
| Temporal | 4.00 | 1.04 | −0.02 | 2.11 |
| Superior | 10.00 | 0.61 | −2.78 | 4.03 |
| Nasal | 8.00 | −0.14 | −1.90 | 2.03 |
| Inferior | 9.00 | 0.18 | −1.49 | 2.03 |
| Clock-hours RNFLT, μm | ||||
| 1 | 15.00 | 0.80 | −3.00 | 4.80 |
| 2 | 14.00 | −0.80 | −3.63 | 2.22 |
| 3 | 8.00 | −0.49 | −2.87 | 1.96 |
| 4 | 10.00 | 0.51 | −1.44 | 3.35 |
| 5 | 14.00 | 1.06 | −1.88 | 4.88 |
| 6 | 17.00 | −1.55 | −4.52 | 2.07 |
| 7 | 15.00 | 0.86 | −3.31 | 4.10 |
| 8 | 10.00 | 0.84 | −1.45 | 2.91 |
| 9 | 4.00 | 0.96 | −0.14 | 2.14 |
| 10 | 7.00 | 1.18 | −0.75 | 3.21 |
| 11 | 14.00 | 1.33 | −2.91 | 5.28 |
| 12 | 17.00 | −0.18 | −5.61 | 5.32 |
| Macular Cube 512×128 Protocol | ||||
| Central Subfield Thickness, μm | 8.04 | −0.71 | −3.52 | 2.06 |
| Macular Cube Volume, mm3 | 0.10 | 0.03 | 0.00 | 0.07 |
| Macular Cube Average Thickness, μm | 1.70 | 0.86 | 0.19 | 1.61 |
| ILM/RPE Thickness, μm | ||||
| Temporal Outer | 8.15 | 0.88 | −1.50 | 3.38 |
| Temporal Inner | 9.71 | 0.20 | −3.14 | 3.55 |
| Superior Outer | 8.65 | 1.16 | −1.26 | 3.64 |
| Superior Inner | 10.09 | −0.04 | −2.86 | 2.86 |
| Nasal Outer | 9.21 | 1.12 | −1.04 | 3.33 |
| Nasal Inner | 10.15 | −0.86 | −4.09 | 2.25 |
| Inferior Outer | 8.39 | 1.02 | −1.75 | 3.75 |
| Inferior Inner | 10.00 | 0.33 | −3.20 | 3.83 |
| Central | 8.04 | −0.71 | −3.52 | 2.06 |
|
| ||||
| Spectralis OCT | ||||
| Macular Thickness,* μm | ||||
| Temporal | 8.43 | −0.10 | −2.77 | 2.57 |
| Superior | 8.73 | 0.34 | −2.53 | 3.21 |
| Nasal | 8.78 | 0.26 | −2.64 | 3.16 |
| Inferior | 8.67 | 0.38 | −1.69 | 2.45 |
| Central | 7.00 | −1.66 | −3.80 | 0.48 |
| Average | 8.32 | −0.16 | −2.86 | 2.55 |
| Macular Volume,* mm3 | ||||
| Temporal | 0.01 | −0.00 | −0.01 | 0.01 |
| Superior | 0.01 | 0.00 | 0.00 | 0.01 |
| Nasal | 0.01 | 0.00 | 0.00 | 0.01 |
| Inferior | 0.01 | 0.00 | 0.00 | 0.00 |
| Central | 0.01 | −0.00 | 0.00 | 0.00 |
| Average | 0.01 | 0.00 | 0.00 | 0.00 |
| Peripapillary Retinal Thickness,* μm | ||||
| Temporal | 15.22 | 3.50 | −0.89 | 7.89 |
| Superior | 19.35 | 5.30 | 0.24 | 10.36 |
| Nasal | 17.82 | 4.64 | 0.04 | 9.25 |
| Inferior | 19.85 | 5.60 | −1.07 | 12.27 |
| Central | 15.49 | 3.33 | −2.68 | 8.60 |
| Average | 17.54 | 4.51 | −1.00 | 10.01 |
| Peripapillary Retinal Volume,* mm3 | ||||
| Temporal | 0.02 | 0.01 | 0.00 | 0.01 |
| Superior | 0.03 | 0.01 | 0.00 | 0.02 |
| Nasal | 0.03 | 0.01 | 0.00 | 0.01 |
| Inferior | 0.03 | 0.01 | 0.00 | 0.02 |
| Central | 0.01 | 0.00 | 0.00 | 0.01 |
| Average | 0.03 | 0.01 | 0.00 | 0.02 |
| Scan Circle Protocol | ||||
| Temporal RNFLT, μm | 1.42 | 0.48 | −0.09 | 1.05 |
| Temporal Superior RNFLT, μm | 2.21 | 0.40 | −0.35 | 1.15 |
| Nasal Superior RNFLT, μm | 2.12 | 0.52 | −0.11 | 1.15 |
| Nasal RNFLT, μm | 1.76 | 0.90 | 0.04 | 1.76 |
| Nasal Inferior RNFLT, μm | 2.16 | 3.48 | 2.20 | 4.76 |
| Temporal Inferior RNFLT, μm | 2.28 | 1.58 | 0.78 | 2.38 |
| Average RNFLT, μm | 1.19 | 1.14 | 0.69 | 1.59 |
CI, confidence interval; ILM/RPE, internal limiting membrane/retinal pigment epithelium; MAR, minimum angle of resolution; N/A, not applicable; RNFLT, retinal nerve fiber layer thickness.
