Version Changes
Revised. Amendments from Version 1
Manuscript text and title have been edited according to reviewer’s recommendations and suggestions. No changes have been made to data and figures, however figure legends have been rewritten to clarify the figures, as requested by reviewers. No further experiments have been added. For further details, see rebuttal to reviewer.
Abstract
The activity of excitatory neurons is controlled by a highly diverse population of inhibitory interneurons. These cells show a high level of physiological, morphological and neurochemical heterogeneity, and play highly specific roles in neuronal circuits. In the mammalian hippocampus, these are divided into 21 different subtypes of GABAergic interneurons based on their expression of different markers, morphology and their electrophysiological properties. Ideally, all can be marked using an antibody directed against the inhibitory neurotransmitter GABA, but parvalbumin, calbindin, somatostatin, and calretinin are also commonly used as markers to narrow down the specific interneuron subtype. Here, we describe a journey to find the necessary immunological reagents for studying GABAergic interneurons of the mouse hippocampus. Based on web searches there are several hundreds of different antibodies on the market directed against these four markers. Searches in the literature databases allowed us to narrow it down to a subset of antibodies most commonly used in publications. However, in our hands the most cited ones did not work for immunofluorescence stainings of formaldehyde fixed tissue sections and cultured hippocampal neurons, and we had to immunostain our way through thirteen different commercial antibodies before finally finding a suitable antibody for each of the four markers. The antibodies were evaluated based on signal-to-noise ratios as well as if positive cells were found in layers of the hippocampus where they have previously been described. Additionally, the antibodies were also tested on sections from mouse spinal cord with similar criteria for specificity of the antibodies. Using the antibodies with a high rating on pAbmAbs, an antibody review database, stainings with high signal-to-noise ratios and location of the immunostained cells in accordance with the literature could be obtained, making these antibodies suitable choices for studying the GABAergic system.
Introduction
Hippocampal networks are composed of a large portion of excitatory principal cells and a smaller cohort of inhibitory interneurons 1. Inhibitory interneurons release γ-aminobutyric acid (GABA), which is the major inhibitory neurotransmitter in the brain. Its principal action is mediated through ubiquitous fast ionotropic GABA A receptors by increasing the membrane permeability to Cl - ions 2. This inhibitory mechanism regulates the excitability of both principal cells and GABAergic interneurons. In this way, GABA is able to efficiently control the rhythms of cortical networks 3, which is believed to be of critical importance for information processing 4 alterations in cortical network rhythms in specific brain networks that may underlie neuropsychiatric disorders, such as schizophrenia, depression and bipolar disorder, is thought to involve a defective GABA system 5.
Inhibitory interneurons of the dentate gyrus is a highly diverse population and early studies identified up to 21 different subtypes in this region alone 6. Immunostaining against GABA have shown discrepancy when compared to in-situ hybridization against glutamate decarboxylase, the enzyme that catalyzes the decarboxylation of glutamate to GABA, indicating that some cells may express very low levels of GABA leaving this as an insufficient choice for immunostaining 7– 9. These 21 subtypes can be distinguished based on axonal distribution, synaptic targets, neuropeptide or calcium-binding protein content and physiological characteristics 10. In order to fully characterize a subtype, all parameters must be taken into account. When immunostaining against neuropeptides or calcium-binding proteins, this is not possible, and immunostaining therefore only allows characterization of subgroups.
One such subgroup is the parvalbumin expressing interneurons. Parvalbumin-labelled cell bodies are found primarily near the granule cell layer and are most prominent at the base of the granule cell layer. However, few are also found near the border of the granule cell and molecular layers and some in the hilus as well 10. Although this is considered the largest group of the subgroups in the hippocampus, in the dentate gyrus these only represent around 20% of the total number of GABAergic interneurons as compared to around 40% in CA1 and CA3 11.
Several distinct populations are found that express the calcium-binding protein calretinin. Most notably, calretinin is also found in mossy cells of the hilus 12, and such mossy cells are particular numerous in the ventral hilus. Calretinin is also found in axon terminals of mossy cells which creates a dense band of labelling in the inner third of the molecular layer 13.
Despite labelling of mossy cells in the hilus, some GABAergic interneurons can also be found in the hilus near the granule layer 14. These can often be distinguished by the more intense labelling when staining for calretinin compared to that of mossy cells.
Another subgroup is the somatostatin expressing interneurons. This subgroup comprises the largest group of GABAergic interneurons in the dentate gyrus and these are almost exclusively found within the hilus where they comprise approximately 55% of the total number of GABAergic interneurons with a slight increase from the dorsal to the ventral part of hippocampus 15. As almost all somatostatin positive interneurons are found within the hilus, little labelling is found within the granule cell layer, except from a large number of axons from hilar somatostatin interneurons that project through this layer 15, 16.
Calbindin has been found to be present in both inhibitory and excitatory neurons with a rather strong staining of granule cells in the dentate gyrus. Misplaced granule cells found in the stratum radiatum of the CA3 subfield are often mistaken for GABAergic interneurons but these are not positive for GABA 1. All other cells in the dentate gyrus should be considered GABAergic interneurons and generally stain for GABA 1. A precise percentage of calbindin interneurons is not available, but around 10–12% of total number of GABAergic interneurons is considered a close estimate 17. Very few calbindin positive interneurons are found in the dentate gyrus compared to the CA-regions and these are difficult to detect due to the strong staining of granule cells, but calbindin positive interneurons can be found in the stratum moleculare and hilus 1.
Importantly, markers of hippocampal GABAergic interneurons do not readily apply to other regions such as the spinal cord GABAergic interneurons. The inhibitory interneurons of the spinal dorsal horn use primarily GABA and/or glycine. GABAergic interneurons are primarily located in laminae I, II and III of the dorsal horn and constitute approximately 25%, 30% and 40% of rat laminae I, II and III neurons, respectively 18, 19. The inhibitory effect of glycine is facilitated by activation of ionotropic ligand-gated glycine receptors that mediate an influx of chloride ions 20 and within lamina I-III glycine immunostaining is largely restricted to GABAergic neurons 18, 19.
