Abstract
In the skin, cannabinoid lipids, whether of endogenous or exogenous origin, are capable of regulating numerous sensory, homeostatic, and inflammatory events. Although many of these effects are mediated by metabotropic cannabinoid receptors, a growing body of evidence has revealed that multiple members of the transient receptor potential (TRP) ion channel family can act as “ionotropic cannabinoid receptors”. Furthermore, many of these same TRP channels are intimately involved in cutaneous processes that include the initiation of pain, temperature, and itch perception, the maintenance of epidermal homeostasis, the regulation of hair follicles and sebaceous glands, and the modulation of dermatitis. Ionotropic cannabinoid receptors therefore represent potentially attractive targets for the therapeutic use of cannabinoids to treat sensory and dermatological diseases. Furthermore, the interactions between neurons and other cell types that are mediated by cutaneous ionotropic cannabinoid receptors are likely to be recapitulated during physiological and pathophysiological processes in the central nervous system and elsewhere, making the skin an ideal setting in which to dissect general complexities of cannabinoid signaling.
Keywords: Transient receptor potential, ion channel, cannabinoids, nociception, pruritis, dermatitis
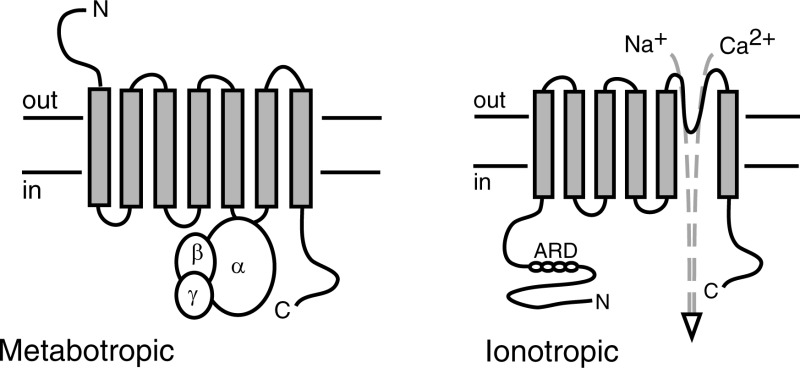
Cannabinoids are a family of lipophilic chemical compounds that either are structurally related to the main psychoactive ingredient in marijuana, Δ9-tetrahydrocannabinol (THC), or bind to the same classically defined pharmacological receptor sites.1 While cannabinoids such as THC are plant-derived, other cannabinoids such as arachidonoyl ethanolamide (anandamide) and 2-arachidonoyl glycerol (2-AG) are produced endogenously in mammalian tissues. In addition, a number of synthetic molecules acting at classically defined cannabinoid receptors have been produced. Cannabinoids evoke diverse biological activities, ranging from well-characterized psychoactive effects to regulation of physiological and pathological processes such as pain, inflammation, feeding, and bone homeostasis. Many recognized actions of cannabinoids have been attributed to seven transmembrane domain-containing G protein-coupled receptors (Figure 1). The best characterized of these so-called metabotropic cannabinoid receptors are CB1 and CB2. In addition, however, cannabinoids can activate nonmetabotropic receptors proteins, including multiple ion channels of the transient receptor potential (TRP) family2 (Figure 1). Although cannabinoid-gated TRP channels may not meet all formal criteria proposed for cannabinoid receptors,3 they will be considered “ionotropic cannabinoid receptors” for the purposes of this Review, which explores the functions of these channels in one realm of biology, namely, cutaneous sensation, homeostasis, and inflammation.
Figure 1.
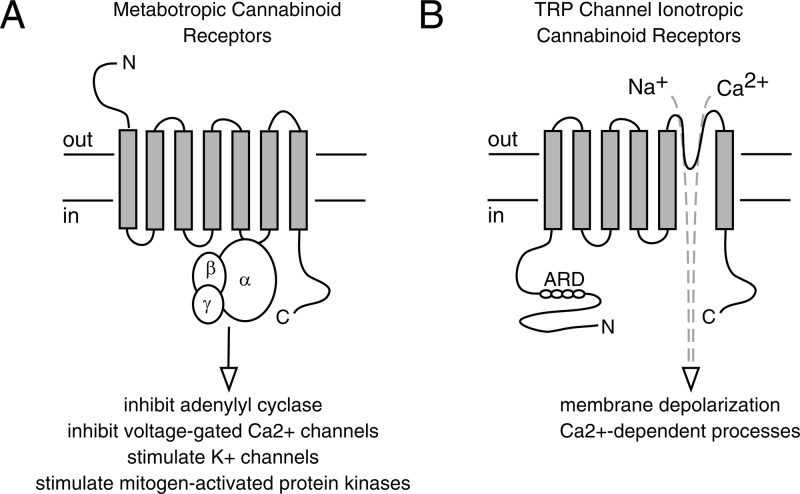
Comparison of metabotropic cannabinoid receptors and TRP channel ionotropic cannabinoid receptors. (A) Metabotropic receptors are seven transmembrane domain-containing proteins that signal via heterotrimeric G proteins. Typical responses resulting from the activation of Gi/Go G proteins by CB1 and CB2 receptors are shown. (B) TRP channel subunits each have six transmembranes domains. Four subunits assemble to form a function channel to mediate influx of sodium and/or calcium ions. ARD, ankyrin repeat domains.
Cannabinoid Effects in Skin
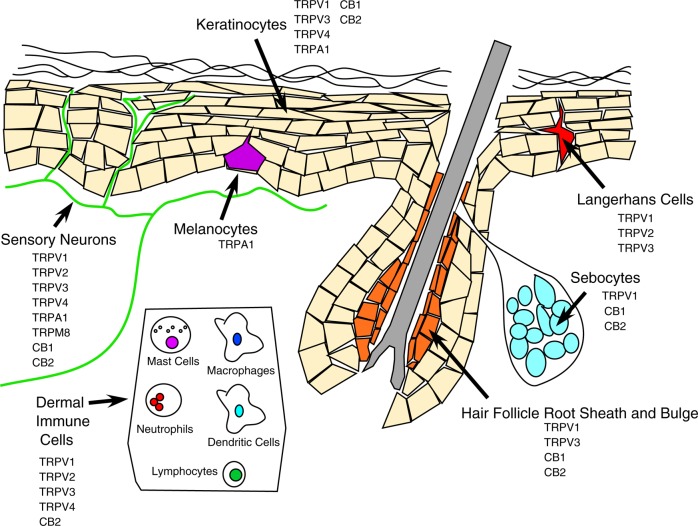
The skin possesses a robust capacity to synthesize and respond to cannabinoids.4,5 As keratinocytes transition from the proliferative basal state, through differentiation and apical migration, to the formation of the dead stratum corneum, the abundance of anandamide increases, due in part to decreases in the expression of the cannabinoid degrading enzyme, fatty acyl amide hydrolase (FAAH). Peripheral sensory neurons in rodents produce anandamide in response to stimulation6 and express FAAH,7 CB1,8 and CB2.9 In human skin, CB1 is expressed in keratinocytes within the more differentiated epidermal layers, hair follicle cells, sebaceous glands, sensory neurons, and immune cells. CB2 is expressed in keratinocytes, sebaceous glands, sensory neurons, and immune cells1,5,10,11 (Figure 2).
Figure 2.
Metabotropic and TRP channel cannabinoid receptor expression across skin cell types. References are listed in the text.