Inner and central sectors of the Early Treatment Diabetic Retinopathy Study grid
Bold, exceeded clinical threshold. Non-equivalence determined by two one-sided t-tests failing to reject the null hypothesis that the pre/post-bed rest differences are within the clinical threshold for equivalence.
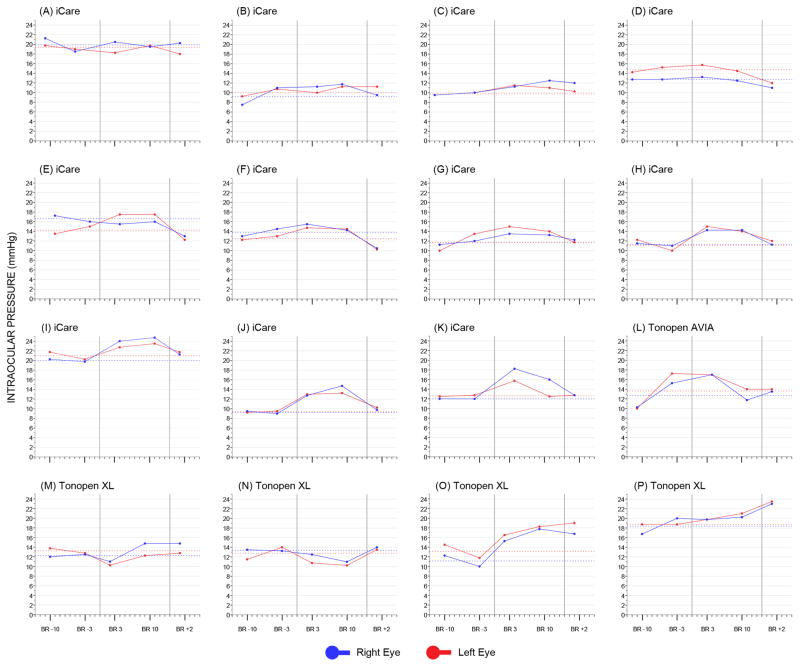
Fig. 1 shows the IOP pattern for each subject. In most cases, the 6° head-down tilt position produced an initial increase in IOP, followed by stabilization on the 2nd week of bed rest and tendency to return to baseline levels post-bed rest (see below and Fig. 1G–L). However, in some cases IOP remained relatively stable (Fig. 1A–D) during the course of the entire study, or it progressively increased (Fig. 1O–P) without the expected decrease post-bed rest. Interestingly, a few subjects displayed decreased in-bed IOP values from baseline (see, for example, Fig. 1M–N).
Fig 1.
Intraocular pressure (IOP) plots of the study subjects. In each plot, the solid vertical lines identify the pre-bed rest (left), in-bed (center) and post-bed rest (right) phases, respectively. The dashed horizontal lines correspond to the baseline IOP of the right and the left eye, respectively, calculated as the mean of the two pre-bed rest visits. BR, bed rest.
