Abstract
Motivational interviewing (MI) is a promising treatment for heavy drinking. Client change talk (CT), a critical component of MI, has been associated with differential brain activation. The goal of this study was to begin to deconstruct how and why CT may affect the brain. Specifically, we sought to determine whether simply repeating statements in favor of change would cause differential brain activation, or whether client statements must be spontaneously generated within a therapeutic milieu in order to influence brain activation. We therefore examined blood oxygenation level dependent (BOLD) response following two types of client language (CT; and sustain talk, ST) across two conditions: (1) Self-Generated: CT and ST were elicited during an MI session vs. (2) Experimenter-Selected: a pre-established list of CT and ST was provided to the individual in the absence of an MI session. Across both conditions, participants’ CT and ST were visually and aurally presented during fMRI. We enrolled 39 recent binge drinkers (41% male; M age = 19.9; n = 18 in Self-Generated group; n = 21 in Experimenter-Selected group). We found that both types of client language (CT and ST) elicited greater BOLD activation in the Self-Generated vs. the Experimenter-Selected group in the left inferior frontal gyrus/anterior insula and superior temporal gyri (p ≤ 0.001). These findings indicate that the nature of client language matters. It appears that it is not just the words themselves, but the origin (naturally generated within a therapeutic session) that influences brain-based effects.
Keywords: alcohol, motivational interviewing, fMRI, client language
1.1 Introduction
Motivational interviewing (MI; Miller & Rollnick, 2013) is a client-centered approach focused on eliciting client language in order to guide clients towards behavior change. Not only is this brief (i.e., 1–2 sessions), empathic, and strength-based intervention highly effective across a number of substance use and health risk behaviors (e.g., Hettema, Steele, & Miller, 2005; Lundahl, Kunz, Brownell, Tollefson, & Burke, 2010), it has been found to be a particularly good fit with wary recipients, such as non-treatment-seeking emerging adults (McCambridge & Strang, 2004). This may be due to the non-judgmental, empathic, and collaborative approach of MI (Miller, Villanueva, Tonigan, & Cuzmar, 2007), whereby the individual’s own values, opinions, and arguments for change are the most valued and reflected part of the therapeutic discussion. However, despite the promise of MI with young problem drinkers (Larimer & Cronce, 2007), the range of observed effect sizes indicates that there is still room for improvement (Carey, Carey, Maisto, & Henson, 2006). Evaluating salient treatment modulators offers one way to examine, and ultimately to target and strengthen, active treatment ingredients.
One innovative way to investigate potential modulators of treatment response is through a translational perspective. Translational investigations actively integrate brain-based approaches with clinical factors to facilitate a more sensitive measure of factors that might influence treatment response (Potenza, Sofuoglu, Carroll, & Rounsaville, 2011; Thayer & Hutchison, 2013). And, functional neuroimaging appears to be a particularly promising avenue to identify salient treatment modulators (Hutchison, 2010). Thus far, initial neurocognitive evaluations of behavioral treatments, including MI, have mirrored the psychosocial literature (Feldstein Ewing, Filbey, Sabbineni, & Hutchison, 2011; Feldstein Ewing, McEachern, et al., 2013; Houck, Moyers, & Tesche, 2013). These studies have shown the importance of client speech in favor of behavior change, or change talk (CT; I’m worried about my drinking), and the risks associated with client speech supporting the behavioral status quo (sustain talk, ST; Drinking is fun.)
While psychosocial evidence highlights the relevance of CT as a treatment target across both the psychosocial (Miller & Rose, 2009) and the neurocognitive fields (Feldstein Ewing, Filbey, Sabbineni, et al., 2011; Feldstein Ewing, McEachern, et al., 2013; Houck et al., 2013), the nature of CT remains under-explored. More specifically, while it is clear that eliciting client statements in favor of change positively impacts treatment outcomes, it is unclear where the power lies - within the change statements themselves, or in their genesis. This is relevant to direct practice, as the MI treatment literature encourages certain clinician approaches and strategies in order to evoke more within-session client CT (Miller & Rollnick, 2013). However, if organic, within-session client CT (Self-Generated CT) catalyzes the same brain-based effects as simply stating or repeating what an interventionist might want to hear (Experimenter-Selected CT; e.g., I will stop drinking), then it stands to reason that a therapeutic session with a behavioral health professional might not, in fact, be necessary to achieve behavior change. This would suggest that problem drinkers could simply repeat therapist-provided statements (i.e., in the absence of a therapeutic interaction) in order to arrive at the same clinical results. Furthermore, at this time, existing behavioral client language coding systems cannot discriminate between organic utterances that stem from the client, and statements that sound like CT, but which may in fact reflect repetitions of provider language (Glynn, Hallgren, Houck, & Moyers, 2012; Moyers, Martin, Manuel, Hendrickson, & Miller, 2005). Thus, a neurocognitive evaluation offers a unique way to access and evaluate whether CT must be elicited within the context of a therapeutic interaction in order to be effective.
