Abstract
Migraine is increasingly understood to be a disorder of the brain. In susceptible individuals, a variety of “triggers” may influence altered central excitability, resulting in the activation and sensitization of trigeminal nociceptive afferents surrounding blood vessels (i.e., the trigeminovascular system), leading to migraine pain. Transient receptor potential (TRP) channels are expressed in a subset of dural afferents, including those containing calcitonin gene related peptide (CGRP). Activation of TRP channels promotes excitation of nociceptive afferent fibers and potentially lead to pain. In addition to pain, allodynia to mechanical and cold stimuli can result from sensitization of both peripheral afferents and of central pain pathways. TRP channels respond to a variety of endogenous conditions including chemical mediators and low pH. These channels can be activated by exogenous stimuli including a wide range of chemical and environmental irritants, some of which have been demonstrated to trigger migraine in humans. Activation of TRP channels can elicit CGRP release, and blocking the effects of CGRP through receptor antagonism or antibody strategies has been demonstrated to be effective in the treatment of migraine. Identification of approaches that can prevent activation of TRP channels provides an additional novel strategy for discovery of migraine therapeutics.
Keywords: TRP channels, migraine, TRPV1, TRPV4, TRPM8, TRPA1
Migraine is a common neurological disorder that ranks among the leading causes of disability in the world. According to the recent Global Burden of Disease Survey 2010 conducted by the World Health Organization,1 migraine, with a global prevalence of 14.7%, ranked as the third most common disease in the world.2 When considered on the basis of years lost to disability (YLDs), migraine accounts for 2.9% of all YLDs, placing it seventh among specific causes of disability.2 Among neurologic disorders, migraine is responsible for more than one-half of YLDs, thus making it the leading neurological disorder causing disability.2 Notably, these rankings of disease burden also take into consideration the treatments that are available, and in use, underscoring the inadequate treatment of headache disorders.2,3 The introduction of triptans represented a remarkable advance in medical management of migraine. Triptans, however, are effective in only a subset of migraineurs, and few appreciable advances in available therapeutics for migraine have been introduced in the last 20 years.4 Migraine remains a critical unmet medical need. Recent advances in our understanding of migraine pathophysiology point to several avenues that could lead to the discovery of novel therapies.
Acute Migraine
A migraine episode generally consists of four phases, beginning with the premonitory phase.5 This initial phase of migraine, reported by a majority of migraine sufferers, can appear hours to days prior to the headache itself and may include symptoms such as sore neck, tiredness, and difficulty in concentrating.5 A sizable minority of migraineurs (15 to 30%) also experience aura up to 1 h prior to the migraine headache.5−8 The aura can include visual disturbances with expanding regions of scintillations or light accompanied by some vision loss termed scotomas. The scotomas normally begin in the center of the visual field and appear to progress toward the periphery.6 Aura may also present with somatosensory, motor, or language disturbances. Aura typically develops slowly over 5–20 min and lasts under 1 h.5 The majority of migraineurs experience migraine without aura (previously “common migraine”). The migraine headache is usually characterized by a moderate to severe throbbing headache with unilateral localization.9 Migraine headache may be exacerbated by movement of the head or by physical activity. It is often accompanied by sensory excitability, expressed as sensitivity to light (photophobia), noise (phonophobia), or smells (osmophobia) as well as nausea and vomiting.6,7 It can last from 4 to 72 h if left untreated.5−7 Migraine may be followed by a postdrome phase lasting hours or even days and often consists of fatigue, irritability, sensory excitability, and impaired concentration, although some individuals may feel euphoric after migraine.5
In spite of the significant socioeconomic impact of migraine, the effective management of acute migraine remains an important unmet medical need. The most commonly employed acute therapies are the triptans and nonsteroidal anti-inflammatory drugs (NSAIDs),4,10 with triptans accounting for up to 80% of medications prescribed for migraine.11 This class of drugs represents the therapy of choice for acute treatment of moderate or severe migraine headache.12 However, triptans are not always effective, as fewer than one-half of patients taking oral triptans report being pain-free at 2 h, and up to one-third of these individuals report headache recurrence within 24 h.13,14 Moreover, triptans are contraindicated in patients with risk factors for cardiovascular disease or with hypertension,15 which account for one-fifth of the migraine population. An important clinical concern is that triptan overuse can lead to medication-overuse headache (MOH).16,17 Consequently, it is recommended that acute treatments not be used more than twice per week, thus rendering these drugs inappropriate as preventive treatments.18 Opioids in combination with NSAIDs or acetaminophen, and barbiturates are also used, principally in the United States. However, as few as 5–8 doses per month of either opioid or barbiturate containing medications may lead to medication overuse headache.19 A recent review of randomized controlled trials (RCT) suggests that high-dose aspirin may be effective against acute migraine attacks,20 and NSAIDs are effective against mild migraine episodes.10 There are also few options available for the acute treatment of severe migraine in the emergency department setting.21 Only parenteral prochlorperazine has a high level of evidence for acute treatment of migraine, although the side effects including akathesia, dyskinesia, and sedation and risk of QT interval prolongation indicate that dopamine antagonists are not ideal for intractable migraine pain. Lysine acetylsalicylic acid (not available in the United States) and metoclopramide have a moderate level of evidence, while only a low level of evidence exists to support the use of intravenous ketorolac.22
Chronic Migraine and Medication Overuse Headache
The rate of occurrences of migraine episodes form a continuum ranging from low-frequency episodic migraine to high-frequency episodic migraine and culminating in chronic migraine.23,24 Chronic migraine is defined by the International Headache Society (IHS) as headaches that occur 15 or more days per month over a period of 3 or more months.17 In addition, headaches occurring on at least 8 of those days must meet the criteria for migraine with aura, migraine without aura, and/or be perceived by the patient to be migraine at onset and be relieved by a triptan or ergot derivative.17 It has been estimated that approximately 14% of patients with episodic migraine will develop chronic migraine, representing 1.3–5.1% of the global population.23−25 As would be expected, chronic migraine is more disabling than episodic migraine, owing to the high frequency of attacks and accompanying comorbidities associated with this condition.23−25 Recent epidemiologic studies identified possible risk factors for the progression to chronic migraine that include female sex, age, low education, low socioeconomic status, and head injury.26−28 Risk factors considered to be modifiable include stressful life events, sleep disturbances, obesity, depression, increased caffeine consumption, and elevated baseline headache frequency (10 or more per month).23,26−28
The excessive use of acute migraine medications represents an especially important risk factor, leading to chronic migraine and medication overuse.23 Approximately 50–75% of patients diagnosed with chronic migraine have a history of overuse of medications, in particular, triptans, analgesics, and barbiturates.23,29−32 Medication overuse headache is diagnosed in those with 15 or more headache days per month and the consumption of simple analgesics (acetaminophen, NSAIDs) for more than 15 days per month or the intake of combination analgesics, opioids, ergots, or triptans on more than 10 days per month.23 Clearly, the acute treatment of frequent migraine episodes is not appropriate for chronic migraine, and prophylactic therapies to manage migraine are needed.
The mechanisms that lead to development of chronic migraine are still not well understood. Consequently, the development of prophylactic agents against this condition is based on treatments that were discovered serendipitously and/or because efficacy was presumed based on pharmacologic class.33−36 Notably, even within class, some drugs are effective (e.g., antiepileptics such as valproate and topiramate)13,37,38 while others (e.g., oxcarbazepine) are not.39 Moreover, many of these treatments are associated with troublesome side effects, including sedation, difficulty in thinking, and weight gain, and are not well tolerated in many patients. Because many patients do not respond to current prophylactic medications, a strong medical need for discovery of new therapeutics exists.13,25,38,40 In addition to antiepileptic drugs such as valproate and topiramate, onabotulinumtoxinA has been shown to be effective in migraine prophylaxis23,41 Double-blind, placebo-controlled trials showed reduced frequency of migraine headaches and improvement in “functioning, vitality, psychological distress, and overall quality of life”.41
Pathophysiology of Migraine
A critical impediment to the development of effective and well-tolerated therapeutics for migraine management has been a relative lack of understanding of the molecular, biochemical, and physiological mechanisms of migraine.34,35 Migraine was initially thought to be primarily of vascular origin, since antimigraine therapies had potent vasoconstrictor properties.42 However, it is becoming increasingly evident that vasodilation of dural vessels during migraine is an epiphenomenon unrelated to the genesis of migraine.43,44 Functional imaging studies have shown that migraine headache can occur in the absence of vasodilation, and appears driven from sites within the brain.45,46 Migraine is now considered to be a neurovascular disorder, where activation and sensitization of primary afferent neurons that innervate the dural vasculature can promote the headache phase.6,7,47,48 Although the events that actually initiate a migraine attack remain unknown, the ultimate activation of the trigeminovascular system is considered to be essential.49,50 Trigeminovascular activation may provoke release of multiple excitatory neurotransmitters including substance P, neurokinin A, and CGRP from dural afferent terminals, resulting in neurogenic vasodilation of dural blood vessels, release of proinflammatory mediators, degranulation of mast cells and plasma protein extravasation.7,51,52 CGRP has been shown to be released in humans during migraine headache and in animal models following dural stimulation (see below53,54). Activation and sensitization of thinly myelinated and unmyelinated nociceptive afferent fibers that innervate the dura can elicit pain.49,50 Additionally, sensitization of the second-order neurons of the trigeminal n. caudalis49,50 can occur resulting in enhanced nociceptive inputs to higher brain centers including the thalamus, hypothalamus and cortical sites, collectively manifesting as migraine pain.49,50,55,56 Peripheral sensitization and central sensitization57,58 amplify signaling along the entire pain transmission pathways. Evidence supports the clinical relevance of centrally sensitized pathways in migraine59,60 and drugs clinically effective in migraine, such as NSAIDs, may elicit their actions primarily through central sites.61 As perivascular stimulation of the dura results in pain referred to the head,7,62 many animal models have applied artificial inflammatory stimuli to the dura to activate and sensitize afferent fibers.63−65 Electrical stimulation of the trigeminal ganglion causes plasma extravasation and mast cell degranulation,66,67 which is blocked by sumatriptan. Unilateral extravasation of proteins has been demonstrated during migraine attack,68 but experimental antimigraine drugs designed to block extravasation, such as an NK1 antagonist,69 an endothelin antagonist,70 and a selective blocker of plasma extravasation,71 all failed against migraine headache in clinical trials.7
Considerable evidence suggests that calcitonin gene-related peptide (CGRP) plays a cardinal role in migraine headache. Blood levels of CGRP, but not of substance P, have been reported to be elevated during migraine attacks,53 though this finding was not confirmed by all studies.72 Additionally, the intravenous infusion of CGRP73 produces migraine headache in migraineurs, but not in normal volunteers.74 Precipitating migraine in susceptible individuals by administration of nitroglycerin causes elevations in levels of CGRP in the jugular venous blood,75−77 although it should be noted that inhibiting CGRP does not block nitroglycerin-induced migraine.78 It is likely that CGRP does not directly activate trigeminal dural afferents but potentiates the release of agents into the perivascular space, possibly because CGRP can act as a mast-cell degranulator and mast cell degranulation has been proposed to contribute to migraine.79−82 Although the development of first-generation triptans was based on their vasoconstrictor properties, triptans likely act by inhibiting the release of pronociceptive transmitters, including CGRP, by activation of 5HT1B/D presynaptic receptors on either the peripheral or central terminals of trigeminal afferents.60,83−86 Clinical investigations have now demonstrated that CGRP receptor antagonists are as efficacious as triptans against migraine.87,88 Their site of action however remains to be determined. CGRP receptor antagonists did not have activity in preclinical models when given into the trigeminal ganglia or onto the dura,89,90 but can inhibit activity of second-order neurons when given into central sites in the nucleus caudalis.91 However, this is in contrast to recent reports of efficacy for CGRP antibodies for migraine,92 which likely do not gain access to the CNS and thus work in the periphery. These observations nonetheless indicate that modulation of trigeminal dural afferents, or of their postsynaptic pathways, can provide effective therapy for migraine, raising possibilities for the discovery of new medications that may provide efficacy in specific populations of migraineurs.