GABAergic interneurons of the spinal dorsal horn can be identified by immunostaining against, for instance, parvalbumin and the neuronal form of nitric oxide synthase (n-NOS) besides GABA and glycine. Parvalbumin is expressed by a subpopulation of spinal cord dorsal horn interneurons that co-express GABA and glycine 21– 23. Conversely, calretinin, somatostatin and calbindin do not co-localize with GABA in interneurons of the dorsal horn, for which reason they are thought to co-localize to excitatory interneurons 21, 23– 25. Thus, care should be taken when extrapolating interneuron markers from one region of the CNS to another. In the present study, we have evaluated a number of different antibodies ( Table 2) against GABAergic markers using both cultured neurons and tissue sections. All tested antibodies have previously been reported to recognize GABAergic interneurons both in peer-reviewed publications and by the manufacturers.
Materials and methods
All experiments were approved by the Danish Animal Experiments Inspectorate under the Ministry of Justice (Permit 2011/561-119) and carried out according to institutional and national guidelines.
For a full list of reagents and chemicals, please see Table 1.
Hippocampal section preparation and immunostaining
Hippocampal sections. Adult C57BL/6j Bomtac (wild type (wt)) mice (Taconic), aged 8 weeks were deeply anesthetized by intraperitoneal injection of 5 mg/ml pentobarbital and perfused transcardially with cold 4% (w/v) formaldehyde (pH 7.4, Hounisen) for five minutes. The brains were hereafter removed and post-fixed in 4% (w/v) formaldehyde overnight at 4°C. The next day the brains were moved to 30% (w/v) sucrose (Merck Millipore) for cryoprotection and left at 4°C for 48 hours, moulded in Tissue-Tek ® (Sakura) and stored at -20°C. Coronal hippocampal sections (10 µm) were cut at -20°C using a Leica CM1900 cryostat (using low-profile disposable blades 819 from Leica Biosystems) and the sections were afterwards stored at -20°C until use.
Immunostaining of tissue. Antigen epitopes shielded by formaldehyde cross-linked lysine side chains were retrieved in a heat-mediated antigen retrieval step using Target Retrieval Solution (Dako), according to manufacturers’ protocol. Hereafter, the sections were washed three times in Tris-buffered saline (TBS; pH 7.4) of ten minutes intervals, and incubated in a solution of TBS containing 0.3% Triton X-100 (Applichem) and 1% bovine serum albumin (BSA; Sigma) for thirty minutes. Following a ten minute washing step in TBS, the sections were incubated with primary antibody ( Table 2) in a 50 mM Tris-based (TB) buffer solution (pH 7.4) containing 1% BSA (Sigma) at 4°C in a moisturized chamber overnight. The next day, the sections were left at room temperature (RT) for one hour, and subsequently washed three times in TBS. Sections were then incubated with secondary antibody ( Table 3) in a 50 mM TB buffer solution containing 1% BSA (Sigma) at RT for four hours. Finally, the sections were washed three times five minutes in TBS, with Hoechst (5 µg/µl, Sigma-Aldrich) being included in the last wash. The sections were hereafter mounted using Fluorescence Mounting Medium (Dako) and stored at 4°C. As negative controls of the immunostaining, simultaneous stainings were done using a similar protocol, except primary antibody was omitted. All immunostatings were tested on at least three different wild type males and repeated at least three times.
Spinal cord section preparation and immunostaining
Spinal cord sections. Adult C57BL/6j Bomtac (wt) mice aged 16 weeks were deeply anaesthetized using 4% isoflurane (IsoFlo ® vet, Abbott) prior to decapitation and hydraulic spinal cord extrusion 26 using ice-cold phosphate-buffered saline (PBS; pH 7.4) as the extrusion liquid. Spinal cords were fixed in 4% (w/v) paraformaldehyde (PFA; Sigma) in PBS (pH 7.4) overnight at 4°C. The spinal cords were then cryoprotected overnight by immersion in 25% (w/v) sucrose in PBS (pH 7.4) at 4°C. Lumbar sections 2–4 of the spinal cords were isolated and embedded in TissueTek ® (Sakura) prior to freezing, which was performed by lowering the tissue into dry-ice cold iso-pentane (VWR BDH Prolabo ®). The tissues were stored at -80°C until further use. Transverse sections of 20 μm thickness were cut at -20°C using the CryoJane ® Tape-Transfer System (Leica Microsystems) on a Leica CM1900 cryostat (using low-profile disposable blades 819 from Leica Biosystems) and the sections were stored at -20°C.
Immunostaining of tissue. This step was done similar to previously described for immunostaining of hippocampal tissue.
Primary hippocampal neurons culture preparation and immunostaining
Culture of primary hippocampal neurons. Postnatal day 0 (P0) C57BL/6j Bomtac (wt) mice pups were sacrificed by decapitation, brains removed and hippocampi dissected into ice cold PBS. The tissue was dissociated for thirty minutes in 20 U/mL activated papain (Worthington Biochemical Corporation). After dissociation, the tissue was washed once in DMEM (Lonza) containing 0.01 mg/mL DNaseI (Sigma) before being triturated in DMEM (Lonza) containing 0.01 mg/mL DNaseI (Sigma). After this, Neurobasal-A medium (Gibco) containing B-27 Supplement (Gibco), 2 mM GlutaMAX (Gibco), 100 μg/mL Primocin (Invivogen) and 20 μM floxuridine + 20 μM uridine (Sigma) was added to the cells and the cells were seeded on poly-D-lysine (Sigma-Aldrich) and laminin (Invitrogen) pre-coated coverslips at a density of 100.000 cells per coverslip and left for fourteen days at 37°C and 5% CO 2, with medium change every second day, before being fixed in PBS containing 4% PFA.