The functional effects of cannabinoids on skin can be divided into four general categories:
(1) Regulation of epidermal homeostasis. Cannabinoids suppress epidermal keratinocyte proliferation and differentiation and promote keratinocyte apoptosis.4,5 One mechanism for these effects involves transcriptional suppression of genes important for keratinocyte differentiation via methylation of their promoters. Whereas transcriptional suppression in this setting by anandamide and cannabidiol (CBD) is sensitive to CB1 antagonism, suppression by cannabigerol (CBG) is not.5,12 Some proliferation and survival effects of anandamide can also be attributed to targets besides CB1 and CB2.13 Cannabinoid effects on epidermal homeostasis may be important in disease states. For example, CB1 activation suppresses the expression of two damage-induced keratins, keratin 6 and keratin 16. This may have relevance to psoriasis, a hyperproliferative disorder in which keratin 6 and keratin 16 are upregulated.14 Additionally, cannabinoids have been shown to modulate tumorigenesis and tumor progression in nonmelanoma skin tumors.15
(2) Regulation of pain sensation.Cannibis has been used since ancient times to treat pain, and there is extensive literature supporting a role for both endocannabinoids and phytocannabinoids as modulators of pain.1,16,17 These effects appear to be mediated in part by actions at both CB1 and CB2. While direct effects on sensory neurons account for some analgesic actions of cannabinoids, these actions may also be indirect. For example, CB2 stimulation in keratinocytes evokes the release of analgesic opioid peptides.9 As detailed below, however, the ability of cannabinoids to evoke pain under some circumstances may involve action at non-CB1/CB2 targets.18,19 Although beyond the scope of this Review, it is also worth noting that endogenous and exogenous cannabinoids can modulate pain and itch through their actions in the spinal cord and brain.16,20,21
(3) Regulation of skin inflammation. Cannabinoids exert anti-inflammatory effects in skin, through both their actions on keratinocyte cytokine production and their modulation of immune cells.22 For example, THC attenuates allergic contact dermatitis in mice sensitized and subsequently challenged with the hapten dinitrofluorobenzene (DNFB). Conversely, pharmacological inhibition or knockout of CB1 and/or CB2 in mice augments DNFB induced dermatitis.23 The levels of both anandamide and 2-AG increase in mouse skin during experimental allergic contact dermatitis. In the case of 2-AG, this effect is even greater in the absence of CB1. Moreover, DNFB treatment decreases CB1 mRNA and increases CB2 mRNA.23 Thus, endocannabinoids and their receptors constitute part of an adaptive system to regulate cutaneous inflammation. As with other cutaneous processes, not all of the anti-inflammatory effects of cannabinoids depend upon CB1 and CB2. For example, THC can inhibit both T Cell production of interferon γ and interferon γ-induced keratinocyte release of the cytokines and chemokines, even in CB1/CB2 double knockout mice,24 while the anti-inflammatory effect of palmitoylethanolamide (PEA) in contact dermatitis appears to involve TRP channels, rather than CB receptors.25
(4) Regulation of skin appendages. Cannabinoids also exert modulatory effects on the development, maintenance, and function of hair follicles and sebaceous glands.4 For example, both anandamide and THC suppress hair shaft elongation and promote regression of cultured human hair follicles. These effects are at least partially CB1 dependent. Both anandamide and 2-AG promote sebum production by cultured human sebocytes through a CB2 dependent mechanism.4 As described below, however, some of these effects of cannabinoids may be mediated, in part, by TRP channels.
The TRP Ion Channel Family
The TRP family of ion channels owes its name to the fact that its first identified member, the Drosophila protein Transient Receptor Potential, is a calcium-permeable ion channel whose genetic absence results in a transient electrophysiological response of the Drosophila photoreceptor cells to light. Humans express 27 TRP channels that can be divided on the basis of their primary structures into 6 subfamilies: TRPA (ankyrin), TRPC (canonical), TRPM (melastatin), TRPML (mucolipin), TRPP (polycystin), and TRPV (vanilloid). The canonical subfamily most closely resembles the original TRP channel identified in Drosophila photoreceptors. The remaining subfamilies are named on the basis of the first member of each to be identified.26
Functional TRP channels consist of four subunits, and may be homo- or heterotetrameric. TRP channel subunits contain six transmembrane domains, with the amino- and carboxyl-termini located in the cytoplasm (Figure 1). Transmembrane domains 5 and 6 from each subunit, together with the intervening loop, line the pore through which ions flow. In some subfamilies (TRPA, TRPC, TRPV), a sequence of 4–16 sequential ankyrin repeat domains is located in the amino terminus. TRP channels are invariably cation selective. However, whereas a few TRP channels are highly calcium or sodium selective, most can be permeated by a range of monovalent and divalent cations.26
Every cell in the body expresses at least one TRP channel subtype. However, the expression of some subtypes is relatively restricted. For example, TRPV1, TRPA1, and TRPM8 are most highly enriched in particular subpopulations of peripheral sensory neurons. TRP channels have been implicated in many physiological processes, ranging from pain, itch and temperature sensation, and regulation of cardiovascular function to regulation of neurotransmitter release, embryological development, and immune function. Accordingly, TRP channel dysfunction has been linked to numerous disease states.26,27
TRP Channels, Cannabinoids, and Skin
As described above, many actions of cannabinoids in skin and elsewhere cannot be explained solely on the basis of actions at metabotropic CB receptors. Several members of the TRP channel family can be activated or inhibited by endocannabinoids, phytocannabinoids, and/or synthetic cannabinoids. The following summary highlights the best-characterized of these interactions and emerging roles for TRP channel ionotropic cannabinoid receptors in skin biology.
TRPV1
Transient receptor potential vanilloid 1 (TRPV1) is a nonselective cation channel that was originally discovered as the pharmacological site of action of pungent vanilloid compounds such as capsaicin. TRPV1 is highly enriched in a subset of peripheral sensory neurons that are important for the detection of painful stimuli. Besides vanilloid compounds, TRPV1 can alternatively be activated by certain other painful stimuli, including protons, ethanol, and noxious heat (>42 °C). Genetic elimination of TRPV1 results in loss of vanilloid-evoked behavioral and physiological pain-related responses, as well as a reduction (but not elimination) of responses to painful heat.28 Approximately 15 years ago, Zygmunt and colleagues made the surprising discovery that anandamide could activate both recombinant and native TRPV1.29 Since then, a number of other cannabinoids of endogenous and exogenous origin have been reported to be capable of activating TRPV1. These include the endocannabinoids, N-arachidonyl dopamine (NADA), N-oleoyl dopamine (NODA),19 and monoacylglycerols such as 2-AG,30 the phytocannabinoid CBD,31 and the synthetic cannabinoid, arachidonoyl-2-chloroethanolamine32 (Table 1).
Table 1. Summary of TRP Channel Ionotropic Cannabinoid Receptors and Their Regulatorsa.
| TRP channel ionotropic cannabinoid receptor | cannabinoid ligands | ref | non-cannabinoid agonists | ref |
|---|---|---|---|---|
| TRPA1 | Δ9-tetrahydrocannabinol, cannabidiol, cannabidiol acid, cannabigerol, cannabichromene, WIN 55,212-2, AM251, AM630 | (2, 46−48) | mustard oil, cinnamaldehyde, acrolein,formaldehyde, icilin, 4-hydroxynonenol, PGJ2, cold?, mechanical?, Ca2+ | (46) |
| TRPM8 | cannabinogerol | (106) | menthol, icilin, cold (<27 °C) | (105) |
| TRPV1 | anandamide, N-arachidonoyl dopamine, N-oleoyl dopamine, 2-arachidonoyl glycerol palmitoylethanolamide, cannabidiol, arachidonoyl-2- chloroethanolamine | (29−33) | capsaicin, resiniferatoxin, protons, ethanol, 2-APB, heat (>45 °C) | (28, 67) |
| TRPV2 | Δ9-tetrahydrocannabinol, cannabidiol, cannabinol | (68, 69) | osmolarity, PI3 kinase activity, probenecid, 2-APB, heat (>52 °C) | (62−67) |
| TRPV3 | cannabidiol, tetrahydrocannabivarin, cannabigerovarin, cannabigerolic acid | (81) | camphor, carvacrol, thymol, 2- APB, incensole acetate, heat (>32 °C) | (67, 75−80) |
| TRPV4 | cannabidivarin, tetrahydrocannabivarin cannabigerovarin, cannabigerolic acid, cannabinol, cannabigerol | (81) | osmolarity, epoxy- eicosatetraenoic acids, 4α-PDD, bisandrographolide A, GSK1016790A, heat (>27 °C) | (95, 96) |
Under cannabinoid ligands, agonists are in normal text and desensitizing agents are in italic text. 2-APB, 2-aminoethoxydiphenyl borate; PGJ2, prostaglandin J2; 4αPDD, 4α-phorbol didecanoate.
TRPV1 Involvement in Pain and Itch Sensation
As described above, sensory neuron-expressed TRPV1 is an important mediator of cutaneous pain in response to certain chemical and thermal stimuli. In addition, recent studies have implicated TRPV1 as an indirect mediator of histaminergic itch.33
TRPV1 as a Trigger for Neurogenic Inflammation
It has long been recognized that capsaicin evokes not only the perception of pain, and associated nocifensive behaviors, but also strong local edema and flare responses. This inflammatory process has been named neurogenic inflammation, since it arises from the release of two potent vasoactive neuropeptides, calcitonin gene related polypeptide (CGRP) and substance P (SP), from a subpopulation of peptidergic nociceptor terminals and involves both vasodilation and plasma extravasation.34 TRPV1 is highly enriched in these peptidergic neurons, and TRPV1 gene deletion eliminates capsaicin-evoked paw swelling in mice.28 Thus, neuronal peptide release represents one important mechanism by which TRPV1 can evoke skin inflammation.