Transient Receptor Potential (TRP) Channels and Headache
TRP channels are a large family of membrane ion channels that have been implicated in a variety of pain states due to their response to stimuli such as temperature, changes in extracellular osmolarity, pH, and an extensive list of natural products.93−95 There are six subfamilies of TRP channels, designated with different lettering systems and grouped according to their primary amino acid sequences including TRPC, TRPM, TRPV, TRPA, TRPP, and TRPML.96,97 TRP channels are nonselective ion channels and contribute to membrane depolarization and activation of second messenger signaling cascades due to the influx of Na+ and Ca2+.93 They are proposed to contribute to an extensive list of sensory encoding including processes in the visual, gustatory, auditory, and somatosensory systems.98 A growing list of studies implicates TRP channels in the pathophysiology of headache and suggests that this family of channels might represent novel targets for headache therapeutics.
Transient Receptor Potential V1 (TRPV1)
TRPV1 is highly expressed on peripheral nociceptors;99,100 its expression in the central nervous system, however, is controversial. Neuronal TRPV1 expression has been reported101 to be largely restricted to nociceptors in primary sensory ganglia, with CNS expression limited to only discrete brain regions including the caudal hypothalamus, consistent with a role of this channel in thermoregulation (see below). The restricted peripheral distribution was conserved across species in rat, monkey, and human brain.101 The TRPV1 channel is activated by noxious heat and by chemicals such as capsaicin, an extract of chili peppers that produces burning sensations in humans.102 Its activity is potentiated by low extracellular pH, implicating this channel in pain due to ischemic and inflammatory events.103 TRPV1 channels are therefore transducers that can result in activation of nociceptors that can produce pain.104,105 Importantly, the TRPV1 channel is also a molecular integrator of nociceptive signaling. Activation of multiple transducers found on nociceptors including bradykinin, serotonin, prostaglandin, and prokineticin receptors can result in activation of intracellular signaling pathways to sensitize the channel so that it is responsive at lower stimulus intensities.106 It should also be noted that TRPV1 is distributed on the central terminals of nociceptive fibers where its activity may promote activation and sensitization of postsynaptic pain transmission pathways.107−109
Relevant to migraine, single nucleotide polymorphisms have been found in the TRPV1 gene in Spanish migraine patients but how these channel mutations may contribute to migraine is not clear.110 Preclinically, TRPV1 has been shown on dural nerve fibers111 as well as on trigeminal ganglion cell bodies retrogradely labeled from the dura.112 Capsaicin can dilate dural vessels in a TRPV1-dependent mechanism,113 and application of capsaicin to the rat dura produces ERK activation in trigeminal ganglion neurons114 and behavioral responses consistent with headache.115,116 Sumatriptan can also inhibit the TRPV1 channel117 as well as attenuate the positive modulation of TRPV1-mediated behavioral responses by 5HT.118 Modulation of TRPV1 may therefore be among the mechanisms by which sumatriptan has efficacy for migraine. Mechanisms contributing to activation of TRPV1 within the dura before or during a migraine are less well-defined. The channel is known to be activated by endocannabinoids such as anandamide,119 endovanilloids such as N-arachidonoyl dopamine (NADA),120 and various lipid products of the lipoxygenase pathway,121 as well as modulated by bradykinin,122 nerve-growth factor,122 and prostaglandins.123 Whether activation/modulation of dural TRPV1 by any of these substances contributes to migraine has yet to be determined.
The potential of TRPV1 for discovery of therapeutics has been an intense focus of the pharmaceutical industry since the molecular identification of the channel.102 The structure of the channel along with its activation mechanisms have recently been revealed using electron cryomicroscopy124,125 which may aid in the development of novel agents targeting the channel. Potential therapies emanating from targeting TRPV1 include pain, urge incontinence, asthma, cough, irritable bowel syndrome, and others (see refs (126) and (127)). Activation of TRPV1 initially produces sensations of heat and pain but can lead additionally to long lasting desensitization of afferent fibers preventing nociceptive signaling. The desensitization strategy has been exploited in numerous TRPV1 therapeutics including capsaicin cream (Zostrix) as well as high concentration patches (Qutenza). An alternate strategy is to block transduction at the channel with antagonists. Unlike agonists that promote inhibition of function in the entire nociceptor through desensitization,127 TRPV1 antagonists only block activation of the channel and do not prevent the actions of other potential pronociceptive mediators. Multiple molecules have been developed as TRPV1 antagonists,128,129 and some of these have advanced to human trials (e.g., refs (130) and (131)). These studies have identified important potential limitations132 to the use of TRPV1 antagonists including hyperthermia and blockade of thermosensation that may be dangerous to activities of daily living in patients.133 Studies are onging to develop TRPV1 antagonists that may block the channel without producing a blockade of thermosensation or of hyperthermia.127
TRPV1-based therapies have nonetheless been developed for migraine. The intranasal TRPV1 agonist Civamide showed efficacy for migraine and reduces the frequency of cluster headache attacks.134 Intranasal capsaicin also showed efficacy for migraine,135 although the site of action is unknown. However, side effects due to TRPV1 activation within the nasal cavity134 may ultimately limit the clinical potential of these agents. TRPV1 antagonists have also been tested in several preclinical models related to headache, but the results have been inconsistent. The TRPV1 antagonist capsazepine given systemically blocked dural vessel dilation due to capsaicin but not electrical stimulation,113 while another TRPV1 antagonist, SB-705498, also given systemically, decreased neuronal activity in the n. caudalis following stimulation of the dura with mechanical, electrical, and chemical stimuli.136 In contrast, systemic administration of the TRPV1 antagonist A993610 had no effect in similar experiments.137 In a recent phase II clinical study, the TRPV1 antagonist SB-705498 was inferior to placebo against migraine headache, photophobia, and phonophobia.138 Ultimately, the future of TRPV1-based therapies for migraine and other applications is unclear.
Transient Receptor Potential V4 (TRPV4)
Dural afferents have been shown in many studies to be mechanically sensitive,139−142 and they are also activated by changes in extracellular osmolarity,140 implicating the mechano- and osmo-sensitive channel TRPV4143−146 in processes contributing to headache. TRPV4 mRNA is expressed in trigeminal ganglion neurons,147 and functional effects of TRPV4 activation can be measured in trigeminal neurons in vitro.148,149 Mice lacking TRPV4 exhibited a loss in both osmotic and pressure sensation.150,151 TRPV4 can be sensitized by pro-inflammatory mediators, and its mechanosensitivity may be enhanced under inflammatory conditions. Administration of inflammatory soup into the paw results in hypersensitivity to osmotic and mechanical stimulation that is present in wild-type but not TRPV4-null mice.152 Intradermal injection of mildly hypertonic solutions causes paw flinching in rats that is increased 7-fold following sensitization with PGE2.153 This effect is decreased following antisense-mediated knockdown of TRPV4 expression and is not observed in TRPV4 knockout mice.154Hypotonic solutions excite 54% of saphenous nerve c-fibers in vivo following sensitization by PGE2, an effect that is decreased by antisense knockdown of TRPV4 expression.155 This effect is likely mediated via direct actions on neurons, since consistent effects are observed using intracellular Ca2+ measurements in trigeminal ganglion cultures taken from TRPV4 wild-type but not TRPV4 knockout mice.156 Finally, support for a role of TRPV4 in mechanosensation in sensitized states is provided by a direct interaction between TRPV4, α1 integrin, and Src tyrosine kinase, which are additional factors thought to be important for mechanosensation.152
Other studies have indirectly implicated TRPV4 in migraine. Sensitization of threshold mechanical responses of dural afferents in vivo was found following activation of the protease-activated receptor 2 (PAR2).157 PAR2 is activated by its N-terminus, which is cleaved by extracellular proteases including tryptase. One likely source of these proteases (in addition to other pro-inflammatory mediators) is mast cells, which have been previously implicated in the pathogenesis of migraine.51,158,159 Consistent with a downstream role for TRPV4 following mast-cell degranulation and release of proteases is a prior study indicating that PAR2 agonists sensitize TRPV4 currents in DRG neurons and lead to mechanical hyperalgesia in the hindpaw and hypersensitivity to colorectal distension that is dependent on expression of this channel.160−162
The most direct evidence supporting a role for TRPV4 in headache thus far comes from a recent study that found TRPV4-like currents on retrogradely labeled dural afferents in response to hypotonic solutions and the TRPV4 activator 4αPDD.163 Activation of dural TRPV4 in rats also produced behavioral responses consistent with headache.163 Whether changes in osmolarity contribute to headache is not known but changes in intracranial pressure due to coughing, sneezing, routine physical activity, or simple changes in position or posture as well as mechanical stimulation of the dura following sudden head movements is known to worsen headache in migraine patients.59 TRPV4 may contribute to these responses and thus TRPV4-based therapies may provide relief to mechanically induced pain in migraine patients but this awaits further investigation.
Transient Receptor Potential Melastatin 8 (TRPM8)
TRPM8 is activated by cool temperatures (below 26 °C) and also by the chemical activators menthol and icilin. The mRNA for TRPM8 is expressed in a small fraction of sensory neurons,164,165 including those of the trigeminal ganglion,166 and mice deficient in TRPM8 have deficits in cold sensation, implicating this channel as the endogenous sensor of external cold temperature.167−169 Trigeminal expression of TRPM8 mediates sensory input from cold stimuli in and around the oral cavity170 and was shown to mediate sensitivity to volatile odorants.171 However, TRPM8 is also expressed on sensory afferents innervating deep tissues such as the colon and bladder172,173 that are not exposed to cold temperatures, suggesting an endogenous sensory role for this channel. The endogenous activator or sensitizer is unclear but may be various lipid mediators or the growth factor artemin.174,175 What information is signaled by TRPM8-expressing neurons in response to these mediators is not yet known.
Little preclinical data exists thus far for a role of TRPM8 in headache and the only studies performed have examined expression, but not function, of TRPM8 on dural afferents. One study found minimal expression of TRPM8 on dural afferents,112 while another found region-specific innervation,176 so whether and where TRPM8 is expressed on these afferents is still unclear. Despite the lack of preclinical data, TRPM8 has turned out to be one of the most consistent findings among the genome-wide association studies performed on migraine patients. TRPM8 gene variants have been found in seven separate groups of migraine patients,177−182 making this one of the most replicated findings in all of the genetic analyses performed on humans to date. To date, it is unknown how these genetic variants alter channel function or expression and what role this may play in migraine. The mutations are found both in/around coding regions as well as in the 5′ untranslated regions (5′UTR), so it is not known whether they impact channel function or expression. These data nonetheless provide much excitement for future TRPM8-based migraine therapeutics.