Thus, this preliminary study sought to determine how the nature and origin of CT may influence brain response within non-treatment-seeking heavy drinkers, who have been shown to be highly responsive to MI (Larimer & Cronce, 2007). Consistent with our empirically-informed working translational model (Feldstein Ewing, Karoly, & Houck, in press), we suggest that the pattern of activation would follow the process of self-reappraisal and perception observed within Bem’s (1967) historic work in this area. Concretely, we believe that as a person generates (and hears) their language and reasons for changing their drinking behavior (CT), they may reevaluate their own alcohol use, including the experienced benefits and costs of drinking, and its potential fit within their self-view and self-image. In terms of relevant brain regions, during this discussion, we suggest that individuals are likely to be engaging areas both important to self-awareness and introspection, as well as regions critical to reward. Prior studies within both the addiction literature more broadly, as well as the emerging field of treatment response, suggest that those regions are likely to include the inferior frontal gyrus (IFG), (anterior) insula (Feldstein Ewing, Filbey, Sabbineni, et al., 2011; Krishnan-Sarin et al., 2013; Seo, Choi, Chung, Rho, & Chae, 2014; Stewart et al., in press), and superior temporal gyrus (Feldstein Ewing, McEachern, et al., 2013; Goudriaan, de Ruiter, van den Brink, Oosterlaan, & Veltman, 2010; Schacht, Anton, & Myrick, 2013).
Understanding how, where, and why MI activates relevant brain regions is critical to targeting and strengthening areas of response to make treatment more effective for this population. Thus, for this study, our goal was to begin to deconstruct these theoretical relationships, to concretely evaluate brain-based modulators. Thus, we posited that CT spontaneously elicited by the individual within a therapeutic context (Self-Generated CT) would generate greater blood oxygenation level dependent (BOLD) activity in relevant self-awareness regions (e.g., inferior frontal gyrus, insula, superior temporal gyri), as compared to having clients read a list of pre-provided set of statements that “sound like” CT in the absence of an MI session (Experimenter-Selected CT).
2.1 Materials and Methods
2.2 Participants
Following other studies examining MI with heavy drinking emerging adults (Walters, Vader, Harris, Field, & Jouriles, 2009), introductory psychology students were recruited to participate in return for class credit. All procedures were approved by the university Institutional Review Board and under the protection of a federal Certificate of Confidentiality. Similar to other studies, participants were required to be 18 to 25 years of age (Carey et al., 2006; Carey, Henson, Carey, & Maisto, 2007), report at least 4 episodes of binge drinking in the past month (defined as ≥4 drinks/occasion for females; ≥5 drinks/occasion for men) (Carey et al., 2006), provide written consent, and meet fMRI safety criteria prior to entering the fMRI scanner (e.g., absence of non-removable metal implants, not claustrophobic, not pregnant or breastfeeding, breath alcohol of 0 as verified by breathalyzer) (Filbey et al., 2008). To facilitate generalizability, exclusionary criteria were kept purposefully broad. Thus, youth were neither screened for nor excluded on the basis of potential co-occurring neuropsychiatric disorders, somatic conditions, or co-occurring substance use. Participants received $60 in return for participation.
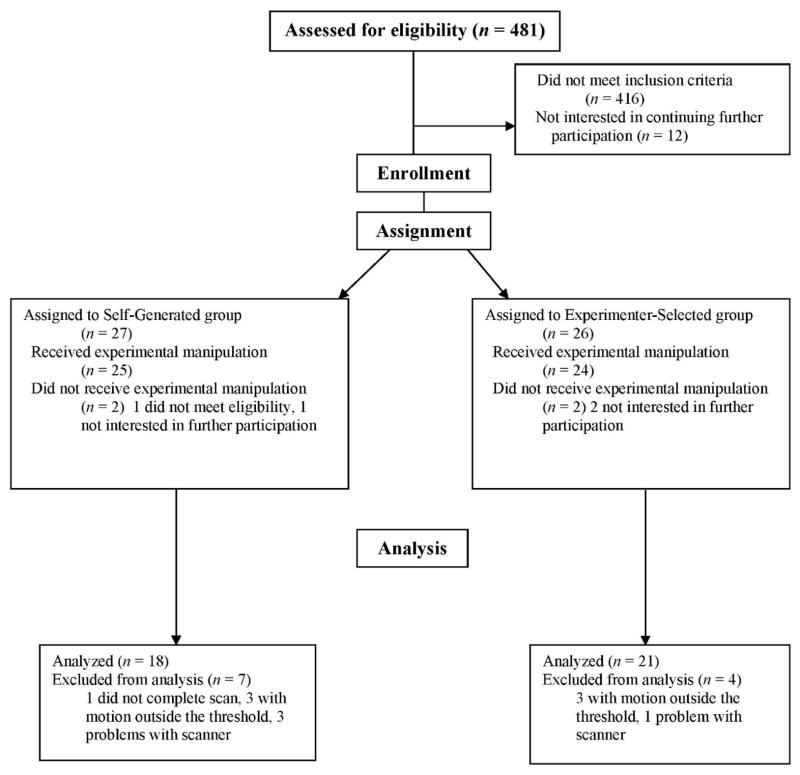
Sixty-five participants were eligible, with 53 assigned to either the Self-Generated condition (n = 26) and Experimenter-Selected condition (n = 27; see Figure 1). Of the 48 with scan data, 39 had minimal head motion within the selected threshold (< 3 mm translational and < 3 degrees rotation). All analyses were therefore conducted with the final sample of 39. This sample was, on average, 19.9 years old (SD = 1.64), 40.9% male, 52.9% Caucasian, 30.7% Hispanic, and 7.9% bi- or multi-racial (see Table 1 for demographics). There were no significant demographic differences between groups.
Fig. 1.
Flow of participants through each stage of the experiment.
Table 1.