Immunostaining of cultured hippocampal neurons. Neurons fixed in 4% PFA was briefly washed in PBS prior to three consecutive washes in PBS containing 0.1% Triton X-100 of ten minute intervals. Hereafter, the cells were washed once in PBS before being incubated in PBS containing 10% FBS (Gibco) for thirty minutes at RT. After this, the cells were incubated with primary antibody ( Table 2) overnight at 4°C. The next day, the immunostaining were left at RT for one hour before continuing the immunostaining protocol. Hereafter, the cells were washed three times five minutes in PBS containing 0.1% Triton-X 100. Subsequently, the cells were incubated with secondary antibodies ( Table 3) for four hours at RT. The coverslips were then washed two times five minutes in PBS followed by a five minute wash in PBS containing Hoechst (5 µg/µl, Sigma-Aldrich) before being mounted using Fluorescence Mounting Medium (Dako) and stored at 4°C. As negative controls of the immunostaining, simultaneous stainings were done using a similar protocol, except primary antibody was omitted.
Confocal microscopy of hippocampal tissue, spinal cord tissue and cultured hippocampal neurons
Confocal microscopy. The samples were analysed on a Zeiss confocal LSM 780 microscope (Carl Zeiss) using 20X/0.8 M27 and 63X/1.20 W Korr (Water immersion correction ring) objectives. Appropriate filters were used upon excitation of the different fluorophores to match their maximum fluorescence emission. The channels used were H258 and A568 and they were configured to obtain the best signal during image acquisition of the samples in order to prevent bleed through between the different probes. The range indicator was used to adjust gain and offset so acquired images were optimally held within the dynamic range of the detector. Frame size was selected to be “optimal” and an averaging of 16 was selected upon image acquisition in order to acquire an appropriate number of pixels and to achieve a maximum of signal-to-noise-ratio, respectively. Image acquisition was performed with foci adjusted with respect to the 568 nm fluorophores, as they were used to visualize the markers of interneurons; parvalbumin, calretinin, calbindin and somatostatin. Processing of the acquired images were performed in Zen 2011 (Carl Zeiss) Image Processing. All images presented were subjected to similar brightness and contrast adjustments.
Table 1. List of chemicals and reagents.
The use of each chemical can be found in the materials and methods section. The products are listed in alphabetic order.
| Reagent | Working Concentration | Manufacturer | Catalog number |
|---|---|---|---|
| Bovine Serum Albumin (BSA) | 1% w/v in TBS or TB buffer | Sigma ® | A4503 |
| B-27 ® Supplement | 1x | Gibco
® by Life
Technologies |
17504-044 |
| Deoxyribonuclease 1 (DNAse1) | 0.01 mg/mL | Sigma ® | DN25 |
| DMEM | 1x | Lonza | BE12-604F/U1 |
| D-PBS | 1x | Gibco
® by Life
Technologies |
14190-094 |
| Fetal bovine serum (FBS) | 1x | Gibco
® by Life
Technologies |
10270-106 |
|
Fluorescence Mounting
Medium |
n/a | Dako | S3023 |
|
Floxuridine +
Uridine |
20 μM
20 μM |
Sigma
®
Sigma ® |
F0503
U3750 |
| Formaldehyde | 4% | Hounisen | 1000.5000 |
| GlutaMAX TM Supplement | 2 mM | Gibco
® by Life
Technologies |
35050-061 |
| Hoechst | 5 μg/μL | Sigma-Aldrich ® | 861405 |
| IsoFlo ® vet | 4% gas | Abbott | 002185 |
| Iso-Pentane | n/a | VWR BDH Prolabo ® | 24872.298 |
| Laminin | 20 μg/mL | Invitrogen | 23017-015 |
| Neurobasal-A ® Medium | n/a | Gibco
® by Life
Technologies |
10888-022 |
| Pentobarbital 50 mg/mL | 5 mg/mL | The pharmacy at Aarhus
University |
|
| Paraformaldehyde | 4% w/v in PBS, pH 7.4 | Sigma Aldrich ® | P6148 |
| Papain | 20 U/mL | Worthington Biochemical
Corporation |
LS003126 |
| Poly-D-Lysine | 0.1 mg/mL | Sigma-Aldrich ® | P6407 |
| Primocin TM | 100 μg/mL | Invivogen | ant-pm-2 |
| Sucrose | 30% w/v in PBS | Merck Millipore | 1.07687.1000 |
| Target Retrieval Solution | 1x | Dako | S1699 |
| Tissue-Tek ® O.C.T TM compound | n/a | Sakura | 4583 |
| Tris Base buffer (TB buffer) | 50 mM Tris Base | Calbiochem | 648311 |
| Tris-buffered saline (TBS) | 50 mM Tris Base
150 mM NaCL |
Calbiochem
Merck Millipore |
648311
1.06404.1000 |
| Triton ® X-100 | 0.3% in TBS for IHC
0.1% in PBS for ICC |
Applichem | A1388 |
Table 2. Primary antibodies used for immunostaining of 1hippocampal sections, 2hippocampal neurons and 3spinal cord sections.