TRPV1 as a Modulator of Dermatitis
Animal studies have linked TRPV1 to two common skin conditions, atopic dermatitis and allergic contact dermatitis. Some studies have pointed toward a pro-inflammatory role for TRPV1 in these conditions. For example, the TRPV1 antagonist PAC-14028 reduced serum IgG and IgE rises, dermal mast cell degranulation, skin thickening, and scratching behavior in NC/Nga mice treated serially with Dermatophagoides farinae (Df) or systemically sensitized and then topically challenged with the contact allergen, oxazolone (Oxa).35,36 TRPV1 expression in skin and its phosphorylation were both increased in the Df model, which was considered by the authors to be a mouse model of atopic dermatitis. The same group also showed that PAC-14028 improved recovery of the skin barrier following exposure to either Df or Oxa.36 Curiously, a different conclusion arose from a study of Oxa-induced dermatitis in TRPV1 knockout mice.37 These investigators observed augmented Oxa-induced ear edema in C57BL/6 mice lacking TRPV1, compared with wild-type C57BL/6 controls, despite similar levels of neutrophil recruitment. In wild-type mice, inactivation of TRPV1 expressing sensory terminals with the vanilloid, resiniferatoxin, similarly augmented Oxa-induced ear edema. The anti-inflammatory role of TRPV1 inferred from these results could not be attributed to either CGRP or SP release. The authors concluded, instead, that the TRPV1 must also trigger the release of one or more anti-inflammatory agents from peptidergic nerve terminals. Why might interference with TRPV1 function suppress Oxa-induced inflammation in one study and augment it in another? Lifelong absence of TRPV1 in the knockout studies might have resulted in compensatory effects in other systems that regulate cutaneous inflammation. Another possibility is that PAC-14028 may act on non-TRPV1 targets. The use of immunologically distinct mouse strains and differences in the doses and anatomical sites of Oxa administration between studies may represent two additional confounding variables.
Cannabinoids and TRPV1 in Skin
Certain dose-dependent cannabinoid effects in skin have been attributed to both direct and indirect modulation of TRPV1. For example, at 100 nM, both anandamide and THC were found to inhibit heat- or capsaicin-evoked CGRP release from rat skin, an effect that could be inhibited by CB1 antagonism or CB1 gene knockout. At 100 μM, however, anandamide or THC alone were sufficient to stimulate CGRP release, effects that could be inhibited by the TRPV1 antagonist, BCTC, or by TRPV1 gene knockout.38 Similar effects of anandamide on TRPV1 were observed by other investigators, who concluded that these effects depend upon the TRPV1 phosphorylation state.6 Furthermore, both NADA and NODA produce thermal hyperalgesia in rodents by acting at TRPV1.19 Another endocannabinoid, PEA was found to inhibit DNFB-evoked allergic contact dermatitis in mice through a mechanism that was suppressed by the TRPV1 antagonist capsazepine.25 In the same study, PEA inhibited cytokine expression in human keratinocyte-derived HaCaT cells, an effect that could be blocked by the TRPV1 antagonist, iodoresiniferatoxin. Anandamide has also been shown to suppress proliferation and promote cell death in human keratinocytes in culture, both through actions on CB1 and by activating TRPV1-mediated calcium influx.13
Together, the findings described above support a model in which cannabinoids indirectly suppress TRPV1 effects on pain and inflammation, by acting at CB1, but directly activate TRPV1 at higher concentrations. However, even the effect of CB1 on TRPV1 may not always be inhibitory. In one study, CB1 knockout mice exhibited deficits in capsaicin-evoked nociceptive responses, CGRP release, and afferent firing similar to those seen in TRPV1 KO mice. Inverse agonists of CB1 also suppressed capsaicin-evoked calcium influx in sensory neuron-derived cells. The authors interpreted these findings as indicating that tonic CB1 activity somehow maintains TRPV1 in a state competent for agonist activation.39 CB2 agonists were found to suppress TRPV1 mediated responses in human DRG neurons.10 Thus, cannabinoids modulate TRPV1 by multiple CB-dependent and -independent mechanisms.
TRPV1 in Human Skin
Capsaicin-evoked flare responses, heat-evoked pain, and UVB-induced heat hyperalgesia can all be inhibited in human skin by selective TRPV1 antagonists.40 Moreover, topical anandamide has been shown to trigger vasodilation in human skin through a mechanism that could be blocked by the TRPV1 antagonist, capsazepine. Interestingly, although anandamide could evoke pain at high concentrations, its vasodilatory effect was achieved at concentrations that were not associated with pain, suggesting a disconnect between influences on afferent versus efferent neuronal activities.18 TRPV1-like immunoreactivity has been observed in human epidermal nerve fibers.41 Other human skin cell types, besides sensory neurons, also appear to express this channel (Figure 2). For example, TRPV1-like immunoreactivity has been reported in human mast cells, hair follicle outer root sheath epithelial cells, and keratinocytes.42,43 In cultured human hair follicles, TRPV1 activation suppressed hair shaft elongation, promoted apoptosis, inhibited proliferation, and promoted transition to catagen,42 all reminiscent of the effects of cannabinoids. TRPV1 expression has also been reported in human dendritic cells (DCs), where its activation suppresses differentiation,44 providing an additional potential site of action during allergic contact dermatitis. Finally, cutaneous TRPV1 expression levels change in some human dermatological conditions. For example, TRPV1 mRNA was found to be upregulated in rosacea in humans, although neither the cellular source of the mRNA nor TRPV1 protein levels were assessed in that study.45
TRPA1
Another TRP channel target of cannabinoids is transient receptor potential ankyrin 1 (TRPA1), so-named because of the existence of 16 ankyrin repeats in its amino terminal domain.46 TRPA1 is most robustly expressed in a subset of TRPV1-expressing peptidergic sensory neurons (Figure 2). Most of the identified TRPA1 agonists are irritant electrophiles such as allyl isothiocyanate (mustard oil), cinnamaldehyde, and formaldehyde. Electrophiles activate TRPA1 by covalently alkylating intracellular cysteine residues located among the ankyrin repeats. Endogenous electrophillic TRPA1 agonists include 4-hydroxynonenol and prostaglandin J2. TRPA1 can alternatively be activated by noncovalent chemical agonists. Examples include intracellular calcium ions and, most relevant to this Review, the phytocannabinoids, THC, CBD, CBD acid, cannabigerol, and cannabichromene, and the synthetic cannabinoids, WIN 55,212-2, AM251, and AM6302,47,48 (Table 1).
TRPA1 Involvement in Pain and Itch Sensation
TRPA1 participates in both acute pain sensation and hyperalgesia. Pain related responses triggered by covalent TRPA1 agonists are reduced by pharmacological antagonism of TRPA1 or by genetic deletion of this channel in mice.46 TRPA1 also appears to act as a mediator of painful mechanosensation and cold nociception, following inflammation.49,50 In humans, a gain-of-function mutation in TRPA1 results in the familial episodic pain syndrome, a condition characterized by bouts of pain triggered by physiological stress.27 There is also strong evidence for the participation of TRPA1 in the perception of both acute and chronic itch. This results in part from sensory neuron-expressed TRPA1 acting downstream of signaling by G protein-coupled receptors for itch-producing peptides or monoamines.51 Neuron-expressed TRPA1 can also act downstream of receptors for thymic stromal lymphopoietin (TSLP), a factor released from keratinocytes following stimulation with histamine.52
TRPA1 as a Positive Regulator of Neurogenic Inflammation and Dermatitis
TRPA1 agonists produce robust neurogenic inflammation in skin, which can be ablated by either pharmacological antagonism or genetic elimination of TRPA1.53,54 There is also a positive role for TRPA1 in allergic contact dermatitis. In the Oxa model, the TRPA1 antagonist HC-030031 inhibited eosinophil and lymphocyte infiltration, epidermal thickening, interleukin 1β production, and plasma extravasation. Oxa increased skin expression of 4-hydroxynonenol, providing a potential means of TRPA1 activation in this model. Furthermore, TRPA1 knockout mice exhibited reduced epidermal thickening and reduced skin expression of inflammatory cytokines in this model.55 TRPA1 antagonism similarly ameliorated dermatitis in an interleukin 13 overexpressing transgenic mouse model of atopic dermatitis.56 Cutaneous TRPA1 apparently also plays a pro-inflammatory role in some nonallergic skin conditions. For example, in the acetone–ether–water model of chronic dry skin, genetic elimination of TRPA1 not only diminished itch-related behavioral responses, but also suppressed epidermal thickening and the cutaneous upregulation of numerous genes, including keratin 6, aquaporin 3, and IL33.52
Nonneuronal Roles for TRPA1 in Skin
Neurogenic mechanisms undoubtedly account for some of the proinflammatory cutaneous effects of TRPA1 described above. However, TRPA1 expression has also been reported in human57 and mouse58 keratinocytes (Table 1). This raises the possibility that alterations in inflammatory responses in TRPA1 knockout mice might additionally reflect changes in the innate immune responses of these cells. TRPA1 mRNA and/or immunoreactivity have also been observed in human dermal fibroblasts and melanocytes.57,59,60 In human melanocytes, TRPA1 regulates cytokine expression levels46 and mediates the early phase melanin synthesis response to ultraviolet B irradiation, implying a role for this channel in photoprotection.59 Whether TRPA1 might also contribute to the pathophysiology of melanoma remains unclear.60
Cannabinoids and TRPA1–TRPV1 Interactions
Although TRPA1 and TRPV1 are capable of functioning as independent channels, they are coexpressed in a subset of peripheral sensory neurons, and growing evidence suggests that these channels can functionally interact. For example, TRPV1 and TRPA1 can cross-desensitize one another when acted upon by their respective agonists.61 Cannabinoids that preferentially activate TRPA1 versus TRPV1 have helped to dissect these processes. For example, WIN 55,212-2 activates TRPA1 to trigger desensitization of TRPV1, whereas the TRPV1-selective cannabinoid agonist arachidonoyl-2-chloroethanolamine desensitizes TRPA1. The mechanisms underlying this reciprocal cross-desensitization are complex, and likely not identical. TRPA1 and TRPV1 can bind to one another, though whether this binding involves the formation of heterotetramers versus binding between two different homotetrameric channels remains unclear. Regardless, the functional interaction of TRPV1 and TRPA1 offers a potential mechanism by which cannabinoids might regulate not only pain and itch perception, but also cutaneous inflammation.