A question that has yet to be answered is whether future therapeutics should be TRPM8 agonists or antagonists. This likely depends on how the mutations impact channel function/expression, that is, whether channel expression/function is increased or decreased. Adding to this uncertainty is that TRPM8 is implicated as a sensor of environmental cold (as well as cold allodynia following nerve injury, see also ref (183)), but activation of TRPM8 is required for the well-known property of agonists such as menthol to be analgesic.184 Thus, TRPM8 activation can be either pro- or antinociceptive and it is not yet clear which approach would be more likely have potential efficacy for migraine. Future work further clarifying how TRPM8 mutations impact the channel may provide better clues to which direction pharmacological approaches should take, activating or blocking channel function.
Transient Receptor Potential A1 (TRPA1)
One of the most intriguing TRP channels proposed to contribute to migraine is TRPA1. This channel was originally named ANKTM1 due to the presence of numerous N-terminal ankyrin repeats in the protein185 but was later renamed as the only member of the TRPA subfamily. There are numerous cysteine residues in these ankyrin repeats at the N-terminus186−189 that appear to contribute to activation of the channel when covalently modified by various substances (see below for examples). TRPA1 is expressed on peripheral sensory neurons,190 including those that innervate the colon191 and neurons innervating the airways that signal irritation of the respiratory system.192,193 It is often coexpressed in sensory neurons with TRPV1, and like TRPV1 its activation can also promote the release of substance P and CGRP.106 Although it was originally proposed to be a sensor of noxious cold,185 this has been a controversial issue194 and most studies now focus on a sensory role of this channel outside of temperature. TRPA1 has also been linked to mechanosensation, although it appears to mostly contribute to mechanical hypersensitivity of afferents after inflammation.195 It should be noted that TRPA1 is expressed on the central terminals of primary afferents where its activation may enhance release of transmitter from afferents.196 Activation of peripheral sensory neurons is thought to release both lipoxygenase and cytochrome P450-epoxygenase products at the central terminals and both can activate TRPA1.197,198 Consistent with a role for central terminal TRPA1 in pain, intrathecal administration of TRPA1 antagonists has shown efficacy in several preclinical pain models.196
Numerous studies have focused on a role for TRPA1 and pain,195 and it is now also believed to contribute to the sensation of itch.199 Activation of the channel can be demonstrated by a variety of exogenous and endogenous substances including the environmental irritants formaldehyde,200 acrolein,201 chlorine,202 and cigarette smoke extract,203 natural products such as isothiocyanates,190 cinnamaldehyde,204 and allicin,205 and endogenous byproducts of oxidative and nitrative stress such as nitro-oleic acid,206 4-hydroxynonenal,207 and reactive prostaglandins.208 It is reasonable to conclude that some of these TRPA1 activators may have a role in promoting migraine and, indeed, some of the environmental TRPA1 activators are well-known migraine triggers.209−212 Interestingly, a gain-of-function mutation in TRPA1 has been identified in humans with familial episodic pain syndrome, a rare disorder characterized by pain in the upper limbs, often preceded by a prodrome phase, and triggered by fasting and physical stress.213 Triggers for this disorder are among those commonly reported to trigger migraine, although headache is not reported in these patients. This study nonetheless links a mutation in TRPA1 to a pain state induced by common migraine triggers and suggests that there may be some role for the channel in other types of pain due to these stimuli.
There is a growing list of preclinical evidence implicating TRPA1 in the pathophysiology of headache. TRPA1 immunoreactivity112 and functional TRPA1-like currents214 have been found on identified dural afferents in rodents. Distribution of the channel has been reported in both unmyelinated and thinly myelinated axons with terminations in superficial lamina of the trigeminal nucleus caudalis providing an anatomical basis for TRPA1-dependent orofacial nociception.215 Application of the TRPA1 agonist mustard oil as well as the environmental irritant acrolein to the nasal cavity can increase blood flow in the dura, an effect that is blocked by dural application of either a CGRP receptor or a TRPA1 antagonist.216 These findings suggest that TRPA1 may contribute to environmental irritant-induced neuronal activation, and the mechanism may be via access of irritants to the meninges through the nasal route and subsequent activation of TRPA1 on meningeal afferents. However, these studies do not provide functional evidence that activation of meningeal TRPA1 contributes to headache. In a preclinical behavioral model of migraine, application of the TRPA1 activator mustard oil to the dura produced cutaneous facial and hindpaw allodynia as well as a decrease in exploratory behavior, both of which were blocked following oral administration of a TRPA1 antagonist.214 Thus, preclinical data support the possibility that activation of TRPA1 within the dura contributes to increased dural blood flow, neurogenic vasodilation (via CGRP release), and headache.
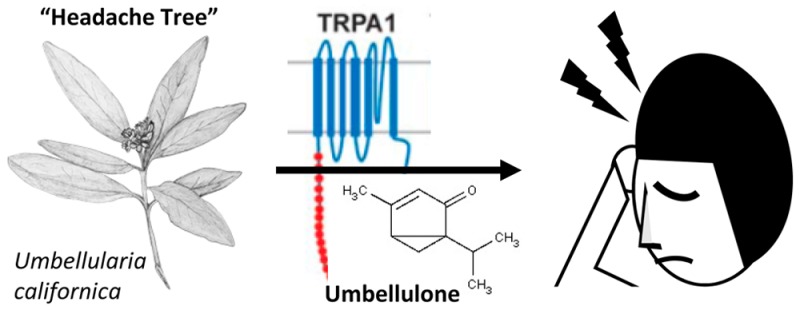
A series of recent studies has taken a backward translation approach using the observation that exposure of susceptible individuals to the “headache tree” Umbellularia californica can trigger violent headache crises.212 One of the major constituents of extracts from the headache tree is a substance known as umbellulone, which can evoke nociceptive behavior, meningeal vasodilation, and calcitonin gene-related peptide (CGRP) release via TRPA1 activation.212 Using the same rat behavioral migraine model described above, dural application of umbellulone produced cutaneous facial and hindpaw allodynia as well as a decrease in exploratory behavior similar to that seen with mustard oil, and these responses were blocked by an oral TRPA1 antagonist.214 The ability of umbellulone to gain access to the meninges via intranasal delivery in humans is unclear, but intranasal delivery of both mustard oil and umbellulone in preclinical studies caused meningeal vasodilatation due to TRPA1 activation.201 Together with the studies described above, these data argue that environmental irritants produce headaches via activation of TRPA1 in the meninges. They also suggest that endogenous activation of TRPA1 may contribute to headache and that TRPA1-based therapeutics may have efficacy for environmental irritant induced headaches as well as other headache disorders such as migraine.
A similar backward translation approach stemmed from the observation that the feverfew herb is commonly used as a migraine therapeutic in humans. One of the major constituents of feverfew, parthenolide, was recently identified as a partial agonist for TRPA1.217 In contrast to other TRPA1 agonists, parthenolide can desensitize the channel, leading to a decrease in neuropeptide release from TRPA1-containing nerve endings, and pretreatment of rats with parthenolide attenuated subsequent headache-like responses to dural application of mustard oil,217 indicating functional desensitization of the channel. These studies raise the question of whether antagonists or desensitizing agonists are a better therapeutic strategy, but in addition they further implicate TRPA1 in headache using preclinical experiments based on human observations.
Conclusion
Migraine is a disabling neurological disorder, ranking among the top 10 in causes of disability worldwide. In addition to the moderate to severe headache pain, heightened sensitivity to environmental sensory stimuli including light, sound, and odors, along with nausea and vomiting contribute to this often debilitating condition. For some individuals, migraine progresses to a chronic condition, where the afflicted individual can expect to have headaches more than half the time if not continuously. Clearly, this condition significantly impacts quality of life in a profound and negative way. Yet in spite of the serious impact of migraine, and especially chronic migraine, novel therapeutics against this disorder have not been introduced into the market recently, and chronic migraine is still treated on a trial by error basis with therapeutics discovered serendipitously.
The past decade has seen a veritable explosion of studies revealing potential mechanisms through which migraine may be generated, providing optimism that the discovery of new medicines will be possible. Migraine is no longer considered primarily a vascular disorder, but a brain disorder. Activation and sensitization of dural afferents likely occur resulting in release of mediators that maintain the sensitized state and sustain pain. It is now clear that blocking the actions of CGRP, released from dural afferent terminals and central endings in the brainstem, is effective in migraine therapy. More recent studies point to a potential role of the TRP channels in activation and sensitization of dural afferents as well as in postsynaptic pathways. A noted example demonstrating the prominent role of TRPA channels is how umbellulone from the “headache tree” can precipitate severe migraine in susceptible individuals. However, the ability of feverfew to treat headache, potentially via desensitization of TRPA1 with parthenolide, raises new questions. Namely, whether continual desensitization of TRPA1 with desensitizing agonists would provide more efficacy or better tolerability than a standard antagonist. This is particularly relevant, as it would inform whether these drugs are designed as prophylactic or abortive agents, for example, desensitizing agonists would more likely be developed as prophylactics. This question should be the subject of future work as the answer is not yet clear. Additionally, given that side effects may limit the therapeutic utility of TRPV1 antagonists, it is important to determine whether continual exposure to TRPA1-modulating drugs has adverse effects. If TRP-based therapies have efficacy equal to that of triptans and opiates, but they do not lead to MOH, they could gain an important place in the migraine therapeutic tool kit. Clearly, many questions remain. Nevertheless, as the role of the TRPA channels as well as possible roles of other TRP channels in producing or maintaining sensitization of trigeminal dural afferent nerves are elucidated, the potential for development of novel, selective, and safer medications against migraine may be realized.