The pAbmAbs rating reflects the average rating of the antibodies as of October 2014.
| Antibody | Host | Clonality | Immunogen | Dilution
factor |
Company | Catalog nr.
batch nr. |
RRID | pAbmAbs rating
(1–5) |
|---|---|---|---|---|---|---|---|---|
|
Anti-
Calbindin 1, 3 |
Rabbit | Polyclonal | Recombinant
mouse calbindin |
1:500 | Millipore | Ab1778
2040376 |
AB_2068336 |
|
|
Anti-
Calbindin 1, 2 |
Mouse | Monoclonal | Bovine kidney
calbindin-D |
1:500 | Sigma-
Aldrich ® |
C9848
052M4833 |
AB_476894 |
|
|
Anti-
Calbindin 1, 2 |
Rabbit | Monoclonal | Chicken gut
calbindin D-28k |
1:200 | Swant | D28K
07 (F) |
n/a |
|
|
Anti-
Calretinin 1, 3 |
Rabbit | Polyclonal | Recombinant
rat calretinin |
1:1000 | Millipore | Ab5054
20 xx 170 |
AB_2068506 |
|
|
Anti-
Calretinin 1, 2 |
Sheep | Polyclonal | Native guinea
pig calretinin |
1:500 | Rockland | 200-601-D13
28000 |
AB_11183443 |
|
|
Anti-
Calretinin 1, 2 |
Mouse | Monoclonal | Recombinant
rat calretinin |
1:1000 | Millipore | Mab1568
2123143 |
AB_94259 |
|
|
Anti-
Calretinin 1, 2 |
Mouse | Monoclonal | Recombinant
human calretinin |
1:200 | Swant | 6B3
010399 |
AB_10000320 |
|
|
Anti-
Parvalbumin 1– 3 |
Rabbit | Polyclonal | Rat
parvalbumin |
1:1000 | Abcam | Ab11427
GR101095-2 |
AB_298032 |
|
|
Anti-
Parvalbumin 1, 2 |
Guinea pig | Polyclonal | Recombinant
rat parvalbumin |
1:250 | Synaptic
systems |
195 004
195004/11 |
AB_2156476 |
|
|
Anti-
Parvalbumin 1, 2 |
Mouse | Monoclonal | Frog muscle
parvalbumin |
1:2000 | Sigma-
Aldrich ® |
P3088
100M4797 |
AB_477329 |
|
|
Anti-
Parvalbumin 1, 2 |
Rabbit | Polyclonal | Synthetic
peptide |
1:250 | Millipore | Ab15736
1869268 |
AB_838238 |
|
|
Anti-
Somatostatin 1– 3 |
Rat | Monoclonal | Synthetic
peptide |
1:1000 | Millipore | Mab354
2060939 |
AB_2255365 |
|
|
Anti-
Somatostatin 1, 2 |
Rabbit | Polyclonal | Synthetic
human peptide |
1:250 | Sigma-
Aldrich ® |
SAB4502861
310328 |
AB_10747468 |
|
Table 3. Secondary antibodies used for immunostaining of 1hippocampal sections, 2hippocampal neurons and 3spinal cord sections.
| Antibody | Host | Fluorescent dye | Dilution factor | Company | Catalog nr. |
|---|---|---|---|---|---|
| α-Rabbit IgG (H+L) 1– 3 | Donkey | Alexa Fluor ® 568 | 1:300 | Molecular probes ® | A-10042 |
| α-Mouse IgG (H+L) 1, 2 | Donkey | Alexa Fluor ® 568 | 1:300 | Molecular probes ® | A-10037 |
| α-Sheep IgG (H+L) 1, 2 | Donkey | Alexa Fluor ® 568 | 1:300 | Molecular probes ® | A-21099 |
| α-Guinea Pig IgG (H+L) 1, 2 | Donkey | CF TM 488A | 1:300 | Sigma | SAB4600033 |
| α-Rat IgG (H+L) 1, 2 | Goat | Alexa Fluor ® 568 | 1:300 | Molecular probes ® | A-11077 |
| α-Rat IgG (H+L) 3 | Donkey | Alexa Fluor ® 594 | 1:300 | Molecular probes ® | A-21209 |
Results and discussion
Interneurons of the hippocampus
Initially, we screened the antibody specificity by staining of cultured hippocampal neurons, evaluating antibodies based on their ability to mark a distinct subset of neurons. Hereafter, when staining hippocampal sections, the antibodies were rated based on the expected localization and abundance of interneurons positive for the specific staining.
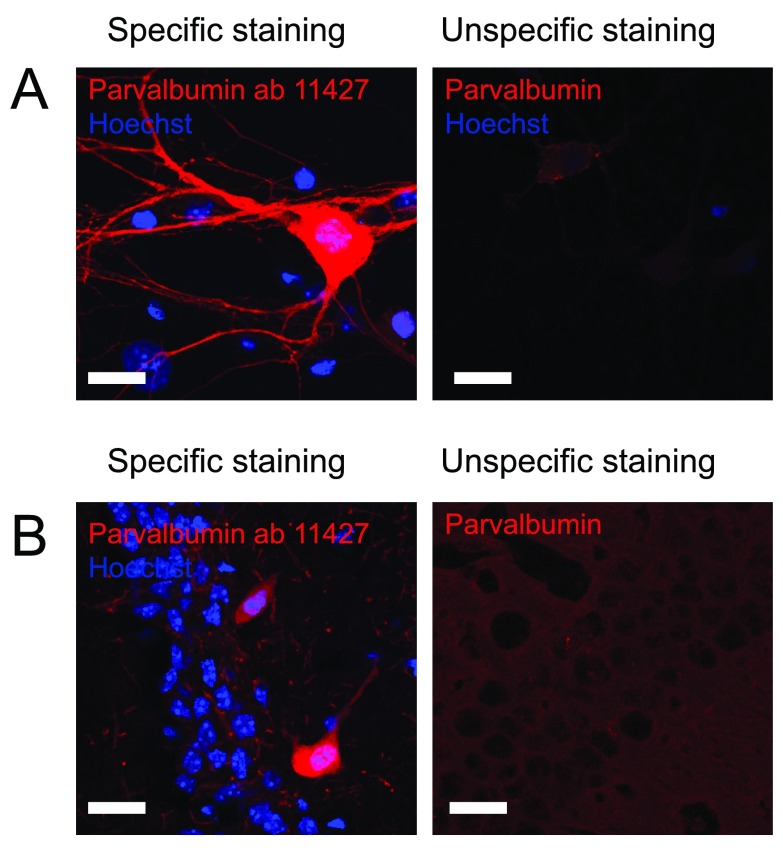
The localization of parvalbumin interneurons within the dentate gyrus is very well described so cells staining positive in layers where parvalbumin interneurons are not expected were considered as unspecific immunostaining. For several of the immunostainings, very little, if any, signal was obtained. However, the anti-parvalbumin ab11427 antibody from Abcam gave a clear and intense staining of parvalbumin interneurons both in culture and in hippocampal tissue sections ( Figure 1 and Table 2). As the positive neurons were found in layers of the dentate gyrus, where parvalbumin positive interneurons have previously been described to be located, at an expected frequency, this was considered a specific staining and was therefore rated with 5 out of 5 stars on pAbmAbs ( www.pAbmAbs.com).