TRPV2
TRPV2 is a nonselective cation channel widely expressed in sensory neurons, immune cells, and a variety of other cell types62−65 (Figure 2). This channel can be activated by very high temperatures (>52 °C), 2-aminoethoxy diphenylborate (2-APB), probenecid, and various stimuli, including hypoosmolarity and formyl peptide receptors, that trigger phosphatidyl inositol 3-kinase (PI3K) signaling.62−64,66,67 PI3K signaling appears to regulate TRPV2 both by promoting its translocation to the cell surface and by activating channels that are on the surface. In addition, however, it was recently shown that TRPV2 can be activated directly by the cannabinoids THC, CBD, and cannabinol (CBN)68,69 (Table 1).
TRPV2 Involvement in Pain Sensation
AlthoughTRPV2 is robustly expressed in peripheral sensory neurons,62 extensive examination of pain responses in TRPV2 knockout mice failed to reveal an obvious role for this channel in thermal or mechanical nociception.70
TRPV2 Involvement in Inflammation
TRPV2 might participate in neurogenic inflammation. CBD could evoke the release of CGRP from cultured rat sensory neurons, and this response could be partially blocked by siRNA knockdown of TRPV2.69 In addition, TRPV2 is expressed in many immune cells found in the skin, and has been implicated in regulation of mast cell degranulation,65 dendritic cell endocytosis,71 and macrophage motility, phagocytosis, and cytokine release.64,72,73 TRPV2 has also been shown to regulate NLRP3 inflammasome activity.74 However, no examinations of cutaneous homeostatic or inflammatory processes in animals lacking TRPV2 or treated with highly selective TRPV2 antagonists have been reported. In humans, TRPV2-like immunoreactivity and mRNA levels were increased in skin of patients with rosacea.45 In another study, TRPV2-like immunoreactivity was observed in human cutaneous peptidergic nerve fibers.41 However, the specificities of the anti-TRPV2 antibodies used in these studies were not established. Thus, while there is abundant circumstantial evidence of TRPV2 involvement in skin biology, more studies will be necessary to clearly define any such roles.
TRPV3
TRPV3 is most highly expressed in skin keratinocytes and in the hair follicle bulge, although there is also evidence for its expression in sensory neurons, brain, and other cell types75−77 (Figure 2). TRPV3 can be activated by warm temperatures (>32 °C) and by chemical stimuli that include 2-APB67and plant-derived compounds such as camphor, carvacrol, and incensole acetate.78−80 With respect to cannabinoids, it was recently reported that CBD and tetrahydrocannabivarin both act as TRPV3 agonists, whereas cannabigerovarin and cannabigerolic acid can both desensitize TRPV381 (Table 1).
TRPV3 Involvement in Pain, Temperature, and Itch Perception
TRPV3 knockout mice were initially observed to exhibit a prolonged latency to heat-evoked behavioral withdrawal and to exhibit delayed selection of preferred temperatures on a thermal gradient.78 However, in later studies conducted on more uniform genetic backgrounds, the thermosensation phenotypes were more modest, were confined to mice on the 129S6 or 129S1/SvlmJ backgrounds, and in one case were seen only in females.82,83 In another study, TRPV3 knockout mice on the ICR genetic background subjected to the acetone–ether–water model of chronic dry skin exhibited reduced scratching behavior, compared with wild-type controls.84 Whether this reflects a role for TRPV3 in the generation of pruritogenic substances or a role in the detection of these substances by sensory neurons was not established. Pain and itch arising from genetic mutation of TRPV3 in humans is discussed below.
TRPV3 Involvement in Epidermal Homeostasis and Dermatitis
Studies of both global and keratinocyte-specific TRPV3 knockout mice have revealed that this channel is critical for normal epidermal differentiation and hair morphology. Late embryonic TRPV3 knockout mice exhibited premature epidermal differentiation, compromised epidermal barrier function, abnormal corneocytes, and reduced epidermal transglutaminase activity. Many of these changes appeared to resolve over time. In addition, throughout life, TRPV3 knockout mice exhibited curly whiskers and abnormalities in body fur.85 The authors of that study went on to show that the TRPV3 knockout cutaneous phenotypes likely arise from the fact that calcium influx through TRPV3 normally triggers the release of the epidermal growth factor receptor ligand transforming growth factor α from keratinocytes. TRPV3 activation has also been reported to inhibit growth of human hair.86
Point mutations in TRPV3 can have profound effects on skin. Missense mutations affecting TRPV3 residue Gly573 (substitutions to Ser and Cys, respectively) were found in two separate hereditary animal models of spontaneous alopecia, the hairless DS-Nh mouse and the SBN/Kob-Ht rat.87 The channels encoded by these mutant genes exhibit a high level of constitutive activity without exogenous stimulation.88 DS-Nh mice also exhibit signs of spontaneous dermatitis, resembling human atopic dermatitis.87 Interestingly, the dermatitis phenotype was incompletely penetrant, and depended heavily on both the genetic background and whether the mice were housed under specific pathogen-free conditions. Genetic background also influenced the ability of the Gly573 Ser mutation to augment the predilection of mice toward chemically evoked allergic contact dermatitis.89 Thus, while TRPV3 gain-of-function mutations can profoundly impact skin homeostasis and inflammation, the precise phenotype achieved appears subject to numerous endogenous and environmental variables.
TRPV3 in Human Skin Disease
Strikingly, the ability of mutations at TRPV3 Gly573 to cause dermatological disease is not confined to rodents. Using whole exome sequencing, Lin et al. discovered that genetic mutations altering the same TRPV3 residue in humans (Gly573Ser, Gly573Cys) or a residue elsewhere in TRPV3 (Trp692 Gly) were responsible for Olmsted syndrome.90 This rare and devastating congenital skin disorder is characterized by extreme epidermal thickening on the palms of the hands and soles of the feet (i.e., palmoplantar keratoderma) as well as keratotic lesions that surround the mouth, nose, anus, and ears. These histological changes are typically accompanied by intense itching. Hair loss and deformities of the digits, including autoamputation, are also observed in some individuals. The Olmsted-associated human TRPV3 mutant variants, like the homologous rodent mutants, exhibited robust spontaneous activity when transfected into cell lines. These mutants also triggered apoptosis in transfected cells, and, accordingly, histological examination revealed an increase in apoptosis in skin from Olmsted syndrome patients.90 It has been speculated that the pathophysiology of Olmsted syndrome might not be explained solely by abnormal TRPV3 function in keratinocytes, but might also involve immune dysfunction arising in other cells, such as cutaneous Langerhans cells.91 More recently, additional TRPV3 mutations were identified in patients with Olmsted syndrome, including several that had been inherited in an autosomal recessive fashion. Some of the affected patients experienced not only plantar keratoderma, but also erythromelalgia, an intermittent reddening of the skin accompanied by intense pain, itch, warmth, and vasodilation.92,93 Thus, TRPV3 mutations can cause a spectrum of severe dermatological, immunological, and sensory phenotypes.