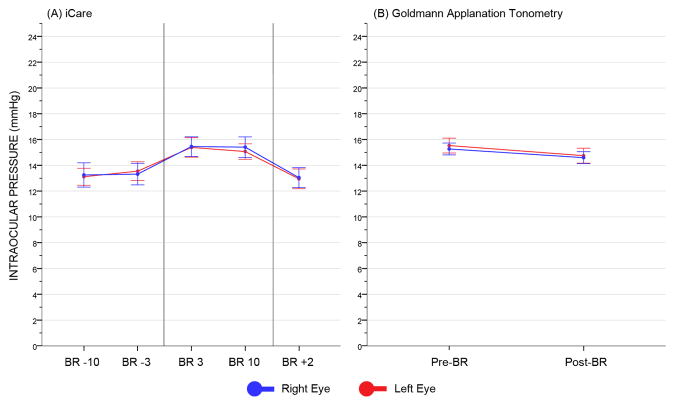
ICare and GAT IOP measures for the entire cohort are presented in Fig. 2. Pre- and post-bed rest GAT IOP values were 15.27 and 14.59 mmHg in the right eye, and 15.53 and 14.75 mmHg in the left eye, respectively, while pre- and post-bed rest iCare measures were 13.28 and 13.05 mmHg in the right eye, and 13.33 and 12.95 mmHg in the left eye, respectively. At BR 3, there was an average increase from baseline in iCare IOP of 2.17 and 2.06 mmHg in the right and left eye, respectively. At BR 10, the change from baseline was +2.13 and +1.74 mmHg in the right and left eye, respectively. Finally, at BR +2, iCare IOP returned to pre-bed rest levels, with mean differences from baseline of −0.24 and −0.38 mmHg in the in the right and left eye, respectively.
Fig 2.
Intraocular pressure plots for iCare (A) and Goldmann applanation tonometer (B). Error bars represent the 95% confidence interval of the mean. In (A), the two vertical lines identify the pre-bed rest (left), in-bed (center) and post-bed rest (right) phases, respectively. BR, bed rest.
DISCUSSION
This study evaluated ocular outcomes in healthy human subjects participating in a 14-day head-down bed rest study designed to simulate the effects of microgravity on the human body. Equivalency tests revealed that nearly all post-bed rest changes were within pre-defined clinically relevant thresholds, suggesting that the experimental conditions did not significantly affect participants’ visual function. However, for some outcomes the pre/post-bed rest difference exceeded the corresponding equivalency threshold. In particular, there was an improvement in near BCVA, as shown by a decrease in logMAR post-bed rest. This is likely explained by a learning effect. In fact, all subjects received weekly BCVA assessment for five consecutive weeks under the same testing conditions, which included identical refraction and visual acuity charts. Therefore, it is plausible that their performance improved over time as a result of test familiarization and possibly memorization. Unfortunately, due to the head-down tilt posture it was not possible to use a computer-based random optotypes presentation system, which would have certainly helped controlling for the learning effect.
Results also indicated the presence of a small myopic shift, as evidenced by a decrease in spherical equivalent post-bed rest. It is known that sustained near-vision activities may lead to transient myopia.[21] In this study, due to the prolonged stay at the FARU (34 days), our subjects routinely performed near-vision activities for recreation purposes, such as laptop use, TV watching, or reading when not engaged in research testing.
With regard to IOP, the analysis revealed an increase in Tonopen XL IOP post-bed rest. It is possible that this effect may be due to the small number of subjects tested with Tonopen XL (n = 4), thus leading to a large 95% CI of the pre/post-bed rest delta that exceeded the pre-defined clinically relevant threshold. When measured on a larger sample using iCare or on the entire study population using GAT, pre- and post-bed rest IOPs were equivalent.