Demographic Characteristics of Participating Sample (N = 39)
| Characteristic | Self-Generated (n = 18)
|
Experimenter-Selected (n = 21)
|
Difference Test | ||
|---|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | ||
| Age | 20.18 (1.55) | 18.00–23.00 | 19.57 (1.72) | 18.00–25.00 | t(36) = − 1.13, ns |
| Gender | Male = 7 (38.9%) | Male = 9 (42.9%) | χ2 (1) = 0.06, ns | ||
| Female = 11 (61.1%) | Female = 12 (57.1%) | ||||
| Race/Ethnicity | Caucasian = 7 (43.8%) | Caucasian = 13 (61.9%) | χ2 (5) = 4.63, ns | ||
| Hispanic = 6 (37.5%) | Hispanic = 5 (23.8%) | ||||
| African-American = 1 (6.3%) | African-American = 0 (0%) | ||||
| Asian = 1 (6.3%) | Asian = 0 (0%) | ||||
| Native American = 0 (0%) | Native American = 1 (4.8%) | ||||
| Bi-/Multi-racial = 1 (6.3%) | Bi-/Multi-racial = 2 (9.5%) | ||||
| Past month Drinking Days (TLFB) | 9.89 (6.58) | 3.00–30.00 | 6.67 (3.77) | 2.00–16.00 | t(37) = − 1.91, ns |
| Past Month Binge Drinking Days (TLFB) | 6.56 (6.99) | 1.00–30.00 | 3.38 (1.56) | 1–9 | t(37) = − 2.01, p < .05 |
| Past Month Drinks per Drinking Days (TLFB) | 5.49 (3.35) | 2.83–17.70 | 5.73 (3.07) | 2.57–14.25 | t(37) = 0.23, ns |
| Hazardous drinking symptoms (AUDIT) | 10.94 (3.73) | 6.00–19.00 | 9.81 (3.97) | 6.00–22.00 | t(37) = − 0.92, ns |
| Alcohol related problems (RAPI) | 9.06 (9.21) | 0.00–36.00 | 5.38 (6.79) | 0.00–29.00 | t(37) = − 1.43, ns |
2.3 Procedures
This study was part of a larger treatment mechanisms investigation (Feldstein Ewing, Houck, Truitt, & McEachern, 2013). Within this component, participants completed a psychosocial assessment and an fMRI paradigm to assess the strength of CT and ST. The Self-Generated group completed a behavioral assessment and MI session during their first appointment. The Experimenter-Selected group completed a behavioral assessment during their first appointment, but did not receive an MI session. All fMRI sessions occurred within 1 week of the behavioral assessment.
2.3.1 Behavioral assessment
At the assessment session, participants completed measures of demographics and alcohol use. The demographics questionnaire collected information on age, gender, education, and race/ethnicity.
Problem Drinking
We evaluated the three drinking behaviors that have best characterized the spectrum of problem drinking within this age group (LaChance, Feldstein Ewing, Bryan, & Hutchison, 2009): average amount consumed per drinking occasion, hazardous drinking symptoms, and alcohol-related consequences.
Past Month Average Drinks Per Drinking Day (DDD)
(TLFB; Sobell & Sobell, 1992). This interviewer-administered measure utilizes a calendar format to yield data regarding the type of alcohol most frequently consumed, quantity of alcohol use (drinks per drinking day), and frequency of alcohol use (alcohol use days) and hazardous drinking (binge drinking days) during the past month.
Hazardous Drinking
The Alcohol Use Disorders Identification Test (AUDIT; Babor, 2006) contains 10 items to assess hazardous drinking symptoms. With a cut score of 10, recent studies (Kelly, Donovan, Chung, Cook, & Delbridge, 2004) have utilized this measure to detect hazardous drinking behaviors and the likelihood of meeting criteria for alcohol use disorders (AUDs).
Alcohol-Related Problems
The Rutgers Alcohol Problem Index (RAPI) is the gold standard measure of alcohol-related problems with young samples (White & Labouvie, 1989). It is the instrument of choice to assess alcohol-related problems across a number of studies with this age group (Walters et al., 2009).
2.3.2 Client statements: Self-Generated Group
All youth in this condition received one 60-minute MI session targeting behavior change around alcohol use. Similar to other MI interventions (Walters et al., 2009), the MI followed a manualized approach (Feldstein Ewing & Moyers, 2008) that emphasized the use of active MI-consistent therapist behaviors, including reflections, open-ended questions, and affirmations. All sessions were conducted by 6 PhD students/PhD-level therapists, and were audio-recorded to monitor intervention fidelity and to facilitate the fMRI paradigm. Following the team’s prior work (Feldstein Ewing, Filbey, Sabbineni, et al., 2011), five unique CT and five unique ST statements were extracted from the MI session to use in the fMRI paradigm.
2.3.3 Client statements: Experimenter-Selected group
The goal of this condition was to have participants provide an audio-recorded set of CT and ST statements that would parallel statements made by their peers. To yield the greatest ecological validity, the five most frequently-occurring CT and ST statements were extracted from the Self-Generated condition (e.g., CT: Drinking is getting in the way of my school; Drinking is bad for my health; Drinking is affecting my relationships; I don’t want to become an alcoholic; I don’t want to lose everything I have worked for; ST: I like drinking – it’s fun; Drinking isn’t causing me any problems; Drinking helps me be more social; I’m happy right now – why should I change?; Drinking helps me when I’m stressed out). Immediately following their completion of the behavioral assessment measures, in this condition, youth were asked to read aloud the 5 provided CT and 5 ST statements to gather the requisite audio-recordings for the fMRI paradigm.
2.3.4 Scan session
Participants abstained from alcohol for 24 hours, and from caffeine and cigarettes for 2 hours prior to scanning (verified by self-report and breath alcohol) to avoid having these substances interfere with estimates of brain activation.