Whether endogenous cannabinoids contribute to the pathophysiology of these conditions, or whether exogenous cannabinoids might be useful to treat patients with TRPV3 mutations, remains to be determined. It is also not yet clear whether abnormalities in TRPV3 sequence, expression, or regulation might lead to other dermatological diseases. Increases in TRPV3 mRNA expression and TRPV3-like immunoreactivity were reported in skin of rosacea patients.45 Furthermore, weak acids, upon entry into keratinocytes, were shown to trigger activation of TRPV3 and promote apoptosis. The authors of that study speculated that this might constitute a mechanistic basis for cosmetic “acid peel” skin exfoliation procedures.94
TRPV4
TRPV4, like TRPV3, is expressed in skin keratinocytes, as well as various other epithelial and endothelial cell types (Figure 2). There is also evidence for TRPV4 expression in both sensory and motor neurons. TRPV4 was originally identified as a channel that could be gated by changes in osmolarity.95,96 Additional endogenous TRPV4 agonists include certain epoxyeichosatetraenoic acid derivatives, and warm temperatures (>27 °C). Exogenous agonists include the phytochemical bisandrographolide A, the phorbol ester 4α-phorbol 12,13-didecanoate (4α-PDD), and a host of molecules identified via high-throughput screening, such as GSK1016790A. Recently, two phytocannabinoids, cannabidivarin and tetrahydrocannabivarin, were shown to stimulate TRPV4, while cannabigerovarin, cannabigerolic acid, cannabinol, and cannabigerol were shown to desensitize this channel81 (Table 1). In addition, anandamide and 2-AG can act as precursors for epoxyeichosatetraenoic acid-derived TRPV4 agonists.95 Studies using TRPV4 agonists and examination of TRPV4 knockout mice have implicated this channel in numerous physiological processes, ranging from vasodilation, reflex bladder contraction, and saliva secretion to pain and osmoregulation.95,97 Genetic mutations in human TRPV4 have been linked to both neurodegenerative disorders (e.g., type IV Charcot-Marie tooth disease) and skeletal disorders (e.g., brachyolmia).27
TRPV4 Involvement in Pain Sensation
Multiple lines of evidence support a role for TRPV4 in pain sensation. TRPV4 knockout mice were found to exhibit reduced acute mechanically evoked pain behaviors.97,98 In addition, genetic elimination or downregulation of TRPV4 suppressed mechanical hypersensitivity in rodents challenged with insults such as chemotherapeutic agents,99 or complete Freund’s adjuvant.100 TRPV4 knockout mice also exhibited modest defects in heat-evoked pain sensation,101 though this phenotype was virtually absent in TRPV3/TRPV4 double knockout mice.82 Thermal and mechanical hypersensitivity following UVB irradiation of the skin were found to be diminished in mice lacking TRPV4 selectively in keratinocytes. One possible contributor to this phenotype was the absence of TRPV4-dependent synthesis and release of endothelin 1 from UVB treated keratinocytes.102
TRPV4 Involvement in Epidermal Homeostasis and Dermatitis
TRPV4 appears to be important for the temperature-dependent formation of normal epithelial tight junctions between skin keratinocytes in both mice and humans. This function involves TRPV4-dependent calcium entry, with subsequent activation of Rho kinases and actin rearrangement. Mice lacking TRPV4 were reported to exhibit impaired epidermal barrier function. They also exhibited a thickened stratum corneum, perhaps as a reaction to the former deficit.103,104 TRPV4 may also participate in certain forms of cutaneous inflammation. For example, the keratinocyte-selective knockout of the TRPV4 gene not only reduces pain arising from UVB irradiation, but also suppresses irradiation induced skin damage and inflammatory cell recruitment. The latter may stem, in part, from reduced UVB induced secretion of cytokines such as interleukin 6 from keratinocytes lacking TRPV4. Correspondingly, human skin overexposed to UV light exhibited upregulation of TRPV4.102 Moreover, in skin from patients with rosacea, although the mRNA levels of TRPV4 were not different from those in healthy skin, an increase in TRPV4-like immunoreactivity was observed in dermal cells that resembled macrophages and T cells.45
TRPM8
TRPM8 is expressed prominently in a subpopulation of nonpeptidergic small-diameter sensory neurons, and can be activated by either cold temperatures or menthol (Figure 2). In mice, this channel is essential for cutaneous discrimination of mildly cold temperatures, and also appears to play both positive and negative roles in cold-evoked pain sensation.105 TRPM8 can also be activated by the cannabinoid CBG106 (Table 1). Immunoreactivity for TRPM8 has been demonstrated in human skin, and is reduced in patients with congenital insensitivity to pain.41 As of yet, TRPM8 has not been linked to skin homeostasis or dermatitis. However, its activation was recently shown to suppress chemically evoked irritation and inhibit TRPV1-mediated CGRP release in colon tissue.107 Thus, potential anti-inflammatory effects of this channel in skin should be explored.
In summary, cannabinoids can engage numerous targets within the skin, including not only metabotropic receptors, but also multiple members of the TRP family of ion channels. Cutaneous ionotropic cannabinoid receptors participate in functions related to pain and itch perception, epidermal homeostasis, and the promotion and suppression of dermatitis in both animal models and humans. This situation creates potential opportunities to intervene therapeutically in sensory and inflammatory skin diseases using the chemically rich pharmacology of cannabinoids. In addition, the experimental accessibility of the skin makes this organ an excellent one in which to uncover principles of intercellular cannabinoid signaling that may be generalizable to the CNS and other less accessible tissues. The value of such an experimentally tractable system is amplified by the multiple examples, described above, in which the effects of a given cannabinoid or a given change in TRP channel activity can produce distinct outcomes, depending on the biological and pharmacological circumstances.
Supported by Grants NIAMS AR-62826 and NIDCR DE022750 to M.J.C. and by the Neurosurgery Pain Research Institute at Johns Hopkins School of Medicine.
The authors declare the following competing financial interest(s): M.J.C. is an inventor on a patent on the use of products related to TRPV1, which is licensed through UCSF and Merck and a member of the Scientific Advisory Board of Hydra Biosciences. These conflicts are being managed by the Johns Hopkins Office on Policy Coordination.
Funding Statement
National Institutes of Health, United States
References
- Pacher P.; Batkai S.; Kunos G. (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 58, 389–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian A. N.; Ruparel N. B.; Jeske N. A.; Patwardhan A.; Hargreaves K. M. (2009) Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends Pharmacol. Sci. 30, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee R. G.; Howlett A. C.; Abood M. E.; Alexander S. P.; Di Marzo V.; Elphick M. R.; Greasley P. J.; Hansen H. S.; Kunos G.; Mackie K.; Mechoulam R.; Ross R. A. (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and Their Ligands: Beyond CB(1) and CB(2). Pharmacol Rev. 62, 588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro T.; Toth B. I.; Hasko G.; Paus R.; Pacher P. (2009) The endocannabinoid system of the skin in health and disease: novel perspectives and therapeutic opportunities. Trends Pharmacol. Sci. 30, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci M.; Pirazzi V.; Pasquariello N.; Maccarrone M. (2011) Endocannabinoid signaling and epidermal differentiation. Eur. J. Dermatol 21(Suppl 2), 29–34. [DOI] [PubMed] [Google Scholar]
- Ahluwalia J.; Urban L.; Bevan S.; Nagy I. (2003) Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur. J. Neurosci 17, 2611–2618. [DOI] [PubMed] [Google Scholar]