The inclusion of in-bed measurements also allowed assessing the IOP pattern in response to bed rest. In general, head-down tilt bed rest induced an acute rise in IOP, in agreement with previous literature,[23] which was followed by stabilization at BR 10 and tendency to return to baseline values post-bed rest; the two eyes appeared to respond similarly to the effects of postural changes (Figs. 1–2). However, a case-by-case analysis revealed other possible IOP patterns. For example, in both eyes of 3 subjects, post-bed rest IOP was lower than at baseline (Fig. 1D–F). It has been hypothesized that compensatory mechanisms, such as decreased aqueous humor production, may be activated when the body is placed in a recumbent position.[14] Persistence of such mechanisms post-bed rest would result in decreased IOP from baseline. However, in our study post-bed rest IOP was measured only on the 3rd day out of bed, to comply with the rigid research protocol. It is conceivable that, by this time, the re-established ambulatory regimen may have allowed the IOP to return to baseline levels, as seen in most of our subjects. On the other hand, inadequate compensatory responses to postural changes might explain why, in 2 cases, IOP tended to increase throughout the study (Fig. 1O–P). However, IOP remained within tolerable limits in each subject (Fig. 1). There is evidence in the literature that the posture-induced increase in IOP correlates with the angle of tilt.[23] This relationship is further confirmed by comparing our measures in the 6° head-down tilt position with IOP values from independent studies in subjects undergoing non-ocular surgeries performed in a steep Trendelenburg position,[1, 3, 7] and with IOP measures under complete body inversion (e.g., headstand position in yoga-practitioners).[23, 25] Finally, Spectralis OCT showed an increase in average, temporal-inferior and nasal-inferior RNFL thickness post-bed rest. However, no visible changes to the optic disc or the RNFL were detected on color stereophotographs. In addition, the analysis showed no post-bed rest changes in Spectralis OCT peripapillary retinal thickness and volume, as well as no changes in Cirrus HD-OCT parameters post-bed rest. This latter finding may be explained by the adoption of larger, more conservative thresholds for Cirrus HD-OCT equivalency tests, reflecting higher test-retest variability than Spectralis-OCT. Important differences in the study design, instrumentation and scanning protocols used may also explain the contrasting findings with recent clinical investigations reporting increased peripapillary total retinal thickness in patients with optic disc edema.[29, 32] Nevertheless, our results confirm that Spectralis OCT may detect subtle changes at the level of the optic nerve head and RNFL thickness otherwise undetectable on ophthalmological exam. In addition, it is interesting to note that the RNFL thickness changes in our study involved the temporal-inferior and nasal-inferior sectors, consistent with the early changes of optic disc edema, in which the swelling typically starts at the inferior pole.[27] RNFL thickening may have occurred as a result of a posture-induced cephalad fluid shift. It is known that the pressure of the internal fluid compartments, such as the aqueous humor or the cerebrospinal fluid, is affected by postural changes.[2] Recent studies also suggested that the translaminar pressure difference (i.e., IOP minus ICP), may be implicated in the pathophysiology of optic neuropathies.[9] Therefore, any disruptions to the translaminar pressure difference may lead to the onset of optic disc changes. RNFL thickening might be explained in our study by an increase in ICP from baseline greater than the average IOP elevation during bed rest, a decrease in IOP not accompanied by a concomitant reduction in ICP post-bed rest, or a combination of both. Unfortunately, ICP measures could not be included as part of the research protocol for this study.
Our results suggest that 14-day head-down bed rest did not produce clinically relevant visual changes. Similar conclusions were drawn by Mekjavic and associates in a previous 35-day 0° bed rest study.[18] Further research is needed to clarify whether more prolonged head-down bed rest can produce ocular findings similar to those observed in the astronauts. For example, subtle ocular structural and functional changes have been recently reported in a healthy human subject enrolled in a 30-day 6° head-down bed rest study.[31] Our study also demonstrated significant RNFL thickening post-bed rest without any visible signs of optic disc edema. However, the bed rest phase (14 days) was significantly shorter than the average duration of the space missions associated with astronauts’ visual impairment (6 months).[13] In addition, although head-down bed rest experiments produce cephalad fluid shifts, they cannot eliminate the gravitational forces on the body. Therefore, they may not perfectly replicate the microgravity environment. Other factors related to the spaceflight environment that were not simulated in the bed rest analog such as increased carbon dioxide levels and increased dietary sodium, when combined with fluid shifting, may have a greater effect on visual outcomes than fluid shifting alone.[16] Also, the 6° head-down tilt likely did not determine internal jugular vein compression, a hypothesized contributing mechanism for intracranial hypertension and the ophthalmological alterations experienced in microgravity.[35] Furthermore, the magnitude of ocular changes may depend on the angle of tilt, which is conventionally set at 6° for long-duration head-down bed rest studies.[22] Another aspect to consider when interpreting our results is the position of the head relative to the body. Although the body was rigorously maintained at −6° tilt during bed rest, the head was at a less steep angle as it was supported by a pillow. Giving safety the highest priority, the pillow was necessary to prevent/reduce musculoskeletal pain, particularly to the upper back, shoulders and neck. As an additional safety precaution, to prevent choking hazard, subjects were allowed to elevate the head on their elbow while eating their meals, for a total of 1.5 hours per day. These factors might have mitigated the effects of head-down bed rest on ocular structures and visual function.