2.3.5 Imaging Parameters
MRI images were collected using a 3T Siemens Trio whole body scanner equipped with Sonata gradient subsystem (40 mT/m amplitude, 200 μs rise time, 100% duty cycle) and a 12-channel receive head phased array coil combined with body coil transmission to achieve greater sensitivity in cortical areas. Whole brain fMRI scans were collected using a gradient echo, echoplanar sequence with ramp sampling correction using the intercomissural line (AC-PC) as a reference (TR: 2.0 s, TE: 27ms, α: 70°, matrix size: 64×64, 32 slices, voxel size: 3×3×4 mm). A tilting acquisition similar to that reported in Filbey et al. (2008) was used to increase signal-to-noise in the orbitofrontal cortex. A high resolution anatomical MRI scan was collected with a T1-weighted multi-echo Magnetization Prepared Rapid Gradient Echo or MPRAGE (MEMPR) with TR/TE/TI=2300/2.74/900 ms, flip angle=8°, FOV=256x256 mm, slab thickness=176 mm, matrix=256×256×176, voxel size=1×1×1 mm, number of echoes=5, pixel bandwidth=650 Hz. A volume set-up established ideal audio levels.
2.3.6 fMRI task
Assessing the effects of CT and ST statements on brain activity were modeled upon a prior fMRI task (Feldstein Ewing, Filbey, Sabbineni, et al., 2011). We were interested in the neural processes during client language (other behavioral factors evaluated in Feldstein Ewing, McEachern, et al., 2013). Thus, all fMRI analyses focused only on the relationship between client language and BOLD activation. In this block design, to ensure an adequate number of trials, and thereby increase the signal-to-noise ratio, we pseudo-randomly presented participants with each of the 5 change talk (CT) and 5 sustain talk (ST) statements twice, yielding 10 CT and 10 ST statements per participant over the course of 4 runs. All runs were counterbalanced by randomly assigning the administration order. For a single run, each trial started with a 16-second audio clip of the participants’ audio-recorded statements simultaneously with a visual presentation of the client’s transcribed CT and ST statements. Statements were presented visually as well as via audio to assist comprehension during moments of loud, ambient noise within the scanner, which can sometimes make hearing auditory stimuli difficult. To serve as an additional level of control, all participants completed an audio set-up (volume adjustment) prior to the scan to ensure their ability to hear the audio stimuli. All participants completed a post-scan debriefing assessment to verify their ability to hear each presented statement. Additional details regarding the fMRI task design are readily available upon author request.
2.3.7 fMRI data analysis
Functional imaging time series were processed using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK). The first seven volumes of each EPI run were discarded to allow the MR signal to reach steady state. Standard pre-processing steps were followed: (1) motion correction using SPM’s realignment module (Friston et al., 1995); subjects with >3 mm translation or 3 degrees rotation were excluded from analyses (n = 9); (2) slice timing correction; (3) normalization into the Montreal Neurological Institute (MNI) standard space (Ashburner & Friston, 1999); and (4) smoothing with a 10 mm (full-width half-maximum) Gaussian kernel.
fMRI signal intensity was scaled by its grand mean and the data were high-pass filtered (filter frequency 1/128.0 Hz). Serial correlations in the data were modeled using the autocorrelation correction with order 1 [AR(1)] (Purdon & Weisskoff, 1998). The regressors (presentation of CT and ST) were created by convolving the stimulus timing files with the canonical hemodynamic response function characterized by two gamma functions, one modeling the peak and the other modeling the undershoot of a typical BOLD impulse response in SPM. The rating period was modeled as a condition of no interest to remove the effect of BOLD signal evoked by the rating task. The remaining conditions were not modeled and formed the implicit baseline for the design matrix. Standard voxel-by-voxel GLM analysis (Friston, Ashburner, Kiebel, Nichols, & Penny, 2007) of the pre-processed functional time series was performed against the resultant design matrix generating beta maps for each condition (CT relative to baseline; ST relative to baseline).
To our knowledge, we are the first to investigate brain mechanisms that underlie change language in this manner. Thus, to evaluate study hypotheses, we conducted independent samples t-tests to determine between-group differences, using an uncorrected p < 0.001, cluster threshold ≥ 720 μl [20 voxels]. The anatomical localization for the regions of activation was confirmed using the Talairach Daemon software and verified visually (Lancaster et al., 2000). Multi-slice overlay of activation maps was obtained using xjView (Cui, Li, & Song, 2010).
3.1 Results
3.2 Alcohol Use and Demographic Variables
Across groups, this sample reported heavy drinking and related symptoms (see Table 1), as indicated by their overall frequency of drinking (past month M = 8.28 drinking days), their frequency of high-risk, binge drinking (past month M = 4.97 binge drinking days), and their average quantity of consumption (past month M = 5.61 drinks per drinking day). In addition, participants showed high levels of hazardous drinking (M = 10.38, as assessed with the AUDIT), and alcohol related problems (M = 7.22, as assessed with the RAPI). Overall, we found no differences in alcohol use or related symptoms, with the exception of binge drinking, whereby the Self-Generated group reported significantly more binge drinking. Importantly, to address this issue, we re-analyzed the data controlling for binge drinking. No significant differences were observed. Thus, the original analyses were retained.