- Lever I. J.; Robinson M.; Cibelli M.; Paule C.; Santha P.; Yee L.; Hunt S. P.; Cravatt B. F.; Elphick M. R.; Nagy I.; Rice A. S. (2009) Localization of the endocannabinoid-degrading enzyme fatty acid amide hydrolase in rat dorsal root ganglion cells and its regulation after peripheral nerve injury. J. Neurosci. 29, 3766–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D.; Rice A. S.; Egertova M.; Elphick M. R.; Winter J.; Michael G. J. (2003) Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience 119, 803–812. [DOI] [PubMed] [Google Scholar]
- Ibrahim M. M.; Porreca F.; Lai J.; Albrecht P. J.; Rice F. L.; Khodorova A.; Davar G.; Makriyannis A.; Vanderah T. W.; Mata H. P.; Malan T. P. Jr. (2005) CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc. Natl. Acad. Sci. U.S.A. 102, 3093–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U.; Otto W. R.; Sanchez-Herrera D.; Facer P.; Yiangou Y.; Korchev Y.; Birch R.; Benham C.; Bountra C.; Chessell I. P.; Anand P. (2008) Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain 138, 667–680. [DOI] [PubMed] [Google Scholar]
- Stander S.; Schmelz M.; Metze D.; Luger T.; Rukwied R. (2005) Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J. Dermatol. Sci. 38, 177–188. [DOI] [PubMed] [Google Scholar]
- Pucci M.; Rapino C.; Di Francesco A.; Dainese E.; D’Addario C.; Maccarrone M. (2013) Epigenetic control of skin differentiation genes by phytocannabinoids. Br. J. Pharmacol. 170, 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth B. I.; Dobrosi N.; Dajnoki A.; Czifra G.; Olah A.; Szollosi A. G.; Juhasz I.; Sugawara K.; Paus R.; Biro T. (2011) Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J. Invest. Dermatol. 131, 1095–1104. [DOI] [PubMed] [Google Scholar]
- Ramot Y.; Sugawara K.; Zakany N.; Toth B. I.; Biro T.; Paus R. (2013) A novel control of human keratin expression: cannabinoid receptor 1-mediated signaling down-regulates the expression of keratins K6 and K16 in human keratinocytes in vitro and in situ. PeerJ. 1, e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M. L.; Blazquez C.; Martinez-Palacio J.; Villanueva C.; Fernandez-Acenero M. J.; Huffman J. W.; Jorcano J. L.; Guzman M. (2003) Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J. Clin. Invest. 111, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B. K.; Vaughan C. W. (2014) Targeting the endogenous cannabinoid system to treat neuropathic pain. Front. Pharmacol. 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. M.; Hohmann A. G. (2005) Cannabinoid mechanisms of pain suppression. Handb. Exp. Pharmacol. 509–554. [DOI] [PubMed] [Google Scholar]
- Movahed P.; Evilevitch V.; Andersson T. L.; Jonsson B. A.; Wollmer P.; Zygmunt P. M.; Hogestatt E. D. (2005) Vascular effects of anandamide and N-acylvanillylamines in the human forearm and skin microcirculation. Br. J. Pharmacol. 146, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. M.; Krey J. F.; Chen J. S.; Vefring E.; Jahnsen J. A.; Bradshaw H.; Huang S. M. (2005) Targeted lipidomics: fatty acid amides and pain modulation. Prostaglandins Other Lipid Mediators 77, 35–45. [DOI] [PubMed] [Google Scholar]
- Hohmann A. G.; Suplita R. L.; Bolton N. M.; Neely M. H.; Fegley D.; Mangieri R.; Krey J. F.; Walker J. M.; Holmes P. V.; Crystal J. D.; Duranti A.; Tontini A.; Mor M.; Tarzia G.; Piomelli D. (2005) An endocannabinoid mechanism for stress-induced analgesia. Nature 435, 1108–1112. [DOI] [PubMed] [Google Scholar]
- Schlosburg J. E.; O’Neal S. T.; Conrad D. H.; Lichtman A. H. (2011) CB1 receptors mediate rimonabant-induced pruritic responses in mice: investigation of locus of action. Psychopharmacology 216, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. W.; Newton C. A. (2007) Therapeutic potential of cannabinoid-based drugs. Adv. Exp. Med. Biol. 601, 395–413. [DOI] [PubMed] [Google Scholar]
- Karsak M.; Gaffal E.; Date R.; Wang-Eckhardt L.; Rehnelt J.; Petrosino S.; Starowicz K.; Steuder R.; Schlicker E.; Cravatt B.; Mechoulam R.; Buettner R.; Werner S.; Di Marzo V.; Tuting T.; Zimmer A. (2007) Attenuation of allergic contact dermatitis through the endocannabinoid system. Science 316, 1494–1497. [DOI] [PubMed] [Google Scholar]
- Gaffal E.; Cron M.; Glodde N.; Tuting T. (2013) Anti-inflammatory activity of topical THC in DNFB-mediated mouse allergic contact dermatitis independent of CB1 and CB2 receptors. Allergy 68, 994–1000. [DOI] [PubMed] [Google Scholar]
- Petrosino S.; Cristino L.; Karsak M.; Gaffal E.; Ueda N.; Tuting T.; Bisogno T.; De Filippis D.; D’Amico A.; Saturnino C.; Orlando P.; Zimmer A.; Iuvone T.; Di Marzo V. (2010) Protective role of palmitoylethanolamide in contact allergic dermatitis. Allergy 65, 698–711. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K.; Montell C. (2007) TRP channels. Annu. Rev. Biochem. 76, 387–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B.; Owsianik G. (2010) Transient receptor potential channelopathies. Pfluegers Arch. 460, 437–450. [DOI] [PubMed] [Google Scholar]
- Caterina M. J.; Julius D. (2001) The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 24, 487–517. [DOI] [PubMed] [Google Scholar]
- Zygmunt P. M.; Petersson J.; Andersson D. A.; Chuang H.; Sorgard M.; Di Marzo V.; Julius D.; Hogestatt E. D. (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400, 452–457. [DOI] [PubMed] [Google Scholar]
- Zygmunt P. M.; Ermund A.; Movahed P.; Andersson D. A.; Simonsen C.; Jonsson B. A.; Blomgren A.; Birnir B.; Bevan S.; Eschalier A.; Mallet C.; Gomis A.; Hogestatt E. D. (2013) Monoacylglycerols activate TRPV1--a link between phospholipase C and TRPV1. PLoS One 8, e81618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T.; Hanus L.; De Petrocellis L.; Tchilibon S.; Ponde D. E.; Brandi I.; Moriello A. S.; Davis J. B.; Mechoulam R.; Di Marzo V. (2001) Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 134, 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruparel N. B.; Patwardhan A. M.; Akopian A. N.; Hargreaves K. M. (2011) Desensitization of transient receptor potential ankyrin 1 (TRPA1) by the TRP vanilloid 1-selective cannabinoid arachidonoyl-2 chloroethanolamine. Mol. Pharmacol. 80, 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim W. S.; Tak M. H.; Lee M. H.; Kim M.; Koo J. Y.; Lee C. H.; Oh U. (2007) TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J. Neurosci. 27, 2331–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppetti P.; Nassini R.; Materazzi S.; Benemei S. (2008) The concept of neurogenic inflammation. BJU Int. 101(Suppl 3), 2–6. [DOI] [PubMed] [Google Scholar]
- Yun J. W.; Seo J. A.; Jang W. H.; Koh H. J.; Bae I. H.; Park Y. H.; Lim K. M. (2011) Antipruritic effects of TRPV1 antagonist in murine atopic dermatitis and itching models. J. Invest. Dermatol. 131, 1576–1579. [DOI] [PubMed] [Google Scholar]
- Yun J. W.; Seo J. A.; Jeong Y. S.; Bae I. H.; Jang W. H.; Lee J.; Kim S. Y.; Shin S. S.; Woo B. Y.; Lee K. W.; Lim K. M.; Park Y. H. (2011) TRPV1 antagonist can suppress the atopic dermatitis-like symptoms by accelerating skin barrier recovery. J. Dermatol. Sci. 62, 8–15. [DOI] [PubMed] [Google Scholar]
- Banvolgyi A.; Palinkas L.; Berki T.; Clark N.; Grant A. D.; Helyes Z.; Pozsgai G.; Szolcsanyi J.; Brain S. D.; Pinter E. (2005) Evidence for a novel protective role of the vanilloid TRPV1 receptor in a cutaneous contact allergic dermatitis model. J. Neuroimmunol. 169, 86–96. [DOI] [PubMed] [Google Scholar]
- Engel M. A.; Izydorczyk I.; Mueller-Tribbensee S. M.; Becker C.; Neurath M. F.; Reeh P. W. (2011) Inhibitory CB1 and activating/desensitizing TRPV1-mediated cannabinoid actions on CGRP release in rodent skin. Neuropeptides 45, 229–237. [DOI] [PubMed] [Google Scholar]
- Fioravanti B.; De Felice M.; Stucky C. L.; Medler K. A.; Luo M. C.; Gardell L. R.; Ibrahim M.; Malan T. P. Jr.; Yamamura H. I.; Ossipov M. H.; King T.; Lai J.; Porreca F.; Vanderah T. W. (2008) Constitutive activity at the cannabinoid CB1 receptor is required for behavioral response to noxious chemical stimulation of TRPV1: antinociceptive actions of CB1 inverse agonists. J. Neurosci. 28, 11593–11602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe M. J.; Chizh B. A. (2009) Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Discovery Today 14, 56–67. [DOI] [PubMed] [Google Scholar]