Author Contributions
All authors contributed equally to the preparation of this manuscript.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Vos T.; Flaxman A. D.; Naghavi M.; Lozano R.; Michaud C.; Ezzati M.; Shibuya K.; Salomon J. A.; Abdalla S.; Aboyans V.; Abraham J.; Ackerman I.; Aggarwal R.; Ahn S. Y.; Ali M. K.; AlMazroa M. A.; Alvarado M.; Anderson H. R.; Anderson L. M.; Andrews K. G.; Atkinson C.; Baddour L. M.; Bahalim A. N.; Barker-Collo S.; Barrero L. H.; Bartels D. H.; Basáñez M.-G.; Baxter A.; Bell M. L.; Benjamin E. J.; Bennett D.; Bernabé E.; Bhalla K.; Bhandari B.; Bikbov B.; Abdulhak A. B.; Birbeck G.; Black J. A.; Blencowe H.; Blore J. D.; Blyth F.; Bolliger I.; Bonaventure A.; Boufous S.; Bourne R.; Boussinesq M.; Braithwaite T.; Brayne C.; Bridgett L.; Brooker S.; Brooks P.; Brugha T. S.; Bryan-Hancock C.; Bucello C.; Buchbinder R.; Buckle G.; Budke C. M.; Burch M.; Burney P.; Burstein R.; Calabria B.; Campbell B.; Canter C. E.; Carabin H.; Carapetis J.; Carmona L.; Cella C.; Charlson F.; Chen H.; Cheng A. T.-A.; Chou D.; Chugh S. S.; Coffeng L. E.; Colan S. D.; Colquhoun S.; Colson K. E.; Condon J.; Connor M. D.; Cooper L. T.; Corriere M.; Cortinovis M.; de Vaccaro K. C.; Couser W.; Cowie B. C.; Criqui M. H.; Cross M.; Dabhadkar K. C.; Dahiya M.; Dahodwala N.; Damsere-Derry J.; Danaei G.; Davis A.; De Leo D.; Degenhardt L.; Dellavalle R.; Delossantos A.; Denenberg J.; Derrett S.; Des Jarlais D. C.; Dharmaratne S. D.; Dherani M.; Diaz-Torne C.; Dolk H.; Dorsey E. R.; Driscoll T.; Duber H.; Ebel B.; Edmond K.; Elbaz A.; Ali S. E.; Erskine H.; Erwin P. J.; Espindola P.; Ewoigbokhan S. E.; Farzadfar F.; Feigin V.; Felson D. T.; Ferrari A.; Ferri C. P.; Fèvre E. M.; Finucane M. M.; Flaxman S.; Flood L.; Foreman K.; Forouzanfar M. H.; Fowkes F. G. R.; Franklin R.; Fransen M.; Freeman M. K.; Gabbe B. J.; Gabriel S. E.; Gakidou E.; Ganatra H. A.; Garcia B.; Gaspari F.; Gillum R. F.; Gmel G.; Gosselin R.; Grainger R.; Groeger J.; Guillemin F.; Gunnell D.; Gupta R.; Haagsma J.; Hagan H.; Halasa Y. A.; Hall W.; Haring D.; Haro J. M.; Harrison J. E.; Havmoeller R.; Hay R. J.; Higashi H.; Hill C.; Hoen B.; Hoffman H.; Hotez P. J.; Hoy D.; Huang J. J.; Ibeanusi S. E.; Jacobsen K. H.; James S. L.; Jarvis D.; Jasrasaria R.; Jayaraman S.; Johns N.; Jonas J. B.; Karthikeyan G.; Kassebaum N.; Kawakami N.; Keren A.; Khoo J.-P.; King C. H.; Knowlton L. M.; Kobusingye O.; Koranteng A.; Krishnamurthi R.; Lalloo R.; Laslett L. L.; Lathlean T.; Leasher J. L.; Lee Y. Y.; Leigh J.; Lim S. S.; Limb E.; Lin J. K.; Lipnick M.; Lipshultz S. E.; Liu W.; Loane M.; Ohno S. L.; Lyons R.; Ma J.; Mabweijano J.; MacIntyre M. F.; Malekzadeh R.; Mallinger L.; Manivannan S.; Marcenes W.; March L.; Margolis D. J.; Marks G. B.; Marks R.; Matsumori A.; Matzopoulos R.; Mayosi B. M.; McAnulty J. H.; McDermott M. M.; McGill N.; McGrath J.; Medina-Mora M. E.; Meltzer M.; Memish Z. A.; Mensah G. A.; Merriman T. R.; Meyer A.-C.; Miglioli V.; Miller M.; Miller T. R.; Mitchell P. B.; Mocumbi A. O.; Moffitt T. E.; Mokdad A. A.; Monasta L.; Montico M.; Moradi-Lakeh M.; Moran A.; Morawska L.; Mori R.; Murdoch M. E.; Mwaniki M. K.; Naidoo K.; Nair M. N.; Naldi L.; Narayan K. M. V.; Nelson P. K.; Nelson R. G.; Nevitt M. C.; Newton C. R.; Nolte S.; Norman P.; Norman R.; O’Donnell M.; O’Hanlon S.; Olives C.; Omer S. B.; Ortblad K.; Osborne R.; Ozgediz D.; Page A.; Pahari B.; Pandian J. D.; Rivero A. P.; Patten S. B.; Pearce N.; Padilla R. P.; Perez-Ruiz F.; Perico N.; Pesudovs K.; Phillips D.; Phillips M. R.; Pierce K.; Pion S.; Polanczyk G. V.; Polinder S.; Pope Iii C. A.; Popova S.; Porrini E.; Pourmalek F.; Prince M.; Pullan R. L.; Ramaiah K. D.; Ranganathan D.; Razavi H.; Regan M.; Rehm J. T.; Rein D. B.; Remuzzi G.; Richardson K.; Rivara F. P.; Roberts T.; Robinson C.; De Leòn F. R.; Ronfani L.; Room R.; Rosenfeld L. C.; Rushton L.; Sacco R. L.; Saha S.; Sampson U.; Sanchez-Riera L.; Sanman E.; Schwebel D. C.; Scott J. G.; Segui-Gomez M.; Shahraz S.; Shepard D. S.; Shin H.; Shivakoti R.; Singh D.; Singh G. M.; Singh J. A.; Singleton J.; Sleet D. A.; Sliwa K.; Smith E.; Smith J. L.; Stapelberg N. J. C.; Steer A.; Steiner T.; Stolk W. A.; Stovner L. J.; Sudfeld C.; Syed S.; Tamburlini G.; Tavakkoli M.; Taylor H. R.; Taylor J. A.; Taylor W. J.; Thomas B.; Thomson W. M.; Thurston G. D.; Tleyjeh I. M.; Tonelli M.; Towbin J. A.; Truelsen T.; Tsilimbaris M. K.; Ubeda C.; Undurraga E. A.; van der Werf M. J.; van Os J.; Vavilala M. S.; Venketasubramanian N.; Wang M.; Wang W.; Watt K.; Weatherall D. J.; Weinstock M. A.; Weintraub R.; Weisskopf M. G.; Weissman M. M.; White R. A.; Whiteford H.; Wiersma S. T.; Wilkinson J. D.; Williams H. C.; Williams S. R. M.; Witt E.; Wolfe F.; Woolf A. D.; Wulf S.; Yeh P.-H.; Zaidi A. K. M.; Zheng Z.-J.; Zonies D.; Lopez A. D.; Murray C. J. L. (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner T. J.; Stovner L. J.; Birbeck G. L. (2013) Migraine: The Seventh Disabler. Headache 53, 227–229. [DOI] [PubMed] [Google Scholar]
- World Health Organization, and Lifting The Burden (2011) Atlas of Headache Disorders and Resources in the World 2011, WHO, Geneva. [Google Scholar]
- Hoffmann J.; Goadsby P. J. (2014) Emerging targets in migraine. CNS Drugs 28, 11–17. [DOI] [PubMed] [Google Scholar]
- Halpern A. L., and Silberstein S. D. (2005) The Migraine Attack—A Clinical Description. In Imitators of Epilepsy (Kaplan P., and Fisher R., Eds.), pp 121–132, Demos Medical Publishing, New York City. [Google Scholar]
- Goadsby P. J. (2009) Pathophysiology of migraine. Neurol. Clin. 27, 335–360. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J.; Charbit A. R.; Andreou A. P.; Akerman S.; Holland P. R. (2009) Neurobiology of migraine. Neuroscience 161, 327–341. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J.; Lipton R. B.; Ferrari M. D. (2002) Migraine—Current understanding and treatment. N. Engl. J. Med. 346, 257–270. [DOI] [PubMed] [Google Scholar]
- (2004) ICHD-II Classification: Parts 1–3: Primary, Secondary and Other. Cephalalgia 24, 23–136.14687009 [Google Scholar]
- Belvis R.; Mas N.; Aceituno A. (2014) Migraine Attack Treatment A Tailor-made Suit, Not One Size Fits All. Recent Pat. CNS Drug Discovery 9, 26–40. [DOI] [PubMed] [Google Scholar]
- Smitherman T. A.; Burch R.; Sheikh H.; Loder E. (2013) The Prevalence, Impact, and Treatment of Migraine and Severe Headaches in the United States: A Review of Statistics From National Surveillance Studies. Headache 53, 427–436. [DOI] [PubMed] [Google Scholar]
- Reddy D. S. (2013) The pathophysiological and pharmacological basis of current drug treatment of migraine headache. Expert Rev. Clin. Pharmacol. 6, 271–288. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J.; Sprenger T. (2010) Current practice and future directions in the prevention and acute management of migraine. Lancet Neurol. 9, 285–298. [DOI] [PubMed] [Google Scholar]
- Ferrari M. D.; Roon K. I.; Lipton R. B.; Goadsby P. J. (2001) Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet 358, 1668–1675. [DOI] [PubMed] [Google Scholar]
- Talabi S.; Masoumi B.; Azizkhani R.; Esmailian M. (2013) Metoclopramide versus Sumatriptan for treatment of migraine headache: A randomized clinical trial. J. Res. Med. Sci. 18, 695–698. [PMC free article] [PubMed] [Google Scholar]
- Ghiotto N.; Sances G.; Galli F.; Tassorelli C.; Guaschino E.; Sandrini G.; Nappi G. (2009) Medication overuse headache and applicability of the ICHD-II diagnostic criteria: 1-year follow-up study (CARE I protocol). Cephalalgia 29, 233–243. [DOI] [PubMed] [Google Scholar]
- (2013) The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 33, 629–808. [DOI] [PubMed] [Google Scholar]
- Thorlund K.; Mills E. J.; Wu P.; Ramos E.; Chatterjee A.; Druyts E.; Goadsby P. J. (2014) Comparative efficacy of triptans for the abortive treatment of migraine: A multiple treatment comparison meta-analysis. Cephalalgia 34, 258–267. [DOI] [PubMed] [Google Scholar]
- Bigal M. E.; Lipton R. B. (2008) Excessive acute migraine medication use and migraine progression. Neurology 71, 1821–1828. [DOI] [PubMed] [Google Scholar]
- Ingledue V. F.; Mounsey A. (2014) PURLs: Treating migraine: The case for aspirin. J. Fam. Pract. 63, 94–96. [PMC free article] [PubMed] [Google Scholar]
- Orr S. L.; Aubé M.; Becker W. J.; Davenport J.; Dilli E.; Dodick D. W.; Giammarco R.; Gladstone J.; Leroux E.; Pim H.; Dickinson G.; Christie S. N. (2014) Canadian Headache Society Systematic Review & Recommendations on the Treatment of Migraine Pain in Emergency Settings. Cephalalgia 10.1177/0333102414535997. [DOI] [PubMed] [Google Scholar]
- Kelley N. E.; Tepper D. E. (2012) Rescue therapy for acute migraine, part 2: Neuroleptics, antihistamines, and others. Headache 52, 292–306. [DOI] [PubMed] [Google Scholar]
- Diener H. C.; Dodick D. W.; Goadsby P. J.; Lipton R. B.; Olesen J.; Silberstein S. D. (2012) Chronic migraine--classification, characteristics and treatment. Nat. Rev. Neurol. 8, 162–171. [DOI] [PubMed] [Google Scholar]
- Katsarava Z.; Manack A.; Yoon M.-S.; Obermann M.; Becker H.; Dommes P.; Turkel C.; Lipton R.; Diener H. (2011) Chronic migraine: Classification and comparisons. Cephalalgia 31, 520–529. [DOI] [PubMed] [Google Scholar]
- Diener H. C.; Holle D.; Dodick D. (2011) Treatment of chronic migraine. Curr. Pain Headache Rep. 15, 64–69. [DOI] [PubMed] [Google Scholar]
- Ashina S.; Lyngberg A.; Jensen R. (2010) Headache characteristics and chronification of migraine and tension-type headache: A population-based study. Cephalalgia 30, 943–954. [DOI] [PubMed] [Google Scholar]
- Bigal M. E.; Lipton R. B. (2009) The epidemiology, burden, and comorbidities of migraine. Neurol. Clin. 27, 321–334. [DOI] [PubMed] [Google Scholar]
- Bigal M. E.; Lipton R. B.; Holland P. R.; Goadsby P. J. (2007) Obesity, migraine, and chronic migraine: possible mechanisms of interaction. Neurology 68, 1851–1861. [DOI] [PubMed] [Google Scholar]
- Bahra A.; Walsh M.; Menon S.; Goadsby P. J. (2003) Does chronic daily headache arise de novo in association with regular use of analgesics?. Headache 43, 179–190. [DOI] [PubMed] [Google Scholar]