Figure 1. Staining against parvalbumin interneurons.
Figure 1 shows immunostaining against parvalbumin on A) cultured hippocampal neurons and B) hippocampal tissue. Left pictures shows an example of an immunostaining considered to be specific while right picture shows an example where immunostaining using other primary antibodies did not meet the criteria and therefore was considered unspecific. Scale bar represents 20 µm.
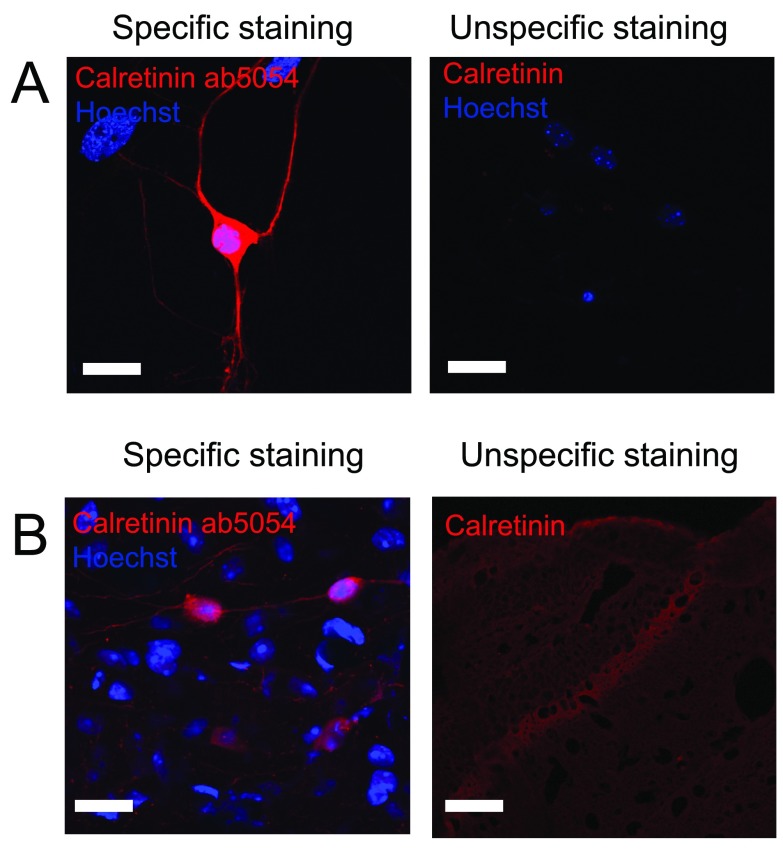
Unlike parvalbumin, calretinin is found not only in interneurons but also in mossy cells within the dentate gyrus. These can often be distinguished based on the intensity of the labelling. When rating these antibodies, the correct localization of positive neurons was therefore considered not only in relation to interneurons but also to mossy cells. Both antibodies from Millipore showed high specificity against calretinin, and especially the anti-calretinin ab5054 antibody gave a very specific staining with a high signal-to-noise ratio and was therefore given 5 out of 5 stars on pAbmAbs ( Figure 2 and Table 2).
Figure 2. Staining against calretinin.
Figure 2 shows immunostaining against calretinin on A) cultured hippocampal neurons and B) hippocampal tissue. Left pictures shows an example of an immunostaining considered to be specific while right picture shows an example where immunostaining using other primary antibodies did not meet the criteria and therefore was considered unspecific. Scale bar represents 20 µm.
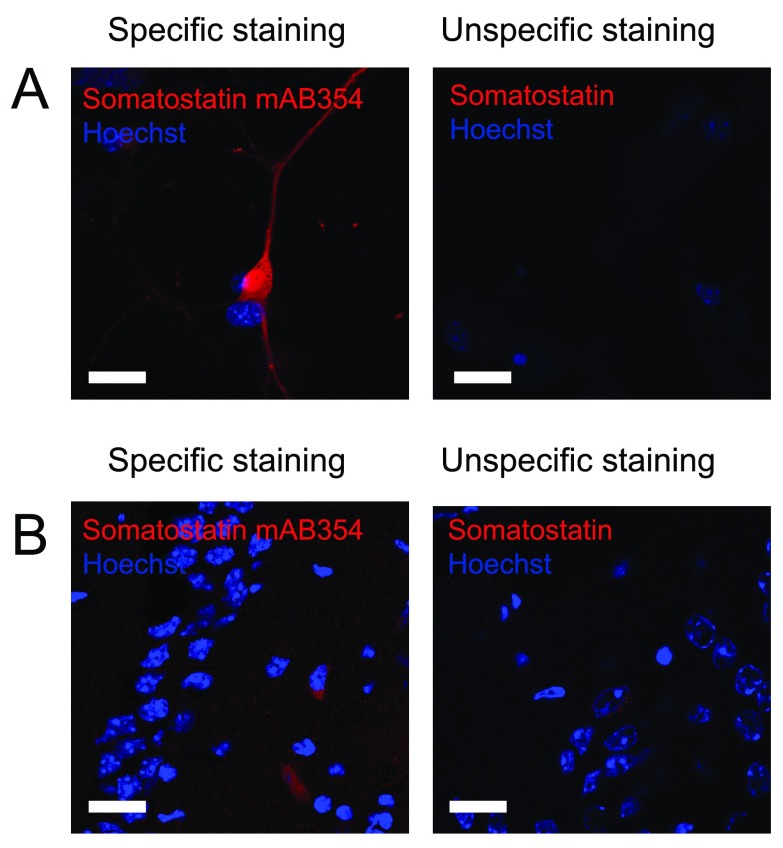
Similarly, antibodies against somatostatin were evaluated based on signal-to-noise and localization of neurons positive for somatostatin. In most cases, staining against somatostatin gave a high background with very low signal. However, using the anti-somatostatin mab364 antibody from Millipore we observed a clear staining with a good signal-to-noise ratio ( Figure 3 and Table 2) and therefore it received a rating of 5 out of 5 stars. The neurons positive for somatostatin were, as expected, found in the hilus of the dentate gyrus.