- Axelsson H. E.; Minde J. K.; Sonesson A.; Toolanen G.; Hogestatt E. D.; Zygmunt P. M. (2009) Transient receptor potential vanilloid 1, vanilloid 2 and melastatin 8 immunoreactive nerve fibers in human skin from individuals with and without Norrbottnian congenital insensitivity to pain. Neuroscience 162, 1322–1332. [DOI] [PubMed] [Google Scholar]
- Bodo E.; Biro T.; Telek A.; Czifra G.; Griger Z.; Toth B. I.; Mescalchin A.; Ito T.; Bettermann A.; Kovacs L.; Paus R. (2005) A hot new twist to hair biology: involvement of vanilloid receptor-1 (VR1/TRPV1) signaling in human hair growth control. Am. J. Pathol. 166, 985–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stander S.; Moormann C.; Schumacher M.; Buddenkotte J.; Artuc M.; Shpacovitch V.; Brzoska T.; Lippert U.; Henz B. M.; Luger T. A.; Metze D.; Steinhoff M. (2004) Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp. Dermatol. 13, 129–139. [DOI] [PubMed] [Google Scholar]
- Toth B. I.; Benko S.; Szollosi A. G.; Kovacs L.; Rajnavolgyi E.; Biro T. (2009) Transient receptor potential vanilloid-1 signaling inhibits differentiation and activation of human dendritic cells. FEBS Lett. 583, 1619–1624. [DOI] [PubMed] [Google Scholar]
- Sulk M.; Seeliger S.; Aubert J.; Schwab V. D.; Cevikbas F.; Rivier M.; Nowak P.; Voegel J. J.; Buddenkotte J.; Steinhoff M. (2012) Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J. Invest. Dermatol. 132, 1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B.; Appendino G.; Owsianik G. (2012) The transient receptor potential channel TRPA1: from gene to pathophysiology. Pfluegers Arch. 464, 425–458. [DOI] [PubMed] [Google Scholar]
- Jordt S. E.; Bautista D. M.; Chuang H. H.; McKemy D. D.; Zygmunt P. M.; Hogestatt E. D.; Meng I. D.; Julius D. (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265. [DOI] [PubMed] [Google Scholar]
- Patil M.; Patwardhan A.; Salas M. M.; Hargreaves K. M.; Akopian A. N. (2011) Cannabinoid receptor antagonists AM251 and AM630 activate TRPA1 in sensory neurons. Neuropharmacology 61, 778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Camino D.; Murphy S.; Heiry M.; Barrett L. B.; Earley T. J.; Cook C. A.; Petrus M. J.; Zhao M.; D’Amours M.; Deering N.; Brenner G. J.; Costigan M.; Hayward N. J.; Chong J. A.; Fanger C. M.; Woolf C. J.; Patapoutian A.; Moran M. M. (2010) TRPA1 contributes to cold hypersensitivity. J. Neurosci. 30, 15165–15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrus M.; Peier A. M.; Bandell M.; Hwang S. W.; Huynh T.; Olney N.; Jegla T.; Patapoutian A. (2007) A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol. Pain 3, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. R.; Gerhold K. A.; Bifolck-Fisher A.; Liu Q.; Patel K. N.; Dong X.; Bautista D. M. (2011) TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci. 14, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. R.; Nelson A. M.; Batia L.; Morita T.; Estandian D.; Owens D. M.; Lumpkin E. A.; Bautista D. M. (2013) The ion channel TRPA1 is required for chronic itch. J. Neurosci. 33, 9283–9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista D. M.; Jordt S. E.; Nikai T.; Tsuruda P. R.; Read A. J.; Poblete J.; Yamoah E. N.; Basbaum A. I.; Julius D. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282. [DOI] [PubMed] [Google Scholar]
- McNamara C. R.; Mandel-Brehm J.; Bautista D. M.; Siemens J.; Deranian K. L.; Zhao M.; Hayward N. J.; Chong J. A.; Julius D.; Moran M. M.; Fanger C. M. (2007) TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. U.S.A. 104, 13525–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.; Escalera J.; Balakrishna S.; Fan L.; Caceres A. I.; Robinson E.; Sui A.; McKay M. C.; McAlexander M. A.; Herrick C. A.; Jordt S. E. (2013) TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 27, 3549–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh M. H.; Oh S. Y.; Lu J.; Lou H.; Myers A. C.; Zhu Z.; Zheng T. (2013) TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J. Immunol 191, 5371–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atoyan R.; Shander D.; Botchkareva N. V. (2009) Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J. Invest. Dermatol. 129, 2312–2315. [DOI] [PubMed] [Google Scholar]
- Kwan K. Y.; Glazer J. M.; Corey D. P.; Rice F. L.; Stucky C. L. (2009) TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J. Neurosci. 29, 4808–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellono N. W.; Kammel L. G.; Zimmerman A. L.; Oancea E. (2013) UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc. Natl. Acad. Sci. U.S.A. 110, 2383–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehler B.; Scholze A.; Schaefer M.; Hill K. (2012) TRPA1 is functionally expressed in melanoma cells but is not critical for impaired proliferation caused by allyl isothiocyanate or cinnamaldehyde. Naunyn-Schmiedeberg’s Arch. Pharmacol. 385, 555–563. [DOI] [PubMed] [Google Scholar]
- Akopian A. N. (2011) Regulation of nociceptive transmission at the periphery via TRPA1-TRPV1 interactions. Curr. Pharm. Biotechnol 12, 89–94. [DOI] [PubMed] [Google Scholar]
- Caterina M. J.; Rosen T. A.; Tominaga M.; Brake A. J.; Julius D. (1999) A capsaicin receptor homologue with a high threshold for noxious heat. Nature 398, 436–441. [DOI] [PubMed] [Google Scholar]
- Kanzaki M.; Zhang Y. Q.; Mashima H.; Li L.; Shibata H.; Kojima I. (1999) Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat. Cell Biol. 1, 165–170. [DOI] [PubMed] [Google Scholar]
- Nagasawa M.; Nakagawa Y.; Tanaka S.; Kojima I. (2007) Chemotactic peptide fMetLeuPhe induces translocation of the TRPV2 channel in macrophages. J. Cell Physiol. 210, 692–702. [DOI] [PubMed] [Google Scholar]
- Stokes A. J.; Shimoda L. M.; Koblan-Huberson M.; Adra C. N.; Turner H. (2004) A TRPV2-PKA signaling module for transduction of physical stimuli in mast cells. J. Exp. Med. 200, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S.; Kim K. Y.; Yoo S.; Lee S. H.; Hwang S. W. (2007) Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci. Lett. 425, 120–125. [DOI] [PubMed] [Google Scholar]
- Hu H. Z.; Gu Q.; Wang C.; Colton C. K.; Tang J.; Kinoshita-Kawada M.; Lee L. Y.; Wood J. D.; Zhu M. X. (2004) 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J. Biol. Chem. 279, 35741–35748. [DOI] [PubMed] [Google Scholar]
- Neeper M. P.; Liu Y.; Hutchinson T. L.; Wang Y.; Flores C. M.; Qin N. (2007) Activation properties of heterologously expressed mammalian TRPV2: evidence for species dependence. J. Biol. Chem. 282, 15894–15902. [DOI] [PubMed] [Google Scholar]
- Qin N.; Neeper M. P.; Liu Y.; Hutchinson T. L.; Lubin M. L.; Flores C. M. (2008) TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J. Neurosci. 28, 6231–6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park U.; Vastani N.; Guan Y.; Raja S. N.; Koltzenburg M.; Caterina M. J. (2011) TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J. Neurosci. 31, 11425–11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szollosi A. G.; Olah A.; Toth I. B.; Papp F.; Czifra G.; Panyi G.; Biro T. (2013) Transient receptor potential vanilloid-2 mediates the effects of transient heat shock on endocytosis of human monocyte-derived dendritic cells. FEBS Lett. 587, 1440–1445. [DOI] [PubMed] [Google Scholar]
- Link T. M.; Park U.; Vonakis B. M.; Raben D. M.; Soloski M. J.; Caterina M. J. (2010) TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat. Immunol. 11, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro K.; Sasano T.; Tojo K.; Namekata I.; Kurokawa J.; Sawada N.; Suganami T.; Kamei Y.; Tanaka H.; Tajima N.; Utsunomiya K.; Ogawa Y.; Furukawa T. (2010) Role of transient receptor potential vanilloid 2 in LPS-induced cytokine production in macrophages. Biochem. Biophys. Res. Commun. 398, 284–289. [DOI] [PubMed] [Google Scholar]
- Compan V.; Baroja-Mazo A.; Lopez-Castejon G.; Gomez A. I.; Martinez C. M.; Angosto D.; Montero M. T.; Herranz A. S.; Bazan E.; Reimers D.; Mulero V.; Pelegrin P. (2012) Cell volume regulation modulates NLRP3 inflammasome activation. Immunity 37, 487–500. [DOI] [PubMed] [Google Scholar]