Our study has limitations. First, ophthalmological testing was performed on a weekly basis, with only two in-bed visits. Although weekly examinations may be sufficient for monitoring purposes, the acute physiological changes and adaptation mechanisms related to postural shifts (i.e., head-down tilt placement and return to the upright position, respectively) could not be fully characterized. For example, it would have been desirable to obtain multiple IOP measurements per day, particularly on the first day in and out of bed. Unfortunately, the multidisciplinary nature of this study limited the subjects’ time and availability for ocular testing. In addition, ocular measures were collected on BR 10 rather than on BR 14, the last day of bed rest, due to rigid protocol constraints.
Second, multiple handheld tonometers were used. Unfortunately, the study could not be postponed until iCare was available. Although Tonopen could have been used for the rest of the study, iCare was adopted to maximize the safety and comfort of the subjects, and to ensure reproducible IOP measurements. However, each subject received IOP measurements with the same instrument at all time points. In addition, the tonometers were not considered interchangeable for the purpose of the analysis, which was performed separately for each device.
Third, it would have been interesting to evaluate our ocular outcomes after 14-day head-down bed rest in men and women separately, as the ophthalmological changes occurred after long duration spaceflight were reported in male astronauts only.[13] Although the gender composition of our cohort resembled that of the whole astronauts’ population, characterized by male predominance, our participants could not be stratified by gender due to the small number of women, which could have significantly affected the results of the equivalency tests. However, the study was not designed to evaluate the effect of gender and bed rest on ocular outcomes.
Finally, most clinically relevant thresholds adopted for equivalency tests were based on published test-retest variability data from larger samples of healthy individuals using the same instrumentation as in our study (see, for example, Cirrus HD-OCT parameters). We are aware that changes exceeding test-retest variability may not necessarily be of clinical importance. However, this approach avoided predefining arbitrary thresholds not supported by published data. Also, it should be noted that changes above test-retest variability should never be ignored by clinicians, but carefully evaluated and possibly incorporated in their decision-making process.
In conclusion, our study found a few functional and structural changes after 14-day head-down tilt bed rest, notably an increase in near BCVA possibly due to learning effect and SD-OCT RNFL thickening without signs of optic disc edema, which may have resulted from cephalad fluid shift in response to bed rest. In addition, the 6° head-down tilt position determined, on average, a small non-progressive increase in IOP, which tended to return to baseline levels post-bed rest. Further investigations using a different duration of bed rest and/or a different angle of tilt are necessary to study the ophthalmological changes observed in spaceflight.
Acknowledgments
Supported by NASA Flight Analogs Project 516724.03.04.01; NIH/NCRR 1UL1RR029876-01.