Figure 3. Staining against somatostatin.
Figure 3 shows immunostaining against somatostatin on A) cultured hippocampal neurons and B) hippocampal tissue. Left pictures shows an example of an immunostaining considered to be specific while right picture shows an example where immunostaining using other primary antibodies did not meet the criteria and therefore was considered unspecific. Scale bar represents 20 µm.
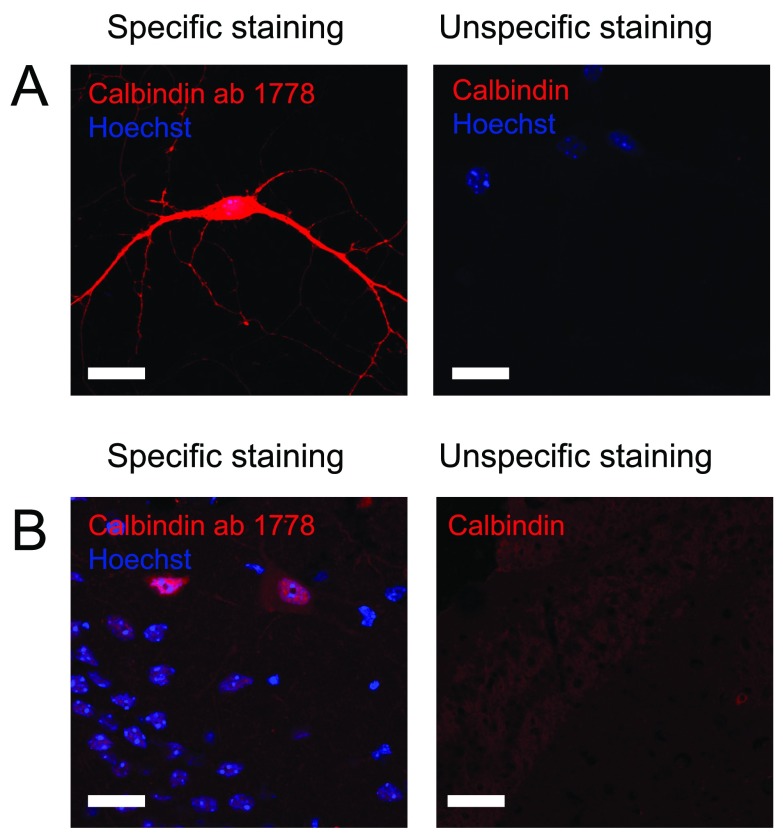
Like calretinin, calbindin is also expressed by non-inhibitory cells. When looking at the dentate gyrus, expression of calbindin by principal cells within the granule cell layer gives a weak immunostaining which might seem like unspecific binding, however that is not the case. Interneurons positive for calbindin can be recognized based on the location as well as increased intensity of the immunostaining. Due to the very low number of calbindin-interneurons in the hilus, this immunostaining can be hard to detect. Many of the antibodies we tested showed very little if any difference in staining intensity between interneurons and granule cells. However, using the anti-calbindin ab1778 antibody from Millipore we were able to distinguish between interneurons and granule cells ( Figure 4 and Table 2). Since this antibody also shows very little background staining it was rated 5 stars on pAbmAbs.
Figure 4. Staining against calbindin.
Figure 4 shows immunostaining against calbindin on A) cultured hippocampal neurons and B) hippocampal tissue. Left pictures shows an example of an immunostaining considered to be specific while right picture shows an example where immunostaining using other primary antibodies did not meet the criteria and therefore was considered unspecific. Scale bar represents 20 µm.
Interneurons of the spinal cord
Parvalbumin positive cells of the spinal cord dorsal horn also represent a subgroup of GABAergic interneurons and immunostaining against parvalbumin can accordingly be used as a marker of GABAergic interneurons. When staining against parvalbumin with the anti-parvalbumin ab11427 antibody from Abcam they appeared to be largely restricted to laminae II-III of the dorsal horn, which is in accordance with previous findings 27. The parvalbumin positive cells of laminae II-III were rather small and showed intense immunoreactivity in the nucleus and in the soma, as previously described 22, making it easy to distinguish them from background staining. This antibody also appeared to stain neuronal processes of the dorsal horn and columns as well as the nuclei of ventral horn motor neurons, as previously described 27– 29. Although this antibody can be used to identify intense immunoreactive parvalbumin positive cells and function as a great marker of the parvalbumin positive subpopulation of GABAergic neurons of the spinal dorsal horn in locations previously described, it showed some background staining of spinal cord cryo-sections and was rated 4 out of 5 stars on pAbmAbs.
Unlike interneurons of the hippocampus, calretinin can only be used as a marker of interneurons that do not contain GABA in the spinal cord 24. The anti-calretinin AB5054 antibody from Merck Millipore works well for IHC of spinal cord cryo-sections (data not shown) and was rated 5 out of 5 stars on pAbmAbs, as it showed very low background staining and intense staining of a dense well-defined band of small calretinin immunoreactive cells in the superficial laminae of the dorsal horn and of large cells in lamina V-VI. These observations correlates with previous description of calretinin immunoreactivity in the spinal cord 24, and indicates high specificity of the antibody.