3.3 Group Differences in Change Language: Self-Generated vs. Experimenter-Selected
3.3.1 Change Talk (CT)
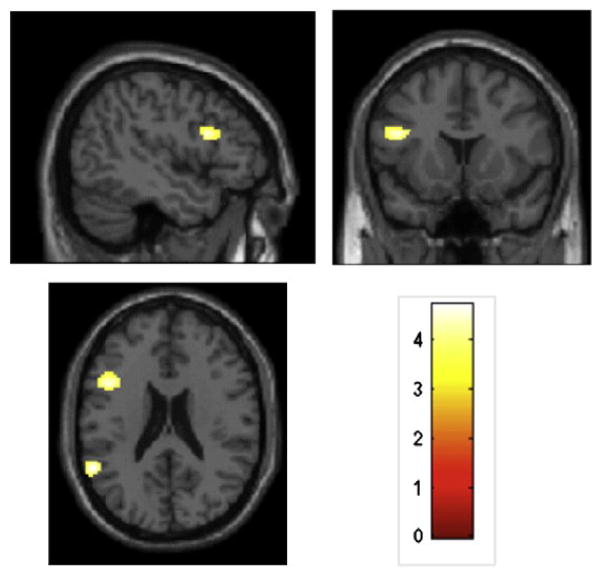
We observed significant differences between the Self-Generated and Experimenter-Selected groups during CT, indicating a group-based differential pattern of response to statements in favor of behavior change (CT). Specifically, during CT, the Self-Generated group showed significantly greater activation than the Experimenter-Selected group in the fronto-insular cortex (inferior frontal gyrus, anterior insula), occipital lobe (fusiform gyrus, middle and inferior occipital gyri), and parietal lobe (supramarginal gyrus), with additional clusters in the middle and superior temporal gyri (see Table 2 for areas and effect sizes; Figure 2). In contrast, during CT, the Experimenter-Selected group showed significantly greater activation than the Self-Generated group in just the precentral gyrus.
Table 2.
Regions of increased BOLD response to Change Talk (CT) and Sustain Talk (ST) in the Self-Generated (n =18) vs. Experimenter-Selected group (n =21).
| # voxels | Localization | BA | x (mm) | y (mm) | z (mm) | Max t | d |
|---|---|---|---|---|---|---|---|
| Change Talk Condition (Self-Generated>Experimenter-Selected) | |||||||
|
| |||||||
| 326 | R middle temporal gyrus | 21 | 56 | −38 | 0 | 4.7 | 1.54 |
| 139 | R superior temporal gyrus | 38 | 54 | 16 | −18 | 4.58 | 1.51 |
| 88 | L inferior frontal gyrus | 47 | −42 | 24 | 0 | 4.11 | 1.35 |
| 248 | L middle occipital gyrus | 18 | −28 | −90 | 14 | 4.09 | 1.35 |
| 154 | R inferior occipital gyrus | 18 | 32 | −88 | −14 | 4.02 | 1.32 |
| R fusiform gyrus | 19 | 38 | −66 | −8 | 3.97 | ||
| R fusiform gyrus | 19 | 38 | −78 | −10 | 3.53 | ||
| 199 | L supramarginal gyrus | 40 | −66 | −46 | 30 | 3.79 | 1.25 |
| L superior temporal gyrus | 39 | −64 | −56 | 24 | 3.65 | ||
| L supramarginal gyrus | 40 | −58 | −48 | 24 | 3.56 | ||
| 94 | L middle temporal gyrus | 21 | −54 | −26 | −4 | 3.78 | 1.24 |
|
| |||||||
| Sustain Talk Condition (Self-Generated>Experimenter-Selected) | |||||||
|
| |||||||
| 133 | L supramarginal gyrus | 40 | −56 | −50 | 22 | 4.72 | 1.55 |
| 181 | L inferior frontal gyrus | 9 | −46 | 14 | 22 | 4.42 | 1.45 |
| 236 | R middle temporal gyrus | 21 | 54 | −34 | −6 | 4.23 | 1.39 |
| R fusiform gyrus | 37 | 50 | −46 | −12 | 3.9 | ||
| 49 | L inferior frontal gyrus | 47 | −40 | 26 | 2 | 4.17 | 1.37 |
| 33 | L thalamus | - | −10 | −8 | 16 | 3.68 | 1.21 |
| 42 | L brainstem/Red nucleus | - | −4 | −26 | −8 | 3.65 | 1.20 |
Note. Results have been reported at uncorrected p ≤ 0.001, extent threshold ≥ 20 voxels. BA = Brodmann area; R= right; L = left.
Fig. 2.
Regions of significantly increased BOLD activation in the Self-Generated as compared to the Experimenter-Selected group for Change Talk (uncorrected p b 0.001, extent threshold N 20 voxels). Activations have been overlaid onto contiguous slices of the T1 canonical template spaced 3 mm apart. Activations are observed in the inferior frontal gyrus and middle and superior temporal gyri. The color bar indicates t values.
3.3.2 Sustain Talk (ST)
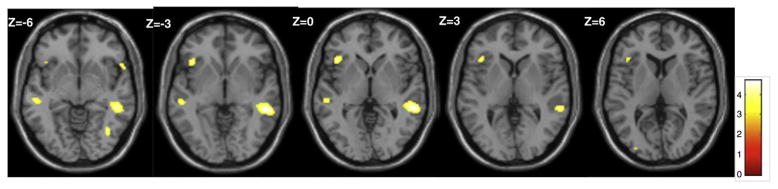
We also observed significant differences between the Self-Generated and Experimenter-Selected groups during ST, demonstrating a group-based differential pattern of response to statements in favor of sustaining drinking behavior (ST). During ST, the Self-Generated group had significantly greater activity than the Experimenter-Selected group in the supramarginal, fusiform gyrus, middle temporal gyri, frontal lobe (IFG), and sub-lobar regions (thalamus; see Table 2 for areas and effect sizes; Figure 3). During ST, the Experimenter-Selected group did not show any areas of greater activation than the Self-Generated group (see Table 2).