- Bigal M. E.; Borucho S.; Serrano D.; Lipton R. B. (2009) The acute treatment of episodic and chronic migraine in the USA. Cephalalgia 29, 891–897. [DOI] [PubMed] [Google Scholar]
- Bigal M. E.; Serrano D.; Buse D.; Scher A.; Stewart W. F.; Lipton R. B. (2008) Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache 48, 1157–1168. [DOI] [PubMed] [Google Scholar]
- Diamond S.; Bigal M. E.; Silberstein S.; Loder E.; Reed M.; Lipton R. B. (2007) Patterns of diagnosis and acute and preventive treatment for migraine in the United States: Results from the American Migraine Prevalence and Prevention study. Headache 47, 355–363. [DOI] [PubMed] [Google Scholar]
- Galletti F.; Cupini L. M.; Corbelli I.; Calabresi P.; Sarchielli P. (2009) Pathophysiological basis of migraine prophylaxis. Prog. Neurobiol. 89, 176–192. [DOI] [PubMed] [Google Scholar]
- Ayata C.; Jin H.; Kudo C.; Dalkara T.; Moskowitz M. A. (2006) Suppression of cortical spreading depression in migraine prophylaxis. Ann. Neurol. 59, 652–661. [DOI] [PubMed] [Google Scholar]
- Ayata C. (2009) Spreading depression: From serendipity to targeted therapy in migraine prophylaxis. Cephalalgia 29, 1095–1114. [DOI] [PubMed] [Google Scholar]
- Dodick D. W. (2009) Tonabersat for migraine prevention: New life or last gasp?. Lancet Neurol. 8, 693–695. [DOI] [PubMed] [Google Scholar]
- Diener H. (2003) Pharmacological approaches to migraine. J. Neural Transm., Suppl. 35–63. [DOI] [PubMed] [Google Scholar]
- Silberstein S. D. (2000) Practice parameter: Evidence-based guidelines for migraine headache (an evidence-based review). Neurology 55, 754–762. [DOI] [PubMed] [Google Scholar]
- Silberstein S.; Saper J.; Berenson F.; Somogyi M.; McCague K.; D’Souza J. (2008) Oxcarbazepine in migraine headache: a double-blind, randomized, placebo-controlled study. Neurology 70, 548–555. [DOI] [PubMed] [Google Scholar]
- Nelles G.; Delbrück A.; Schulze L.; Kademann B.; Bornhoevd K.; Schäfer S.; Schäuble B. (2009) Topiramate for Migraine Prevention in a Naturalistic Setting: Results From an Open Label, Flexible Dose Study. Headache 49, 1454–1465. [DOI] [PubMed] [Google Scholar]
- Diener H.-C.; Holle D.; Dodick D. (2011) Treatment of Chronic Migraine. Curr. Pain Headache Rep. 15, 64–69. [DOI] [PubMed] [Google Scholar]
- Schytz H. W.; Birk S.; Wienecke T.; Kruuse C.; Olesen J.; Ashina M. (2009) PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 132, 16–25. [DOI] [PubMed] [Google Scholar]
- Humphrey P. P.; Goadsby P. J. (1994) The mode of action of Sumatriptan is vascular? A debate. Cephalalgia 14, 401–410discussion 393.. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J. (2009) The vascular theory of migraine—A great story wrecked by the facts. Brain 132, 6–7. [DOI] [PubMed] [Google Scholar]
- Cohen A. S.; Goadsby P. J. (2004) Functional neuroimaging of primary headache disorders. Curr. Neurol. Neurosci. Rep. 4, 105–110. [DOI] [PubMed] [Google Scholar]
- Schoonman G. G.; van der Grond J.; Kortmann C.; van der Geest R. J.; Terwindt G. M.; Ferrari M. D. (2008) Migraine headache is not associated with cerebral or meningeal vasodilatation—A 3T magnetic resonance angiography study. Brain 131, 2192–2200. [DOI] [PubMed] [Google Scholar]
- Pietrobon D.; Striessnig J. (2003) Neurobiology of migraine. Nat. Rev. Neurosci. 4, 386–398. [DOI] [PubMed] [Google Scholar]
- Olesen J. (2008) The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol. Ther. 120, 157–171. [DOI] [PubMed] [Google Scholar]
- Noseda R.; Burstein R. (2013) Migraine pathophysiology: Anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 154(Suppl 1), S44–53. [DOI] [PubMed] [Google Scholar]
- Bernstein C.; Burstein R. (2012) Sensitization of the trigeminovascular pathway: Perspective and implications to migraine pathophysiology. J. Clin. Neurol. 8, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D.; Burstein R.; Strassman A. M. (2006) Mast cell involvement in the pathophysiology of migraine headache: A hypothesis. Headache 46(Suppl 1), S13–18. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J.; Edvinsson L. (1993) The trigeminovascular system and migraine: Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann. Neurol. 33, 48–56. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J.; Edvinsson L.; Ekman R. (1990) Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 28, 183–187. [DOI] [PubMed] [Google Scholar]
- Zagami A. S.; Goadsby P. J.; Edvinsson L. (1990) Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides 16, 69–75. [DOI] [PubMed] [Google Scholar]
- Burstein R.; Collins B.; Jakubowski M. (2004) Defeating migraine pain with triptans: A race against the development of cutaneous allodynia. Ann. Neurol. 55, 19–26. [DOI] [PubMed] [Google Scholar]
- Noseda R.; Jakubowski M.; Kainz V.; Borsook D.; Burstein R. (2011) Cortical projections of functionally identified thalamic trigeminovascular neurons: Implications for migraine headache and its associated symptoms. J. Neurosci. 31, 14204–14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf C. J.; Thompson S. W.; King A. E. (1988) Prolonged primary afferent induced alterations in dorsal horn neurones, an intracellular analysis in vivo and in vitro. J. Physiol. (Paris) 83, 255–266. [PubMed] [Google Scholar]
- Woolf C. J. (1983) Evidence for a central component of post-injury pain hypersensitivity. Nature 306, 686–688. [DOI] [PubMed] [Google Scholar]
- Burstein R.; Cutrer M. F.; Yarnitsky D. (2000) The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain 123(Pt 8), 1703–1709. [DOI] [PubMed] [Google Scholar]
- Dodick D.; Silberstein S. (2006) Central sensitization theory of migraine: Clinical implications. Headache 46(Suppl 4), S182–191. [DOI] [PubMed] [Google Scholar]
- Jakubowski M.; Levy D.; Kainz V.; Zhang X. C.; Kosaras B.; Burstein R. (2007) Sensitization of central trigeminovascular neurons: blockade by intravenous naproxen infusion. Neuroscience 148, 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff h. g. (1948) Headache and other head pain, Oxford University Press, London. [Google Scholar]
- Burstein R.; Jakubowski M. (2004) Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann. Neurol. 55, 27–36. [DOI] [PubMed] [Google Scholar]
- De Felice M.; Eyde N.; Dodick D.; Dussor G. O.; Ossipov M. H.; Fields H. L.; Porreca F. (2013) Capturing the aversive state of cephalic pain preclinically. Ann. Neurol. 74, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmayer R. M., Ossipov M. H., and Porreca F. (2012) An experimental model of headache-related pain, In Methods in molecular biology (Luo Z. D., Ed.), 2012/02/22 ed., pp 109–120, Springer, New York. [DOI] [PubMed] [Google Scholar]
- Dimitriadou V.; Buzzi M. G.; Moskowitz M. A.; Theoharides T. C. (1991) Trigeminal sensory fiber stimulation induces morphological changes reflecting secretion in rat dura mater mast cells. Neuroscience 44, 97–112. [DOI] [PubMed] [Google Scholar]
- Markowitz S.; Saito K.; Moskowitz M. A. (1987) Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J. Neurosci. 7, 4129–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knotkova H.; Pappagallo M. (2007) Imaging intracranial plasma extravasation in a migraine patient: A case report. Pain Med. 8, 383–387. [DOI] [PubMed] [Google Scholar]
- Diener H. C. (2003) RPR100893, a substance-P antagonist, is not effective in the treatment of migraine attacks. Cephalalgia 23, 183–185. [DOI] [PubMed] [Google Scholar]
- May A.; Gijsman H. J.; Wallnofer A.; Jones R.; Diener H. C.; Ferrari M. D. (1996) Endothelin antagonist bosentan blocks neurogenic inflammation, but is not effective in aborting migraine attacks. Pain 67, 375–378. [DOI] [PubMed] [Google Scholar]
- Roon K. I.; Olesen J.; Diener H. C.; Ellis P.; Hettiarachchi J.; Poole P. H.; Christianssen I.; Kleinermans D.; Kok J. G.; Ferrari M. D. (2000) No acute antimigraine efficacy of CP-122,288, a highly potent inhibitor of neurogenic inflammation: results of two randomized, double-blind, placebo-controlled clinical trials. Ann. Neurol. 47, 238–241. [PubMed] [Google Scholar]
- Tvedskov J. F.; Lipka K.; Ashina M.; Iversen H. K.; Schifter S.; Olesen J. (2005) No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann. Neurol. 58, 561–568. [DOI] [PubMed] [Google Scholar]
- Lassen L. H.; Haderslev P. A.; Jacobsen V. B.; Iversen H. K.; Sperling B.; Olesen J. (2002) CGRP may play a causative role in migraine. Cephalalgia 22, 54–61. [DOI] [PubMed] [Google Scholar]
- Olesen J.; Iversen H. K.; Thomsen L. L. (1993) Nitric oxide supersensitivity: a possible molecular mechanism of migraine pain. NeuroReport 4, 1027–1030. [DOI] [PubMed] [Google Scholar]
- Fanciullacci M.; Alessandri M.; Figini M.; Geppetti P.; Michelacci S. (1995) Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain 60, 119–123. [DOI] [PubMed] [Google Scholar]
- Juhasz G.; Zsombok T.; Modos E. A.; Olajos S.; Jakab B.; Nemeth J.; Szolcsanyi J.; Vitrai J.; Bagdy G. (2003) NO-induced migraine attack: strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain 106, 461–470. [DOI] [PubMed] [Google Scholar]
- Vanmolkot F.; Van der Schueren B.; de Hoon J. (2006) Sumatriptan causes parallel decrease in plasma CGRP concentration and migraine headache during nitroglycerin-induced migraine attack. Cephalalgia 26, 1037–1038author reply 1038–1039.. [DOI] [PubMed] [Google Scholar]
- Tvedskov J. F.; Tfelt-Hansen P.; Petersen K. A.; Jensen L. T.; Olesen J. (2010) CGRP receptor antagonist olcegepant (BIBN4096BS) does not prevent glyceryl trinitrate-induced migraine. Cephalalgia 30, 1346–1353. [DOI] [PubMed] [Google Scholar]
- Levy D.; Burstein R.; Strassman A. M. (2005) Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine. Ann. Neurol. 58, 698–705. [DOI] [PubMed] [Google Scholar]
- Geppetti P.; Capone J. G.; Trevisani M.; Nicoletti P.; Zagli G.; Tola M. R. (2005) CGRP and migraine: neurogenic inflammation revisited. J. Headache Pain 6, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messlinger K.; Lennerz J. K.; Eberhardt M.; Fischer M. J. (2012) CGRP and NO in the trigeminal system: mechanisms and role in headache generation. Headache 52, 1411–1427. [DOI] [PubMed] [Google Scholar]