- Peier A. M.; Reeve A. J.; Andersson D. A.; Moqrich A.; Earley T. J.; Hergarden A. C.; Story G. M.; Colley S.; Hogenesch J. B.; McIntyre P.; Bevan S.; Patapoutian A. (2002) A heat-sensitive TRP channel expressed in keratinocytes. Science 296, 2046–2049. [DOI] [PubMed] [Google Scholar]
- Smith G. D.; Gunthorpe M. J.; Kelsell R. E.; Hayes P. D.; Reilly P.; Facer P.; Wright J. E.; Jerman J. C.; Walhin J. P.; Ooi L.; Egerton J.; Charles K. J.; Smart D.; Randall A. D.; Anand P.; Davis J. B. (2002) TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418, 186–190. [DOI] [PubMed] [Google Scholar]
- Xu H.; Ramsey I. S.; Kotecha S. A.; Moran M. M.; Chong J. A.; Lawson D.; Ge P.; Lilly J.; Silos-Santiago I.; Xie Y.; DiStefano P. S.; Curtis R.; Clapham D. E. (2002) TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418, 181–186. [DOI] [PubMed] [Google Scholar]
- Moqrich A.; Hwang S. W.; Earley T. J.; Petrus M. J.; Murray A. N.; Spencer K. S.; Andahazy M.; Story G. M.; Patapoutian A. (2005) Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307, 1468–1472. [DOI] [PubMed] [Google Scholar]
- Moussaieff A.; Rimmerman N.; Bregman T.; Straiker A.; Felder C. C.; Shoham S.; Kashman Y.; Huang S. M.; Lee H.; Shohami E.; Mackie K.; Caterina M. J.; Walker J. M.; Fride E.; Mechoulam R. (2008) Incensole acetate, an incense component, elicits psychoactivity by activating TRPV3 channels in the brain. FASEB J. 22, 3024–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.; Delling M.; Jun J. C.; Clapham D. E. (2006) Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 9, 628–635. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L.; Orlando P.; Moriello A. S.; Aviello G.; Stott C.; Izzo A. A.; Di Marzo V. (2012) Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 204, 255–266. [DOI] [PubMed] [Google Scholar]
- Huang S. M.; Li X.; Yu Y.; Wang J.; Caterina M. J. (2011) TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol. Pain 7, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T.; Petrus M. J.; Dubin A. E.; Patapoutian A. (2011) TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat. Commun. 2, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Kasai E.; Imura K.; Yasui K.; Shichijou M.; Oshima I.; Hirasawa T.; Sakata T.; Yoshioka T. (2012) TRPV3 as a therapeutic target for itch. J. Invest. Dermatol. 132, 2109–2112. [DOI] [PubMed] [Google Scholar]
- Cheng X.; Jin J.; Hu L.; Shen D.; Dong X. P.; Samie M. A.; Knoff J.; Eisinger B.; Liu M. L.; Huang S. M.; Caterina M. J.; Dempsey P.; Michael L. E.; Dlugosz A. A.; Andrews N. C.; Clapham D. E.; Xu H. (2010) TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 141, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbiro I.; Lisztes E.; Toth B. I.; Czifra G.; Olah A.; Szollosi A. G.; Szentandrassy N.; Nanasi P. P.; Peter Z.; Paus R.; Kovacs L.; Biro T. (2011) Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J. Invest. Dermatol. 131, 1605–1614. [DOI] [PubMed] [Google Scholar]
- Asakawa M.; Yoshioka T.; Matsutani T.; Hikita I.; Suzuki M.; Oshima I.; Tsukahara K.; Arimura A.; Horikawa T.; Hirasawa T.; Sakata T. (2006) Association of a mutation in TRPV3 with defective hair growth in rodents. J. Invest. Dermatol. 126, 2664–2672. [DOI] [PubMed] [Google Scholar]
- Xiao R.; Tian J.; Tang J.; Zhu M. X. (2008) The TRPV3 mutation associated with the hairless phenotype in rodents is constitutively active. Cell Calcium 43, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura K.; Yoshioka T.; Hirasawa T.; Sakata T. (2009) Role of TRPV3 in immune response to development of dermatitis. J. Inflammation 6, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z.; Chen Q.; Lee M.; Cao X.; Zhang J.; Ma D.; Chen L.; Hu X.; Wang H.; Wang X.; Zhang P.; Liu X.; Guan L.; Tang Y.; Yang H.; Tu P.; Bu D.; Zhu X.; Wang K.; Li R.; Yang Y. (2012) Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am. J. Hum. Genet. 90, 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danso-Abeam D.; Zhang J.; Dooley J.; Staats K. A.; Van Eyck L.; Van Brussel T.; Zaman S.; Hauben E.; Van de Velde M.; Morren M. A.; Renard M.; Van Geet C.; Schaballie H.; Lambrechts D.; Tao J.; Franckaert D.; Humblet-Baron S.; Meyts I.; Liston A. (2013) Olmsted syndrome: exploration of the immunological phenotype. Orphanet J. Rare Dis. 8, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchatelet S.; Guibbal L.; de Veer S.; Fraitag S.; Nitschke P.; Zarhrate M.; Bodemer C.; Hovnanian A. (2014) Olmsted syndrome with erythromelalgia caused by recessive TRPV3 mutations. Br. J. Dermatol. 10.1111/bjd.12951. [DOI] [PubMed] [Google Scholar]
- Eytan O.; Fuchs-Telem D.; Mevorach B.; Indelman M.; Bergman R.; Sarig O.; Goldberg I.; Adir N.; Sprecher E. (2014) Olmsted Syndrome Caused by a Homozygous Recessive Mutation in TRPV3. J. Invest. Dermatol. 10.1038/jid.2014.37. [DOI] [PubMed] [Google Scholar]
- Cao X.; Yang F.; Zheng J.; Wang K. (2012) Intracellular proton-mediated activation of TRPV3 channels accounts for the exfoliation effect of alpha-hydroxyl acids on keratinocytes. J. Biol. Chem. 287, 25905–25916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerts W.; Nilius B.; Owsianik G. (2010) The vanilloid transient receptor potential channel TRPV4: from structure to disease. Prog. Biophys. Mol. Biol. 103, 2–17. [DOI] [PubMed] [Google Scholar]
- Liedtke W.; Choe Y.; Marti-Renom M. A.; Bell A. M.; Denis C. S.; Sali A.; Hudspeth A. J.; Friedman J. M.; Heller S. (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103, 525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W.; Friedman J. M. (2003) Abnormal osmotic regulation in trpv4–/– mice. Proc. Natl. Acad. Sci. U.S.A. 100, 13698–13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M.; Mizuno A.; Kodaira K.; Imai M. (2003) Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 278, 22664–22668. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N.; Dina O. A.; Yeh J. J.; Parada C. A.; Reichling D. B.; Levine J. D. (2004) Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J. Neurosci. 24, 4444–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Williams S. H.; McNulty A. L.; Hong J. H.; Lee S. H.; Rothfusz N. E.; Parekh P. K.; Moore C.; Gereau R. W. t.; Taylor A. B.; Wang F.; Guilak F.; Liedtke W. (2013) Temporomandibular joint pain: a critical role for Trpv4 in the trigeminal ganglion. Pain 154, 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.; Iida T.; Mizuno A.; Suzuki M.; Caterina M. J. (2005) Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J. Neurosci. 25, 1304–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C.; Cevikbas F.; Pasolli H. A.; Chen Y.; Kong W.; Kempkes C.; Parekh P.; Lee S. H.; Kontchou N. A.; Yeh I.; Jokerst N. M.; Fuchs E.; Steinhoff M.; Liedtke W. B. (2013) UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc. Natl. Acad. Sci. U.S.A. 110, E3225–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida N.; Sokabe T.; Kashio M.; Haruna K.; Mizuno Y.; Suga Y.; Nishikawa K.; Kanamaru A.; Hongo M.; Oba A.; Tominaga M. (2012) Importance of transient receptor potential vanilloid 4 (TRPV4) in epidermal barrier function in human skin keratinocytes. Pfluegers Arch 463, 715–725. [DOI] [PubMed] [Google Scholar]
- Sokabe T.; Tominaga M. (2010) The TRPV4 cation channel: A molecule linking skin temperature and barrier function. Commun. Integr. Biol. 3, 619–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy D. D. (2013) The molecular and cellular basis of cold sensation. ACS Chem. Neurosci. 4, 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L.; Vellani V.; Schiano-Moriello A.; Marini P.; Magherini P. C.; Orlando P.; Di Marzo V. (2008) Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J. Pharmacol. Exp. Ther. 325, 1007–1015. [DOI] [PubMed] [Google Scholar]
- Ramachandran R.; Hyun E.; Zhao L.; Lapointe T. K.; Chapman K.; Hirota C. L.; Ghosh S.; McKemy D. D.; Vergnolle N.; Beck P. L.; Altier C.; Hollenberg M. D. (2013) TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc. Natl. Acad. Sci. U.S.A. 110, 7476–7481. [DOI] [PMC free article] [PubMed] [Google Scholar]