Footnotes
Presented at the Association for Research in Vision and Ophthalmology Annual Meeting, Fort Lauderdale, FL, May 4–10 2012
References
- 1.Awad H, Santilli S, Ohr M, Roth A, Yan W, et al. The effects of steep trendelenburg positioning on intraocular pressure during robotic radical prostatectomy. Anesth Analg. 2009;109:473–8. doi: 10.1213/ane.0b013e3181a9098f. [DOI] [PubMed] [Google Scholar]
- 2.Berdahl JP, Allingham RR. Intracranial pressure and glaucoma. Curr Opin Ophthalmol. 2010;21:106–11. doi: 10.1097/ICU.0b013e32833651d8. [DOI] [PubMed] [Google Scholar]
- 3.Borahay MA, Patel PR, Walsh TM, Tarnal V, Koutrouvelis A, et al. Intraocular pressure and steep Trendelenburg during minimally invasive gynecologic surgery: is there a risk? J Minim Invasive Gynecol. 2013;20:819–24. doi: 10.1016/j.jmig.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Martin E, Pinilla I, Idoipe M, Fuertes I, Pueyo V. Intra and interoperator reproducibility of retinal nerve fibre and macular thickness measurements using Cirrus Fourier-domain OCT. Acta Ophthalmol. 2011;89:e23–9. doi: 10.1111/j.1755-3768.2010.02045.x. [DOI] [PubMed] [Google Scholar]
- 5.Garway-Heath DF, Kotecha A, Lerner F, Dayanir V, Brandt JD, et al. Measurement of intraocular pressure. In: Weinreb RN, Brandt JD, Garway-Heath DF, Medeiros FA, editors. Intraocular pressure: reports and consensus statements of the 4th global AIGS consensus meeting on intraocular pressure. The Hague, The Netherlands: Kugler Publications; 2007. p. 31. [Google Scholar]
- 6.Hagen S, Krebs I, Haas P, Glittenberg C, Falkner-Radler CI, et al. Reproducibility and comparison of retinal thickness and volume measurements in normal eyes determined with two different Cirrus OCT scanning protocols. Retina. 2011;31:41–7. doi: 10.1097/IAE.0b013e3181dde71e. [DOI] [PubMed] [Google Scholar]
- 7.Hoshikawa Y, Tsutsumi N, Ohkoshi K, Serizawa S, Hamada M, et al. The effect of steep Trendelenburg positioning on intraocular pressure and visual function during robotic-assisted radical prostatectomy. Br J Ophthalmol. 2014;98:305–8. doi: 10.1136/bjophthalmol-2013-303536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inniss AM, Rice BL, Smith SM. Dietary support of long-duration head-down bed rest. Aviat Space Environ Med. 2009;80(5, Suppl):A9–14. doi: 10.3357/asem.br04.2009. [DOI] [PubMed] [Google Scholar]
- 9.Jonas JB, Wang N. Cerebrospinal Fluid Pressure and Glaucoma. J Ophthalmic Vis Res. 2013;8:257–63. [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer LA, Sargsyan AE, Hasan KM, Polk JD, Hamilton DR. Orbital and intracranial effects of microgravity: findings at 3-T MR imaging. Radiology. 2012;263:819–27. doi: 10.1148/radiol.12111986. [DOI] [PubMed] [Google Scholar]
- 11.Langenegger SJ, Funk J, Toteberg-Harms M. Reproducibility of retinal nerve fiber layer thickness measurements using the eye tracker and the retest function of Spectralis SD-OCT in glaucomatous and healthy control eyes. Invest Ophthalmol Vis Sci. 2011;52:3338–44. doi: 10.1167/iovs.10-6611. [DOI] [PubMed] [Google Scholar]
- 12.Leung CK, Cheung CY, Weinreb RN, Qiu Q, Liu S, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology. 2009;116:1257–63. 63.e1–2. doi: 10.1016/j.ophtha.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology. 2011;118:2058–69. doi: 10.1016/j.ophtha.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Mader TH, Taylor GR, Hunter N, Caputo M, Meehan RT. Intraocular pressure, retinal vascular, and visual acuity changes during 48 hours of 10 degrees head-down tilt. Aviat Space Environ Med. 1990;61:810–3. [PubMed] [Google Scholar]
- 15.Malihi M, Sit AJ. Effect of head and body position on intraocular pressure. Ophthalmology. 2012;119:987–91. doi: 10.1016/j.ophtha.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Marshall-Bowman K, Barratt MR, Gibson CR. Ophthalmic changes and increased intracranial pressure associated with long duration spaceflight: an emerging understanding. Acta Astronautica. 2013;87:77–87. [Google Scholar]
- 17.Meck JV, Dreyer SA, Warren LE. Long-duration head-down bed rest: project overview, vital signs, and fluid balance. Aviat Space Environ Med. 2009;80(5, Suppl):A1–8. doi: 10.3357/asem.br01.2009. [DOI] [PubMed] [Google Scholar]