- Levy D. (2012) Endogenous mechanisms underlying the activation and sensitization of meningeal nociceptors: the role of immuno-vascular interactions and cortical spreading depression. Curr. Pain Headache Rep. 16, 270–277. [DOI] [PubMed] [Google Scholar]
- Burstein R. (2001) Deconstructing migraine headache into peripheral and central sensitization. Pain 89, 107–110. [DOI] [PubMed] [Google Scholar]
- Burstein R.; Yarnitsky D.; Goor-Aryeh I.; Ransil B. J.; Bajwa Z. H. (2000) An association between migraine and cutaneous allodynia. Ann. Neurol. 47, 614–624. [PubMed] [Google Scholar]
- Bartsch T.; Goadsby P. J. (2003) Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain 126, 1801–1813. [DOI] [PubMed] [Google Scholar]
- Bartsch T.; Goadsby P. J. (2002) Stimulation of the greater occipital nerve induces increased central excitability of dural afferent input. Brain 125, 1496–1509. [DOI] [PubMed] [Google Scholar]
- Olesen J.; Diener H. C.; Husstedt I. W.; Goadsby P. J.; Hall D.; Meier U.; Pollentier S.; Lesko L. M. (2004) Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 350, 1104–1110. [DOI] [PubMed] [Google Scholar]
- Ho T. W.; Mannix L. K.; Fan X.; Assaid C.; Furtek C.; Jones C. J.; Lines C. R.; Rapoport A. M. (2008) Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology 70, 1304–1312. [DOI] [PubMed] [Google Scholar]
- Fischer M. J.; Koulchitsky S.; Messlinger K. (2005) The nonpeptide calcitonin gene-related peptide receptor antagonist BIBN4096BS lowers the activity of neurons with meningeal input in the rat spinal trigeminal nucleus. J. Neurosci. 25, 5877–5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covasala O.; Stirn S. L.; Albrecht S.; De Col R.; Messlinger K. (2012) Calcitonin gene-related peptide receptors in rat trigeminal ganglion do not control spinal trigeminal activity. J. Neurophysiol. 108, 431–440. [DOI] [PubMed] [Google Scholar]
- Sixt M. L.; Messlinger K.; Fischer M. J. (2009) Calcitonin gene-related peptide receptor antagonist olcegepant acts in the spinal trigeminal nucleus. Brain 132, 3134–3141. [DOI] [PubMed] [Google Scholar]
- Dodick D. W.; Goadsby P. J.; Spierings E. L.; Scherer J. C.; Sweeney S. P.; Grayzel D. S. (2014) Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: A phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 13, 885–892. [DOI] [PubMed] [Google Scholar]
- Ramsey I. S.; Delling M.; Clapham D. E. (2006) An introduction to TRP channels. Annu. Rev. Physiol. 68, 619–647. [DOI] [PubMed] [Google Scholar]
- Liu M.; Liu M. C.; Magoulas C.; Priestley J. V.; Willmott N. J. (2003) Versatile regulation of cytosolic Ca2+ by vanilloid receptor I in rat dorsal root ganglion neurons. J. Biol. Chem. 278, 5462–5472. [DOI] [PubMed] [Google Scholar]
- Karai L. J.; Russell J. T.; Iadarola M. J.; Olah Z. (2004) Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J. Biol. Chem. 279, 16377–16387. [DOI] [PubMed] [Google Scholar]
- Vriens J.; Appendino G.; Nilius B. (2009) Pharmacology of vanilloid transient receptor potential cation channels. Mol. Pharmacol. 75, 1262–1279. [DOI] [PubMed] [Google Scholar]
- Holzer P.; Izzo A. A. (2014) The pharmacology of TRP channels. Br. J. Pharmacol. 171, 2469–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazaki M.; Tominaga M. (2004) Nociception and TRP Channels. Curr. Drug Targets: CNS Neurol. Disord. 3, 479–485. [DOI] [PubMed] [Google Scholar]
- Guo A.; Vulchanova L.; Wang J.; Li X.; Elde R. (1999) Immunocytochemical localization of the vanilloid receptor 1 (VR1): Relationship to neuropeptides, the P2 × 3 purinoceptor and IB4 binding sites. Eur. J. Neurosci 11, 946–958. [DOI] [PubMed] [Google Scholar]
- Ichikawa H.; Sugimoto T. (2001) VR1-immunoreactive primary sensory neurons in the rat trigeminal ganglion. Brain Res. 890, 184–188. [DOI] [PubMed] [Google Scholar]
- Cavanagh S. R.; Fitzgerald E. J.; Urry H. L. (2014) Emotion reactivity and regulation are associated with psychological functioning following the 2011 earthquake, tsunami, and nuclear crisis in Japan. Emotion 14, 235–240. [DOI] [PubMed] [Google Scholar]
- Caterina M. J.; Julius D. (2001) The vanilloid receptor: A molecular gateway to the pain pathway. Annu. Rev. Neurosci. 24, 487–517. [DOI] [PubMed] [Google Scholar]
- Jordt S. E.; Tominaga M.; Julius D. (2000) Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. U.S.A. 97, 8134–8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M. J.; Schumacher M. A.; Tominaga M.; Rosen T. A.; Levine J. D.; Julius D. (1997) The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 389, 816–824. [DOI] [PubMed] [Google Scholar]
- Tominaga M.; Caterina M. J.; Malmberg A. B.; Rosen T. A.; Gilbert H.; Skinner K.; Raumann B. E.; Basbaum A. I.; Julius D. (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543. [DOI] [PubMed] [Google Scholar]
- Julius D. (2013) TRP channels and pain. Annu. Rev. Cell Dev. Biol. 29, 355–384. [DOI] [PubMed] [Google Scholar]
- Yeo E. J.; Cho Y. S.; Paik S. K.; Yoshida A.; Park M. J.; Ahn D. K.; Moon C.; Kim Y. S.; Bae Y. C. (2010) Ultrastructural analysis of the synaptic connectivity of TRPV1-expressing primary afferent terminals in the rat trigeminal caudal nucleus. J. Comp. Neurol. 518, 4134–4146. [DOI] [PubMed] [Google Scholar]
- Chun J. N.; Lim J. M.; Kang Y.; Kim E. H.; Shin Y. C.; Kim H. G.; Jang D.; Kwon D.; Shin S. Y.; So I.; Jeon J. H. (2014) A network perspective on unraveling the role of TRP channels in biology and disease. Pfluegers Arch. 466, 173–182. [DOI] [PubMed] [Google Scholar]
- Kim Y. S.; Chu Y.; Han L.; Li M.; Li Z.; Lavinka P. C.; Sun S.; Tang Z.; Park K.; Caterina M. J.; Ren K.; Dubner R.; Wei F.; Dong X. (2014) Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron 81, 873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno O.; Corominas R.; Fernandez-Morales J.; Camina M.; Sobrido M. J.; Fernandez-Fernandez J. M.; Pozo-Rosich P.; Cormand B.; Macaya A. (2012) SNP variants within the vanilloid TRPV1 and TRPV3 receptor genes are associated with migraine in the Spanish population. Am. J. Med. Genet., Part B 159B, 94–103. [DOI] [PubMed] [Google Scholar]
- Shimizu T.; Toriumi H.; Sato H.; Shibata M.; Nagata E.; Gotoh K.; Suzuki N. (2007) Distribution and origin of TRPV1 receptor-containing nerve fibers in the dura mater of rat. Brain Res. 1173, 84–91. [DOI] [PubMed] [Google Scholar]
- Huang D.; Li S.; Dhaka A.; Story G. M.; Cao Y. Q. (2012) Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Mol. Pain 8, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman S.; Kaube H.; Goadsby P. J. (2003) Vanilloid type 1 receptors (VR1) on trigeminal sensory nerve fibres play a minor role in neurogenic dural vasodilatation, and are involved in capsaicin-induced dural dilation. Br. J. Pharmacol. 140, 718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita T.; Shimizu T.; Shibata M.; Toriumi H.; Ebine T.; Funakubo M.; Suzuki N. (2013) Activation of extracellular signal-regulated kinase in the trigeminal ganglion following both treatment of the dura mater with capsaicin and cortical spreading depression. Neurosci. Res. 77, 110–119. [DOI] [PubMed] [Google Scholar]
- Yan J.; Edelmayer R. M.; Wei X.; De Felice M.; Porreca F.; Dussor G. (2011) Dural afferents express acid-sensing ion channels: A role for decreased meningeal pH in migraine headache. Pain 152, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove G. M.; Moskowitz M. A. (1997) Primary afferent neurons innervating guinea pig dura. J. Neurophysiol. 77, 299–308. [DOI] [PubMed] [Google Scholar]
- Evans M. S.; Cheng X.; Jeffry J. A.; Disney K. E.; Premkumar L. S. (2012) Sumatriptan inhibits TRPV1 channels in trigeminal neurons. Headache 52, 773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd D. R.; Chen P. B.; Hargreaves K. M. (2012) Anti-hyperalgesic effects of anti-serotonergic compounds on serotonin- and capsaicin-evoked thermal hyperalgesia in the rat. Neuroscience 203, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt P. M.; Petersson J.; Andersson D. A.; Chuang H.; Sorgard M.; Di Marzo V.; Julius D.; Hogestatt E. D. (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400, 452–457. [DOI] [PubMed] [Google Scholar]
- Huang S. M.; Bisogno T.; Trevisani M.; Al-Hayani A.; De Petrocellis L.; Fezza F.; Tognetto M.; Petros T. J.; Krey J. F.; Chu C. J.; Miller J. D.; Davies S. N.; Geppetti P.; Walker J. M.; Di Marzo V. (2002) An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U.S.A. 99, 8400–8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. W.; Cho H.; Kwak J.; Lee S. Y.; Kang C. J.; Jung J.; Cho S.; Min K. H.; Suh Y. G.; Kim D.; Oh U. (2000) Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 97, 6155–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang H. H.; Prescott E. D.; Kong H.; Shields S.; Jordt S. E.; Basbaum A. I.; Chao M. V.; Julius D. (2001) Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411, 957–962. [DOI] [PubMed] [Google Scholar]
- Moriyama T.; Higashi T.; Togashi K.; Iida T.; Segi E.; Sugimoto Y.; Tominaga T.; Narumiya S.; Tominaga M. (2005) Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol. Pain 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E.; Liao M.; Cheng Y.; Julius D. (2013) TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M.; Cao E.; Julius D.; Cheng Y. (2013) Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A.; Cortright D. N.; Blum C. A.; Eid S. R. (2007) The vanilloid receptor TRPV1:10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discovery 6, 357–372. [DOI] [PubMed] [Google Scholar]
- Brederson J. D.; Kym P. R.; Szallasi A. (2013) Targeting TRP channels for pain relief. Eur. J. Pharmacol. 716, 61–76. [DOI] [PubMed] [Google Scholar]
- Wong G. Y.; Gavva N. R. (2009) Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res. Rev. 60, 267–277. [DOI] [PubMed] [Google Scholar]
- Szallasi A. (2011) New developments in the medicinal chemistry of vanilloid TRPV1 and related receptors. Curr. Top. Med. Chem. 11, 2116–2117. [DOI] [PubMed] [Google Scholar]
- Gavva N. R.; Treanor J. J.; Garami A.; Fang L.; Surapaneni S.; Akrami A.; Alvarez F.; Bak A.; Darling M.; Gore A.; Jang G. R.; Kesslak J. P.; Ni L.; Norman M. H.; Palluconi G.; Rose M. J.; Salfi M.; Tan E.; Romanovsky A. A.; Banfield C.; Davar G. (2008) Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain 136, 202–210. [DOI] [PubMed] [Google Scholar]