Fig. 3.
Regions of significantly increased BOLD activation in the Self-Generated as compared to the Experimenter-Selected group for Sustain Talk (uncorrected p b 0.001, extent threshold N 20 voxels). Activations are observed in the inferior frontal and supramarginal gyri. The color bar indicates t values.
3.4 Manipulation Check
To check the experimental manipulation, following their fMRI scan, participants in the Experimenter-Selected condition (only) completed a Likert-scaled measure indicating their agreement with each of the 10 statements provided to them in their fMRI paradigm [5 CT and 5 ST; “On a scale of 1 (totally disagree) to 10 (totally agree), please rate how much you agree with each of the following statements”]. Participants reported equal agreement for both the provided CT (M = 6.18) and ST statements (M = 6.73). To determine how their level of agreement with the statements might correspond with brain activation, we regressed the BOLD activation during CT with level of agreement with the provided statements. We found an area of positive activation in the lateral superior temporal gyrus (BA 38; coordinates = 44, 18, −28), suggesting that greater agreement with CT corresponded with greater activation for the Experimenter-Selected group. We also assessed BOLD activation during ST and level of agreement. We found no areas of significant activation, indicating that participant agreement with the ST statements did not appear to drive the observed outcomes for the Experimenter-Selected group.
4.1 Conclusions
The goal of this study was to employ a creative, translational approach to evaluate how the nature and origin of change talk (CT) may differentially influence brain response. Several studies have found that contemplating personally-relevant, substance-related consequences is associated with both behavioral and brain-based changes (Kober, Kross, Mischel, Hart, & Ochsner, 2010), and that this self-referential processing may occur in areas important to self-awareness, including the inferior frontal gyrus (IFG), (anterior) insula (Feldstein Ewing, Filbey, Sabbineni, et al., 2011; Krishnan-Sarin et al., 2013; Seo et al., 2014; Stewart et al., in press), and superior temporal gyrus (Feldstein Ewing, McEachern, et al., 2013; Goudriaan et al., 2010; Schacht et al., 2013).
Thus, for this study, we posited that change statements spontaneously generated by the client within the context of a true MI session (Self-Generated CT) would result in significantly greater BOLD activation in areas related to self-awareness (e.g., IFG/anterior insula, middle and superior temporal gyri), as compared with statements that “sound like” CT, but were not generated by the client during a therapeutic session (Experimenter-Selected CT). Supporting and extending our study hypotheses, we found that the Self-Generated group not only had significantly greater activation for CT, but also for ST, in the left IFG/anterior insula as well as middle and superior temporal gyri. We believe that these results reflect the neurocognitive processes that take place during a therapeutic intervention.
More concretely, the greater activation of these regions for the Self-Generated group are in line with recent translational theory (Feldstein Ewing, Filbey, Hendershot, McEachern, & Hutchison, 2011) and empirical data indicating the involvement of introspective areas, such as the frontal lobe and insula, in behavioral treatment outcomes (DeVito et al., 2012; Vollstadt-Klein et al., 2011). These studies suggest that having a participant generate their own thoughts and reasons for change within the context of a behavioral intervention may more strongly activate neurocognitive areas important in introspection and personal connection than if they simply repeat similar statements. Building upon the psychosocial literature, these data also indicate the importance of client-generated language (i.e., self-generated CT and ST) by demonstrating elevated activation patterns in relevant neural substrates.
While the Experimenter-Selected group did not show significant neural response during ST, they did show significantly greater activation than the Self-Generated group during ST in the precentral gyrus. Potentially due to its involvement in motor function, greater precentral gyrus activity is often attributed to greater effort (Han et al., 2011; Tapert et al., 2004). In this case, this activation may suggest the greater effort required by participants to process unfamiliar, potentially less personally-relevant third-party ST, as contrasted with their self-generated reasons for staying the same.
Together, these data also have important implications for direct practice. To that end, this study indicates that when participants do not produce their own self-relevant change language in the context of a true therapeutic relationship, such as stating what therapists may want to hear or parroting change language, relevant brain-based changes may not be initiated. Taken a step further, these brain-based data may suggest that MI-inconsistent therapist behaviors (e.g., confront, warn, advise without permission) (Moyers & Martin, 2006) that do not successfully engage clients in the active process of self-reflection are unlikely to generate the observed pattern of brain-based activation, and ultimately, related behavior change. Instead, an evocative conversation with a therapist, where clients are drawn forth to spontaneously provide self-relevant statements, may be critical to successful clinical treatment outcomes.