In contrast to IHC of hippocampal sections with the anti-somatostatin MAB354 antibody from Millipore, this antibody gave a low signal when staining against somatostatin on spinal cord sections. Using this antibody, it was difficult to identify somatostatin positive cells in the spinal dorsal horn that otherwise previously have been described to be located in a dense band in lamina II of rat 25 and mouse 21 spinal dorsal horn. Therefore, the antibody was rated 2 out of 5 stars on pAbmAbs. This antibody was rated 5 out of 5 for the hippocampal staining, leading to an average rating of 3.5 on pAbmAbs.
Like calretinin and somatostatin, calbindin can be used as a marker of spinal dorsal horn interneurons that do not contain GABA 23. A dense band of calbindin immunoreactivity has previously been shown in lamina II and a more sparse band in lamina I, III and IV of the rat spinal dorsal horn 23. This localization of calbindin immunoreactivity is also seen when using the anti-calbindin AB1778 antibody from Merck Millipore (data not shown). Also, the cells that constitute the central channel and motor neurons of the ventral horn also show calbindin immunoreactivity when staining with this antibody, which is in accordance with previously findings 28, 30. The antibody showed very intense staining of cytoplasm and nuclei, as well as processes of the outer lamina of the dorsal horn and showed low background staining. On the basis of these observations the antibody was rated 5 out of 5 stars on pAbmAbs.
http://dx.doi.org/10.5256/f1000research.5349.d36682
The raw microscopy images for both hippocampal and spinal cord interneurons are shown in the .czi files provided.
Conclusion
In conclusion, staining against interneurons can be a very tedious task and great consideration is needed to ensure that it is actually only interneurons that are being stained. Optimizing protocols for immunostaining can be a, not only time consuming, but also an expensive task in a market full of different antibody options. By creating an information-sharing platform, pAbmAbs allows for a fast and cost-free screening of the current antibodies out there and thereby ensures that only the best antibodies are used. In the current study, we tested antibodies against parvalbumin, calretinin, calbindin and somatostatin, all markers of hippocampal GABAergic interneurons, both in culture and on hippocampal and spinal cord tissue. These antibodies were rated on specificity, and signal-to-noise ratio, for both tissue and culture. When immunostaining tissue, we also looked at the localization of positive cells within the tissue to ensure that only cells in the expected layers of the tissue stained positive for the GABAergic markers. When staining against parvalbumin we found that out of four different antibodies, the anti-parvalbumin ab11427 antibody from Abcam got a high score as it stained cells specifically with a high signal-to-noise ratio with the expected localization within the tissue. When staining against calretinin, the anti-calretinin ab5054 antibody from Millipore obtained the highest score on pAbmAbs. This antibody gave a very nice signal-to-noise ratio compared to the other antibodies tested. The anti-somatostatin mab354 antibody from Millipore was found to be the best antibody for stainings against somatostatin. Similar to the other antibodies with high pAbmAbs ratings, this also had a high signal-to-noise ratio compared to other antibodies tested. Finally, the anti-calbindin ab1178 antibody from Millipore obtained the highest rating out of the antibodies tested against this GABAergic subgroup. Overall, the antibody tested gave varying results when using our protocols. The specificities of the antibodies are therefore reflected on pAbmAbs which, by serving as a database, will help fast and cost-free evaluation of antibodies.
Data availability
F1000Research: Dataset 1. Interneurons of hippocampus and spinal cord, 10.5256/f1000research.5349.d36682 31
Acknowledgements
We thank Helene Andersen, Anja Aagaard and Benedicte Vestergaard for excellent technical assistance.
Funding Statement
This study was funded by the Lundbeck Foundation, Danish Medical Research Council, Fonden til forskning af sindslidelser and Agnes og Poul Friis Fond.
v2; ref status: indexed
References
- 1.Freund TF, Buzsaki G: Interneurons of the hippocampus. Hippocampus. 1996;6(4):347–470. [DOI] [PubMed] [Google Scholar]
- 2.Farrant M, Nusser Z: Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6(3):215–29. 10.1038/nrn1625 [DOI] [PubMed] [Google Scholar]
- 3.Wang XJ, Buzsaki G: Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16(20):6402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzsaki G, Draguhn A: Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. 10.1126/science.1099745 [DOI] [PubMed] [Google Scholar]
- 5.Benes FM, Berretta S: GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. 10.1016/S0893-133X(01)00225-1 [DOI] [PubMed] [Google Scholar]
- 6.Amaral DG: A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182(4 Pt 2):851–914. 10.1002/cne.901820508 [DOI] [PubMed] [Google Scholar]
- 7.Erlander MG, Tillakaratne NJ, Feldblum S, et al. : Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7(1):91–100. 10.1016/0896-6273(91)90077-D [DOI] [PubMed] [Google Scholar]
- 8.Ribak CE, Vaughn JE, Saito K: Immunocytochemical localization of glutamic acid decarboxylase in neuronal somata following colchicine inhibition of axonal transport. Brain Res. 1978;140(2):315–32. 10.1016/0006-8993(78)90463-8 [DOI] [PubMed] [Google Scholar]