- Quiding H.; Jonzon B.; Svensson O.; Webster L.; Reimfelt A.; Karin A.; Karlsten R.; Segerdahl M. (2013) TRPV1 antagonistic analgesic effect: A randomized study of AZD1386 in pain after third molar extraction. Pain 154, 808–812. [DOI] [PubMed] [Google Scholar]
- Szallasi A.; Sheta M. (2012) Targeting TRPV1 for pain relief: Limits, losers and laurels. Expert Opin. Invest. Drugs 21, 1351–1369. [DOI] [PubMed] [Google Scholar]
- Rowbotham M. C.; Nothaft W.; Duan W. R.; Wang Y.; Faltynek C.; McGaraughty S.; Chu K. L.; Svensson P. (2011) Oral and cutaneous thermosensory profile of selective TRPV1 inhibition by ABT-102 in a randomized healthy volunteer trial. Pain 152, 1192–1200. [DOI] [PubMed] [Google Scholar]
- Diamond S.; Freitag F.; Phillips S. B.; Bernstein J. E.; Saper J. R. (2000) Intranasal civamide for the acute treatment of migraine headache. Cephalalgia 20, 597–602. [DOI] [PubMed] [Google Scholar]
- Fusco B. M.; Barzoi G.; Agro F. (2003) Repeated intranasal capsaicin applications to treat chronic migraine. Br J. Anaesth. 90, 812. [DOI] [PubMed] [Google Scholar]
- Lambert G. A.; Davis J. B.; Appleby J. M.; Chizh B. A.; Hoskin K. L.; Zagami A. S. (2009) The effects of the TRPV1 receptor antagonist SB-705498 on trigeminovascular sensitisation and neurotransmission. Naunyn-Schmiedeberg's Arch. Pharmacol. 380, 311–325. [DOI] [PubMed] [Google Scholar]
- Summ O.; Holland P. R.; Akerman S.; Goadsby P. J. (2011) TRPV1 receptor blockade is ineffective in different in vivo models of migraine. Cephalalgia 31, 172–180. [DOI] [PubMed] [Google Scholar]
- Palmer C. B.; Lai R.; Guillard F.; Bullman J.; Baines A.; Napolitano A.; Appleby J. (2009) A randomised, two-period cross-over study to investigate the efficacy of the TRPV1 antagonist SB-705498 in acute migraine. European Jorunal of Pain 13(S1), S202a–S202. [Google Scholar]
- Kaube H.; Hoskin K. L.; Goadsby P. J. (1992) Activation of the trigeminovascular system by mechanical distension of the superior sagittal sinus in the cat. Cephalalgia 12, 133–136. [DOI] [PubMed] [Google Scholar]
- Strassman A. M.; Raymond S. A.; Burstein R. (1996) Sensitization of meningeal sensory neurons and the origin of headaches. Nature 384, 560–564. [DOI] [PubMed] [Google Scholar]
- Levy D.; Strassman A. M. (2002) Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J. Neurophysiol. 88, 3021–3031. [DOI] [PubMed] [Google Scholar]
- Ray B. S.; Wolff H. G. (1940) Experimental studies on headache: Pain sensitive structures of the head and their significance in headache. Arch. Surg. 41, 813–856. [Google Scholar]
- Liedtke W.; Tobin D. M.; Bargmann C. I.; Friedman J. M. (2003) Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 100(Suppl 2), 14531–14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J.; Watanabe H.; Janssens A.; Droogmans G.; Voets T.; Nilius B. (2004) Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. U.S.A. 101, 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H.; Davis J. B.; Smart D.; Jerman J. C.; Smith G. D.; Hayes P.; Vriens J.; Cairns W.; Wissenbach U.; Prenen J.; Flockerzi V.; Droogmans G.; Benham C. D.; Nilius B. (2002) Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J. Biol. Chem. 277, 13569–13577. [DOI] [PubMed] [Google Scholar]
- Liedtke W.; Choe Y.; Marti-Renom M. A.; Bell A. M.; Denis C. S.; Sali A.; Hudspeth A. J.; Friedman J. M.; Heller S. (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103, 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara T.; Li H. S.; Balaban C. D. (2005) Changes in transient receptor potential cation channel superfamily V (TRPV) mRNA expression in the mouse inner ear ganglia after kanamycin challenge. Hear. Res. 201, 132–144. [DOI] [PubMed] [Google Scholar]
- Chen L.; Liu C.; Liu L. (2008) The modulation of voltage-gated potassium channels by anisotonicity in trigeminal ganglion neurons. Neuroscience 154, 482–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.; Liu C.; Liu L. (2008) Changes in osmolality modulate voltage-gated calcium channels in trigeminal ganglion neurons. Brain Res. 1208, 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A.; Matsumoto N.; Imai M.; Suzuki M. (2003) Impaired osmotic sensation in mice lacking TRPV4. Am. J. Physiol.: Cell Physiol. 285, C96–101. [DOI] [PubMed] [Google Scholar]
- Suzuki M.; Mizuno A.; Kodaira K.; Imai M. (2003) Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 278, 22664–22668. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N.; Dina O. A.; Joseph E. K.; Reichling D. B.; Levine J. D. (2008) Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J. Neurosci. 28, 1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N.; Joseph E.; Dina O. A.; Liedtke W.; Levine J. D. (2005) TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain 118, 70–79. [DOI] [PubMed] [Google Scholar]
- Chen X.; Alessandri-Haber N.; Levine J. D. (2007) Marked attenuation of inflammatory mediator-induced C-fiber sensitization for mechanical and hypotonic stimuli in TRPV4–/– mice. Mol. Pain 3, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N.; Yeh J. J.; Boyd A. E.; Parada C. A.; Chen X.; Reichling D. B.; Levine J. D. (2003) Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 39, 497–511. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N.; Dina O. A.; Joseph E. K.; Reichling D.; Levine J. D. (2006) A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J. Neurosci. 26, 3864–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. C.; Levy D. (2008) Modulation of meningeal nociceptors mechanosensitivity by peripheral proteinase-activated receptor-2: the role of mast cells. Cephalalgia 28, 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D.; Burstein R.; Kainz V.; Jakubowski M.; Strassman A. M. (2007) Mast cell degranulation activates a pain pathway underlying migraine headache. Pain 130, 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. C.; Strassman A. M.; Burstein R.; Levy D. (2007) Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J. Pharmacol. Exp. Ther. 322, 806–812. [DOI] [PubMed] [Google Scholar]
- Cenac N.; Altier C.; Chapman K.; Liedtke W.; Zamponi G.; Vergnolle N. (2008) Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 135(937–946), 946 e931–932. [DOI] [PubMed] [Google Scholar]
- Grant A. D.; Cottrell G. S.; Amadesi S.; Trevisani M.; Nicoletti P.; Materazzi S.; Altier C.; Cenac N.; Zamponi G. W.; Bautista-Cruz F.; Lopez C. B.; Joseph E. K.; Levine J. D.; Liedtke W.; Vanner S.; Vergnolle N.; Geppetti P.; Bunnett N. W. (2007) Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J. Physiol. 578, 715–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe W. E.; Brierley S. M.; Martin C. M.; Phillis B. D.; Cruz F. B.; Grady E. F.; Liedtke W.; Cohen D. M.; Vanner S.; Blackshaw L. A.; Bunnett N. W. (2008) Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am. J. Physiol.: Gastrointest. Liver Physiol. 294, G1288–1298. [DOI] [PubMed] [Google Scholar]
- Wei X.; Edelmayer R. M.; Yan J.; Dussor G. (2011) Activation of TRPV4 on dural afferents produces headache-related behavior in a preclinical rat model. Cephalalgia 31, 1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy D. D.; Neuhausser W. M.; Julius D. (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58. [DOI] [PubMed] [Google Scholar]
- Peier A. M.; Moqrich A.; Hergarden A. C.; Reeve A. J.; Andersson D. A.; Story G. M.; Earley T. J.; Dragoni I.; McIntyre P.; Bevan S.; Patapoutian A. (2002) A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715. [DOI] [PubMed] [Google Scholar]
- Nealen M. L.; Gold M. S.; Thut P. D.; Caterina M. J. (2003) TRPM8 mRNA is expressed in a subset of cold-responsive trigeminal neurons from rat. J. Neurophysiol. 90, 515–520. [DOI] [PubMed] [Google Scholar]
- Bautista D. M.; Siemens J.; Glazer J. M.; Tsuruda P. R.; Basbaum A. I.; Stucky C. L.; Jordt S. E.; Julius D. (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208. [DOI] [PubMed] [Google Scholar]
- Colburn R. W.; Lubin M. L.; Stone D. J. Jr.; Wang Y.; Lawrence D.; D’Andrea M. R.; Brandt M. R.; Liu Y.; Flores C. M.; Qin N. (2007) Attenuated cold sensitivity in TRPM8 null mice. Neuron 54, 379–386. [DOI] [PubMed] [Google Scholar]
- Dhaka A.; Murray A. N.; Mathur J.; Earley T. J.; Petrus M. J.; Patapoutian A. (2007) TRPM8 is required for cold sensation in mice. Neuron 54, 371–378. [DOI] [PubMed] [Google Scholar]
- Kim Y. S.; Park J. H.; Choi S. J.; Bae J. Y.; Ahn D. K.; McKemy D. D.; Bae Y. C. (2014) Central connectivity of transient receptor potential melastatin 8-expressing axons in the brain stem and spinal dorsal horn. PloS One 9, e94080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbert M.; Kyereme J.; Schobel N.; Beltran L.; Wetzel C. H.; Hatt H. (2013) Transient receptor potential channels encode volatile chemicals sensed by rat trigeminal ganglion neurons. PloS One 8, e77998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji G.; Yiangou Y.; Corcoran S. L.; Selmer I. S.; Smith G. D.; Benham C. D.; Bountra C.; Agarwal S. K.; Anand P. (2006) Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol. 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington A. M.; Hughes P. A.; Martin C. M.; Yang J.; Castro J.; Isaacs N. J.; Blackshaw L. A.; Brierley S. M. (2011) A novel role for TRPM8 in visceral afferent function. Pain 152, 1459–1468. [DOI] [PubMed] [Google Scholar]
- Sousa-Valente J.; Andreou A. P.; Urban L.; Nagy I. (2014) Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Br. J. Pharmacol. 171, 2508–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippoldt E. K.; Elmes R. R.; McCoy D. D.; Knowlton W. M.; McKemy D. D. (2013) Artemin, a glial cell line-derived neurotrophic factor family member, induces TRPM8-dependent cold pain. J. Neurosci. 33, 12543–12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom J., Holt J. L., Neubert J. K., Caudle R., Ahn A. H.. A high density of TRPM8 expressing sensory neurons in specialized structures of the head. Proceedings of the Society for Neuroscience.Oct 17, 2012, New Orleans, LA.