- 18.Mekjavic PJ, Eiken O, Mekjavic IB. Visual function after prolonged bed rest. J Gravit Physiol. 2002;9:P31–2. [PubMed] [Google Scholar]
- 19.Mudge JF, Baker LF, Edge CB, Houlahan JE. Setting an optimal alpha that minimizes errors in null hypothesis significance tests. PLoS One. 2012;7:e32734. doi: 10.1371/journal.pone.0032734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicogossian AE, Parker JF. Space physiology and medicine (NASA SP-447) Washington, DC: U.S. Government Printing Office; 1982. [Google Scholar]
- 21.Ong E, Ciuffreda KJ. Nearwork-induced transient myopia: a critical review. Doc Ophthalmol. 1995;91:57–85. doi: 10.1007/BF01204624. [DOI] [PubMed] [Google Scholar]
- 22.Pavy-Le Traon A, Heer M, Narici MV, Rittweger J, Vernikos J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986–2006) Eur J Appl Physiol. 2007;101:143–94. doi: 10.1007/s00421-007-0474-z. [DOI] [PubMed] [Google Scholar]
- 23.Prata TS, De Moraes CG, Kanadani FN, Ritch R, Paranhos A., Jr Posture-induced intraocular pressure changes: considerations regarding body position in glaucoma patients. Surv Ophthalmol. 2010;55:445–53. doi: 10.1016/j.survophthal.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Rosner B. Fundamentals of Biostatistics. 5. Pacific Grove, CA: Duxbury Press; 2000. [Google Scholar]
- 25.Sanborn GE, Friberg TR, Allen R. Optic nerve dysfunction during gravity inversion. Visual field abnormalities. Arch Ophthalmol. 1987;105:774–6. doi: 10.1001/archopht.1987.01060060060033. [DOI] [PubMed] [Google Scholar]
- 26.Savini G, Carbonelli M, Parisi V, Barboni P. Repeatability of optic nerve head parameters measured by spectral-domain OCT in healthy eyes. Ophthalmic Surg Lasers Imaging. 2011;42:209–15. doi: 10.3928/15428877-20110224-02. [DOI] [PubMed] [Google Scholar]
- 27.Schirmer CM, Hedges TR., 3rd Mechanisms of visual loss in papilledema. Neurosurg Focus. 2007;23:E5. doi: 10.3171/FOC-07/11/E5. [DOI] [PubMed] [Google Scholar]
- 28.Schuirmann DJ. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987;15:657–80. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- 29.Skau M, Milea D, Sander B, Wegener M, Jensen R. OCT for optic disc evaluation in idiopathic intracranial hypertension. Graefes Arch Clin Exp Ophthalmol. 2011;249:723–30. doi: 10.1007/s00417-010-1527-2. [DOI] [PubMed] [Google Scholar]
- 30.Taibbi G, Cromwell RL, Kapoor KG, Godley BF, Vizzeri G. The effect of microgravity on ocular structures and visual function: a review. Surv Ophthalmol. 2013;58:155–63. doi: 10.1016/j.survophthal.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Taibbi G, Kaplowitz K, Cromwell RL, Godley BF, Zanello SB, Vizzeri G. Effects of 30-day head-down bed rest on ocular structures and visual function in a healthy subject. Aviat Space Environ Med. 2013;84:148–54. doi: 10.3357/asem.3520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vartin CV, Nguyen AM, Balmitgere T, Bernard M, Tilikete C, Vighetto A. Detection of mild papilloedema using spectral domain optical coherence tomography. Br J Ophthalmol. 2012;96:375–9. doi: 10.1136/bjo.2010.199562. [DOI] [PubMed] [Google Scholar]
- 33.Vizzeri G, Weinreb RN, Gonzalez-Garcia AO, Bowd C, Medeiros FA, et al. Agreement between spectral-domain and time-domain OCT for measuring RNFL thickness. Br J Ophthalmol. 2009;93:775–81. doi: 10.1136/bjo.2008.150698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26:192–6. doi: 10.1007/s11606-010-1513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiener TC. Space obstructive syndrome: intracranial hypertension, intraocular pressure, and papilledema in space. Aviat Space Environ Med. 2012;83:64–6. doi: 10.3357/asem.3083.2012. [DOI] [PubMed] [Google Scholar]
- 36.Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, Iliev ME, Frey M, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2009;50:3432–7. doi: 10.1167/iovs.08-2970. [DOI] [PubMed] [Google Scholar]
- 37.Wu H, de Boer JF, Chen TC. Reproducibility of retinal nerve fiber layer thickness measurements using spectral domain optical coherence tomography. J Glaucoma. 2011;20:470–6. doi: 10.1097/IJG.0b013e3181f3eb64. [DOI] [PMC free article] [PubMed] [Google Scholar]