This study offers a preliminary step toward understanding the biological correlates of spontaneous and personally-relevant client language. While this study has several strengths, findings must be interpreted considering the limitations of the study. First, to evaluate study hypotheses, the design of this study required that the Self-Generated group differ from the Experimenter-Selected group in two ways: the Self-Generated participants generated their own CT statements, and also spent 60 minutes with a therapist who was actively working with them to elicit their natural change language. The Experimenter-Selected group had no opportunity to work with someone who actively elicited their change language and/or who provided any context or rationale supporting the reasons/importance of their changing, prior to the presentation of their change statements in the scanner. Thus, only one experimental group received a true clinical treatment session (MI) prior to the scan. Despite our efforts to present an ecologically-valid set of statements in the comparison condition, we were unable to ensure that the presented statements “made sense” to the recipients of the Experimenter-Selected condition. Future work comparing how active ingredients contrast across two different therapeutic approaches, as well as within an MI-inconsistent “sham” session (e.g., one focused on advising participants to stop drinking), represents an important next step. This would facilitate the ability to control for and compare the clinical nature, clinical presentation and rationale, and relevance of the statements between groups. Second, the results may, in part, reflect self- versus other- effects, along with clinically relevant changes. Third, due to institutional issues in approval, participants were run sequentially rather than being randomized to each condition. Simultaneously conducted, randomized controlled trials are preferable for future work. Fourth, while memory is likely to be important in treatment response (Wilcox, Dekonenko, Mayer, Bogenschutz, & Turner, 2014), we did not observe relevant activation in brain memory areas, which we would expect if observed outcomes were being driven by memory processes. Fifth, while matched across all other demographic and clinical variables, the two groups had different levels of binge drinking; matching on this variable to control for this potential issue is an important consideration for future studies. Sixth, although current scanner limitations preclude conducting MI in vivo within the context of fMRI (Feldstein Ewing, Filbey, Sabbineni, et al., 2011), other brain-based methodologies permit a temporally-sensitive evaluation of brain response during therapy (magnetoencephalograpy) (Houck et al., 2013). Our team continues to brainstorm ways to extend this in vivo work to the fMRI modality. Finally, while this is a preliminary examination, our effect sizes indicate that this study is an important first step, suggesting that it is not simply CT, but spontaneous, personally-relevant change talk made in the context of a therapeutic exchange that is critical in client change language.
Contributor Information
Sarah W. Feldstein Ewing, Email: swfeld@salud.unm.edu.
Uma Yezhuvath, Email: uma.yezhuvath@advancemri.com.
Jon M. Houck, Email: jhouck@unm.edu.
Francesca M. Filbey, Email: filbey@utdallas.edu.
References
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Human Brain Mapping. 1999;7(4):254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Indentification Test: Guidelines for Use in Primary Care. 2. The World Health Organization; 2006. [Google Scholar]
- Bem DJ. Self-perception: An alternative intepretation of cogntiive dissonance phenomena. Psychological Review. 1967;74:183–200. doi: 10.1037/h0024835. [DOI] [PubMed] [Google Scholar]
- Carey KB, Carey MP, Maisto SA, Henson JM. Brief motivational interventions for heavy college drinkers: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2006;74(5):943–954. doi: 10.1037/0022-006X.74.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KB, Henson JM, Carey MP, Maisto SA. Which heavy drinking college students benefit from a brief motivational intervention? Journal of Consulting and Clinical Psychology. 2007;75(4):663–669. doi: 10.1037/0022-006X.75.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Li J, Song X. xjView (Version 8) 2010 http://www.alivelearn.net/xjview.
- DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Kober H, Potenza MN. A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug and Alcohol Dependence. 2012;22:228–235. doi: 10.1016/j.drugalcdep.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Hendershot CS, McEachern AD, Hutchison KE. A proposed model of the neurobiological mechanisms underlying psychosocial alcohol interventions: The example of motivational interviewing and functional magnetic resonance imaging. Journal of Studies on Alcohol and Drugs. 2011;72:903–916. doi: 10.15288/jsad.2011.72.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. How psychosocial alcohol interventions work: A preliminary look at what fMRI can tell us. Alcoholism: Clinical and Experimental Research. 2011;35(4):1–9. doi: 10.1111/j.1530-0277.2010.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Houck JM, Truitt D, McEachern AD. The role of impulsivity, anger, verbal ability, and abstract reasoning in emerging adults’ treatment outcomes. Journal of Addiction and Prevention. 2013;1(1):5. [Google Scholar]
- Feldstein Ewing SW, Karoly H, Houck JM. Deconstructing the neural substrates of motivational interviewing: A new look at an unresolved question. In: Feldstein Ewing SW, Witkiewitz K, Filbey FM, editors. Neuroimaging and Psychosocial Addiction Treatment. London, UK: Guilford; (in press) [Google Scholar]
- Feldstein Ewing SW, McEachern AD, Yezuhavth U, Bryan AD, Hutchison KE, Filbey FM. Integrating brain and behavior: Evaluating adolescents’ response to a cannabis intervention. Psychology of Addictive Behaviors. 2013;27:510–525. doi: 10.1037/a0029767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Moyers T. Motivational Interviewing with Neuroimaging; The MINI Study Intervention Manual. Albuquerque, NM: The Mind Research Network; 2008. [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33(6):1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Frith CD, Poline JP, Heather JD, Frackowiak RS. Spatial registration and normalization of images. Human Brain Mapping. 1995;2:165–189. [Google Scholar]
- Friston K, Ashburner J, Kiebel SJ, Nichols TE, Penny WD. Statistical parametric mapping: The analysis of funtional brain images. Academic press; 2007. [Google Scholar]