- 9.Jinno S, Aika Y, Fukuda T, et al. : Quantitative analysis of GABAergic neurons in the mouse hippocampus, with optical disector using confocal laser scanning microscope. Brain Res. 1998;814(1–2):55–70. 10.1016/S0006-8993(98)01075-0 [DOI] [PubMed] [Google Scholar]
- 10.Somogyi P, Klausberger T: Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562(Pt 1):9–26. 10.1113/jphysiol.2004.078915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribak CE, Nitsch R, Seress L: Proportion of parvalbumin-positive basket cells in the GABAergic innervation of pyramidal and granule cells of the rat hippocampal formation. J Comp Neurol. 1990;300(4):449–61. 10.1002/cne.903000402 [DOI] [PubMed] [Google Scholar]
- 12.Blasco-Ibanez JM, Freund TF: Distribution, ultrastructure, and connectivity of calretinin-immunoreactive mossy cells of the mouse dentate gyrus. Hippocampus. 1997;7(3):307–20. [DOI] [PubMed] [Google Scholar]
- 13.Fujise N, Liu Y, Hori N, et al. : Distribution of calretinin immunoreactivity in the mouse dentate gyrus: II. Mossy cells, with special reference to their dorsoventral difference in calretinin immunoreactivity. Neuroscience. 1998;82(1):181–200. 10.1016/S0306-4522(97)00261-3 [DOI] [PubMed] [Google Scholar]
- 14.Gulyas AI, Hajos N, Freund TF: Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci. 1996;16(10):3397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jinno S, Kosaka T: Patterns of expression of neuropeptides in GABAergic nonprincipal neurons in the mouse hippocampus: Quantitative analysis with optical disector. J Comp Neurol. 2003;461(3):333–49. 10.1002/cne.10700 [DOI] [PubMed] [Google Scholar]
- 16.Katona I, Acsady L, Freund TF: Postsynaptic targets of somatostatin-immunoreactive interneurons in the rat hippocampus. Neuroscience. 1999;88(1):37–55. 10.1016/S0306-4522(98)00302-9 [DOI] [PubMed] [Google Scholar]
- 17.Gulyas AI, Toth K, Danos P, et al. : Subpopulations of GABAergic neurons containing parvalbumin, calbindin D28k, and cholecystokinin in the rat hippocampus. J Comp Neurol. 1991;312(3):371–8. 10.1002/cne.903120305 [DOI] [PubMed] [Google Scholar]
- 18.Todd AJ, Sullivan AC: Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol. 1990;296(3):496–505. 10.1002/cne.902960312 [DOI] [PubMed] [Google Scholar]
- 19.Polgar E, Hughes DI, Riddell JS, et al. : Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104(1–2):229–39. 10.1016/S0304-3959(03)00011-3 [DOI] [PubMed] [Google Scholar]
- 20.Zeilhofer HU: The glycinergic control of spinal pain processing. Cell Mol Life Sci. 2005;62(18):2027–35. 10.1007/s00018-005-5107-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinke B, Ruscheweyh R, Forsthuber L, et al. : Physiological, neurochemical and morphological properties of a subgroup of GABAergic spinal lamina II neurones identified by expression of green fluorescent protein in mice. J Physiol. 2004;560(Pt 1):249–66. 10.1113/jphysiol.2004.070540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laing I, Todd AJ, Heizmann CW, et al. : Subpopulations of GABAergic neurons in laminae I–III of rat spinal dorsal horn defined by coexistence with classical transmitters, peptides, nitric oxide synthase or parvalbumin. Neuroscience. 1994;61(1):123–32. 10.1016/0306-4522(94)90065-5 [DOI] [PubMed] [Google Scholar]
- 23.Antal M, Polgár E, Chalmers J, et al. : Different populations of parvalbumin- and calbindin-D28k-immunoreactive neurons contain GABA and accumulate 3H-D-aspartate in the dorsal horn of the rat spinal cord. J Comp Neurol. 1991;314(1):114–24. 10.1002/cne.903140111 [DOI] [PubMed] [Google Scholar]
- 24.Ren K, Ruda MA: A comparative study of the calcium-binding proteins calbindin-D28K, calretinin, calmodulin and parvalbumin in the rat spinal cord. Brain Res Brain Res Rev. 1994;19(2):163–79. 10.1016/0165-0173(94)90010-8 [DOI] [PubMed] [Google Scholar]
- 25.Proudlock F, Spike RC, Todd AJ: Immunocytochemical study of somatostatin, neurotensin, GABA, and glycine in rat spinal dorsal horn. J Comp Neurol. 1993;327(2):289–97. 10.1002/cne.903270210 [DOI] [PubMed] [Google Scholar]
- 26.Meikle AD, Martin AH: A rapid method for removal of the spinal cord. Stain Technol. 1981;56(4):235–7. 10.3109/10520298109067317 [DOI] [PubMed] [Google Scholar]
- 27.Hughes DI, Sikander S, Kinnon CM, et al. : Morphological, neurochemical and electrophysiological features of parvalbumin-expressing cells: a likely source of axo-axonic inputs in the mouse spinal dorsal horn. J Physiol. 2012;590(Pt 16):3927–51. 10.1113/jphysiol.2012.235655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ince P, Stout N, Shaw P, et al. : Parvalbumin and calbindin D-28k in the human motor system and in motor neuron disease. Neuropathol Appl Neurobiol. 1993;19(4):291–9. 10.1111/j.1365-2990.1993.tb00443.x [DOI] [PubMed] [Google Scholar]
- 29.Antal M, Freund TF, Polgar E: Calcium-binding proteins, parvalbumin- and calbindin-D 28k-immunoreactive neurons in the rat spinal cord and dorsal root ganglia: a light and electron microscopic study. J Comp Neurol. 1990;295(3):467–84. 10.1002/cne.902950310 [DOI] [PubMed] [Google Scholar]
- 30.Holm MM, Nieto-Gonzalez JL, Vardya I, et al. : Mature BDNF, but not proBDNF, reduces excitability of fast-spiking interneurons in mouse dentate gyrus. J Neurosci. 2009;29(40):12412–8. 10.1523/JNEUROSCI.2978-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molgaard S, Ulrichsen M, Boggild S, et al. : Dataset 1. Interneurons of hippocampus and spinal cord. F1000Research. 2014. Data Source [Google Scholar]