- Fan X.; Wang J.; Fan W.; Chen L.; Gui B.; Tan G.; Zhou J. (2014) Replication of migraine GWAS susceptibility loci in Chinese Han population. Headache 54, 709–715. [DOI] [PubMed] [Google Scholar]
- Ghosh J.; Pradhan S.; Mittal B. (2013) Genome-wide-associated variants in migraine susceptibility: a replication study from North India. Headache 53, 1583–1594. [DOI] [PubMed] [Google Scholar]
- An X. K.; Ma Q. L.; Lin Q.; Zhang X. R.; Lu C. X.; Qu H. L. (2013) PRDM16 rs2651899 variant is a risk factor for Chinese common migraine patients. Headache 53, 1595–1601. [DOI] [PubMed] [Google Scholar]
- Chasman D. I.; Anttila V.; Buring J. E.; Ridker P. M.; Schurks M.; Kurth T.; International Headache Genetics C. (2014) Selectivity in genetic association with sub-classified migraine in women. PLoS Genet. 10, e1004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasman D. I.; Schurks M.; Anttila V.; de Vries B.; Schminke U.; Launer L. J.; Terwindt G. M.; van den Maagdenberg A. M.; Fendrich K.; Volzke H.; Ernst F.; Griffiths L. R.; Buring J. E.; Kallela M.; Freilinger T.; Kubisch C.; Ridker P. M.; Palotie A.; Ferrari M. D.; Hoffmann W.; Zee R. Y.; Kurth T. (2011) Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat. Genet. 43, 695–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freilinger T.; Anttila V.; de Vries B.; Malik R.; Kallela M.; Terwindt G. M.; Pozo-Rosich P.; Winsvold B.; Nyholt D. R.; van Oosterhout W. P.; Artto V.; Todt U.; Hamalainen E.; Fernandez-Morales J.; Louter M. A.; Kaunisto M. A.; Schoenen J.; Raitakari O.; Lehtimaki T.; Vila-Pueyo M.; Gobel H.; Wichmann E.; Sintas C.; Uitterlinden A. G.; Hofman A.; Rivadeneira F.; Heinze A.; Tronvik E.; van Duijn C. M.; Kaprio J.; Cormand B.; Wessman M.; Frants R. R.; Meitinger T.; Muller-Myhsok B.; Zwart J. A.; Farkkila M.; Macaya A.; Ferrari M. D.; Kubisch C.; Palotie A.; Dichgans M.; van den Maagdenberg A. M. (2012) Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat. Genet. 44, 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton W. M.; Daniels R. L.; Palkar R.; McCoy D. D.; McKemy D. D. (2011) Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PloS One 6, e25894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton W. M.; Palkar R.; Lippoldt E. K.; McCoy D. D.; Baluch F.; Chen J.; McKemy D. D. (2013) A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J. Neurosci. 33, 2837–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story G. M.; Peier A. M.; Reeve A. J.; Eid S. R.; Mosbacher J.; Hricik T. R.; Earley T. J.; Hergarden A. C.; Andersson D. A.; Hwang S. W.; McIntyre P.; Jegla T.; Bevan S.; Patapoutian A. (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112, 819–829. [DOI] [PubMed] [Google Scholar]
- Cvetkov T. L.; Huynh K. W.; Cohen M. R.; Moiseenkova-Bell V. Y. (2011) Molecular architecture and subunit organization of TRPA1 ion channel revealed by electron microscopy. J. Biol. Chem. 286, 38168–38176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman A.; Chuang H. H.; Bautista D. M.; Julius D. (2006) TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. U.S.A. 103, 19564–19568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson L. J.; Dubin A. E.; Evans M. J.; Marr F.; Schultz P. G.; Cravatt B. F.; Patapoutian A. (2007) Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445, 541–545. [DOI] [PubMed] [Google Scholar]
- Wang L.; Cvetkov T. L.; Chance M. R.; Moiseenkova-Bell V. Y. (2012) Identification of in vivo disulfide conformation of TRPA1 ion channel. J. Biol. Chem. 287, 6169–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt S. E.; Bautista D. M.; Chuang H. H.; McKemy D. D.; Zygmunt P. M.; Hogestatt E. D.; Meng I. D.; Julius D. (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265. [DOI] [PubMed] [Google Scholar]
- Bautista D. M.; Pellegrino M.; Tsunozaki M. (2013) TRPA1: A gatekeeper for inflammation. Annu. Rev. Physiol. 75, 181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo O.; Meseguer V.; Belmonte C.; Viana F. (2008) TRPA1 channels mediate cold temperature sensing in mammalian vagal sensory neurons: pharmacological and genetic evidence. J. Neurosci. 28, 7863–7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini R.; Materazzi S.; De Siena G.; De Cesaris F.; Geppetti P. (2010) Transient receptor potential channels as novel drug targets in respiratory diseases. Curr. Opin. Invest. Drugs 11, 535–542. [PubMed] [Google Scholar]
- Caspani O.; Heppenstall P. A. (2009) TRPA1 and cold transduction: An unresolved issue?. J. Gen. Physiol. 133, 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt P. M.; Hogestatt E. D. (2014) Trpa1. Handb. Exp. Pharmacol. 222, 583–630. [DOI] [PubMed] [Google Scholar]
- Koivisto A.; Chapman H.; Jalava N.; Korjamo T.; Saarnilehto M.; Lindstedt K.; Pertovaara A. (2014) TRPA1: A transducer and amplifier of pain and inflammation. Basic Clin. Pharmacol. Toxicol. 114, 50–55. [DOI] [PubMed] [Google Scholar]
- Sisignano M.; Park C. K.; Angioni C.; Zhang D. D.; von Hehn C.; Cobos E. J.; Ghasemlou N.; Xu Z. Z.; Kumaran V.; Lu R.; Grant A.; Fischer M. J.; Schmidtko A.; Reeh P.; Ji R. R.; Woolf C. J.; Geisslinger G.; Scholich K.; Brenneis C. (2012) 5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals. J. Neurosci. 32, 6364–6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregus A. M.; Doolen S.; Dumlao D. S.; Buczynski M. W.; Takasusuki T.; Fitzsimmons B. L.; Hua X. Y.; Taylor B. K.; Dennis E. A.; Yaksh T. L. (2012) Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proc. Natl. Acad. Sci. U.S.A. 109, 6721–6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista D. M.; Wilson S. R.; Hoon M. A. (2014) Why we scratch an itch: The molecules, cells and circuits of itch. Nat. Neurosci. 17, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara C. R.; Mandel-Brehm J.; Bautista D. M.; Siemens J.; Deranian K. L.; Zhao M.; Hayward N. J.; Chong J. A.; Julius D.; Moran M. M.; Fanger C. M. (2007) TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. U.S.A. 104, 13525–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista D. M.; Jordt S. E.; Nikai T.; Tsuruda P. R.; Read A. J.; Poblete J.; Yamoah E. N.; Basbaum A. I.; Julius D. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282. [DOI] [PubMed] [Google Scholar]
- Bessac B. F.; Jordt S. E. (2008) Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre E.; Campi B.; Materazzi S.; Trevisani M.; Amadesi S.; Massi D.; Creminon C.; Vaksman N.; Nassini R.; Civelli M.; Baraldi P. G.; Poole D. P.; Bunnett N. W.; Geppetti P.; Patacchini R. (2008) Cigarette smoke-induced neurogenic inflammation is mediated by α,β-unsaturated aldehydes and the TRPA1 receptor in rodents. J. Clin. Invest. 118, 2574–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M.; Story G. M.; Hwang S. W.; Viswanath V.; Eid S. R.; Petrus M. J.; Earley T. J.; Patapoutian A. (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857. [DOI] [PubMed] [Google Scholar]
- Bautista D. M.; Movahed P.; Hinman A.; Axelsson H. E.; Sterner O.; Hogestatt E. D.; Julius D.; Jordt S. E.; Zygmunt P. M. (2005) Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. U.S.A. 102, 12248–12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark T. E.; Ghatta S.; Bettner W.; Undem B. J. (2009) Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol. Pharmacol. 75, 820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisani M.; Siemens J.; Materazzi S.; Bautista D. M.; Nassini R.; Campi B.; Imamachi N.; Andre E.; Patacchini R.; Cottrell G. S.; Gatti R.; Basbaum A. I.; Bunnett N. W.; Julius D.; Geppetti P. (2007) 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. U.S.A. 104, 13519–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materazzi S.; Nassini R.; Andre E.; Campi B.; Amadesi S.; Trevisani M.; Bunnett N. W.; Patacchini R.; Geppetti P. (2008) Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. U.S A. 105, 12045–12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wantke F.; Focke M.; Hemmer W.; Bracun R.; Wolf-Abdolvahab S.; Gotz M.; Jarisch R.; Tschabitscher M.; Gann M.; Tappler P. (2000) Exposure to formaldehyde and phenol during an anatomy dissecting course: sensitizing potency of formaldehyde in medical students. Allergy 55, 84–87. [DOI] [PubMed] [Google Scholar]
- Irlbacher K.; Meyer B. U. (2002) Nasally triggered headache. Neurology 58, 294. [DOI] [PubMed] [Google Scholar]
- Kelman L. (2007) The triggers or precipitants of the acute migraine attack. Cephalalgia 27, 394–402. [DOI] [PubMed] [Google Scholar]
- Nassini R.; Materazzi S.; Vriens J.; Prenen J.; Benemei S.; De Siena G.; la Marca G.; Andre E.; Preti D.; Avonto C.; Sadofsky L.; Di Marzo V.; De Petrocellis L.; Dussor G.; Porreca F.; Taglialatela-Scafati O.; Appendino G.; Nilius B.; Geppetti P. (2012) The “headache tree” via umbellulone and TRPA1 activates the trigeminovascular system. Brain 135, 376–390. [DOI] [PubMed] [Google Scholar]
- Kremeyer B.; Lopera F.; Cox J. J.; Momin A.; Rugiero F.; Marsh S.; Woods C. G.; Jones N. G.; Paterson K. J.; Fricker F. R.; Villegas A.; Acosta N.; Pineda-Trujillo N. G.; Ramirez J. D.; Zea J.; Burley M. W.; Bedoya G.; Bennett D. L.; Wood J. N.; Ruiz-Linares A. (2010) A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 66, 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmayer R. M.; Le L. N.; Yan J.; Wei X.; Nassini R.; Materazzi S.; Preti D.; Appendino G.; Geppetti P.; Dodick D. W.; Vanderah T. W.; Porreca F.; Dussor G. (2012) Activation of TRPA1 on dural afferents: A potential mechanism of headache pain. Pain 153, 1949–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. S.; Son J. Y.; Kim T. H.; Paik S. K.; Dai Y.; Noguchi K.; Ahn D. K.; Bae Y. C. (2010) Expression of transient receptor potential ankyrin 1 (TRPA1) in the rat trigeminal sensory afferents and spinal dorsal horn. J. Comp. Neurol. 518, 687–698. [DOI] [PubMed] [Google Scholar]
- Kunkler P. E.; Ballard C. J.; Oxford G. S.; Hurley J. H. (2011) TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain 152, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materazzi S.; Benemei S.; Fusi C.; Gualdani R.; De Siena G.; Vastani N.; Andersson D. A.; Trevisan G.; Moncelli M. R.; Wei X.; Dussor G.; Pollastro F.; Patacchini R.; Appendino G.; Geppetti P.; Nassini R. (2013) Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain 154, 2750–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]