- Glynn LH, Hallgren KA, Houck JM, Moyers TB. CACTI: Free, open-source software for the sequential coding of behavioral interactions. PLoS One. 2012;7:e39740. doi: 10.1371/journal.pone.0039740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, de Ruiter MB, van den Brink W, Oosterlaan J, Veltman DJ. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: An fMRI study. Addiction Biology. 2010;15:491–503. doi: 10.1111/j.1369-1600.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DH, Bolo N, Daniels MA, Aranella L, Lyoo IK, Renshaw PF. Brain activity and desire for internet video game play. Comprehensive Psychiatry. 2011;52:88–95. doi: 10.1016/j.comppsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller WR. A meta-analysis of research on motivational interviewing treatment effectiveness (MARMITE) Annual Review of Clinical Psychology. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Houck JM, Moyers TB, Tesche CD. Through a glass darkly: Some insight on change talk via magnetoencephalography. Psychology of Addictive Behaviors. 2013;27:489–500. doi: 10.1037/a0029896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE. Substance use disorders: Realizing the promise of pharmacogenomics and personalized medicine. Annual Review of Clinical Psychology. 2010;6:577–589. doi: 10.1146/annurev.clinpsy.121208.131441. [DOI] [PubMed] [Google Scholar]
- Kelly TM, Donovan JE, Chung T, Cook RL, Delbridge TR. Alcohol use disorders among emergency department-treated older adolescents: A new brief screen (RUFT-Cut) using the AUDIT, CAGE, CRAFFT, and RAPS-QF. Alcoholism: Clinical and Experimental Research. 2004;28(5):746–753. doi: 10.1097/01.alc.0000125346.37075.85. [DOI] [PubMed] [Google Scholar]
- Kober H, Kross EF, Mischel W, Hart CL, Ochsner KN. Regulation of craving by cognitive strategies in cigarette smokers. Drug and Alcohol Dependence. 2010;106:52–55. doi: 10.1016/j.drugalcdep.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Balodis IM, Kober H, Worhunsky PD, Liss T, Xu J, Potenza MN. An exploratory pilot study of the relationship between neural correlates of cognitive control and reduction in cigarette use among treatment-seeking adolescent smokers. Psychology of Addictive Behaviors. 2013;27:526–532. doi: 10.1037/a0032479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaChance HA, Feldstein Ewing SW, Bryan AD, Hutchison KE. What makes group MET work? A randomized controlled trial of college student drinkers in mandated alcohol diversion. Psychology of Addictive Behaviors. 2009;23(4):589–612. doi: 10.1037/a0016633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer ME, Cronce JM. Identification, prevention, and treatment revisited: Individual-focused college drinking prevention strategies 1999–2006. Addictive Behaviors. 2007;32(11):2439–2468. doi: 10.1016/j.addbeh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Lundahl BW, Kunz C, Brownell C, Tollefson D, Burke BL. A meta-analysis of motivational interviewing: Twenty-five years of empirical studies. Research on Social Work Practice. 2010;20(2):137–160. [Google Scholar]
- McCambridge J, Strang J. The efficacy of single-session motivational interviewing in reducing drug consumption and perceptions of drug-related risk and harm among young people: Results from a multi-site cluster randomized trial. Addiction. 2004;99:39–52. doi: 10.1111/j.1360-0443.2004.00564.x. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3. New York: Guilford Press; 2013. [Google Scholar]
- Miller WR, Rose GS. Toward a theory of motivational interviewing. American Psychologist. 2009;64(6):527–537. doi: 10.1037/a0016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Villanueva M, Tonigan JS, Cuzmar I. Are special treatments needed for special populations? Alcoholism Treatment Quarterly. 2007;25(4):63–78. [Google Scholar]
- Moyers TB, Martin T. Therapist influence on client language during motivational interviewing sessions: Support for a potential causal mechanism. Journal of Substance Abuse Treatment. 2006;30:245–251. doi: 10.1016/j.jsat.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. Journal of Substance Abuse Treatment. 2005;28(1):19–26. doi: 10.1016/j.jsat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdon PL, Weisskoff RM. Effect of temporal autocorrelation due to physiological noise and stimulus paradigm on voxel-level false-positive rates in fMRI. Human Brain Mapping. 1998;6(4):239–249. doi: 10.1002/(SICI)1097-0193(1998)6:4<239::AID-HBM4>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuorimaging studies of alcohol cue reactivity: A quantative meta-analysis and systematic review. Addiction Biology. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HJ, Choi YH, Chung YA, Rho W, Chae JH. Changes in cerebral blood flow after cognitive behavior therapy in patients with panic disorder: A SPECT study. Journal of Neuropsychiatric Disease and Treatment. 2014;10:661–669. doi: 10.2147/NDT.S58660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Time-line follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption. Totowa, N.J: Humana Press; 1992. pp. 73–98. [Google Scholar]
- Stewart JL, Connolly GC, May AC, Tapert SF, Wittmann M, Paulus MP. Cocaine dependent individuals with attenuated striatal activation during reinforcement learning are more susceptible to relapse. Psychiatry Research. doi: 10.1016/j.pscychresns.2014.04.014. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Thayer RE, Hutchison KE. Neuroimaging in clinical studies of craving: Importance of reward and control networks. Psychology of Addictive Behaviors. 2013;27:543–546. doi: 10.1037/a0030275. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Buhler M, Von der Goltz C, Hermann D, Mann K, Kiefer F. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: A randomized trial. Biological Psychiatry. 2011;69(11):1060–1066. doi: 10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Walters ST, Vader AM, Harris TR, Field CA, Jouriles EN. Dismantling motivational interviewing and feedback for college drinkers: A randomized clinical trial. Journal of Consulting and Clinical Psychology. 2009;77(1):64–73. doi: 10.1037/a0014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Labouvie EW. Towards the assessment of adolescent problem drinking. Journal of Studies on Alcohol. 1989;50:30–37. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Dekonenko CJ, Mayer AR, Bogenschutz MP, Turner JA. Cognitive control in alcohol use disorder: Deficits and clinical relevance. Rev Neurosci. 2014;25:1–24. doi: 10.1515/revneuro-2013-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]