Abstract
Objectives:
This study aims to conduct a non-invasive measurement of the cutaneous temperature of selected masticatory muscle regions of volunteers with and without myogenous temporomandibular disorder (TMD), using infrared thermography.
Methods:
23 females (10 myogenous TMD volunteers and 13 controls) were recruited and studied. The temperature at the surface of the facial area over the anterior temporalis and masseter muscles was assessed by medical thermography, using regional lateral views and clinical examination.
Results:
The temperature levels measured at the masseter and anterior temporalis muscle regions in myogenous TMD volunteers (32.85 ± 0.85 and 34.37 ± 0.64 ºC, respectively) were significantly lower (p < 0.05) than those measured in controls (33.49 ± 0.92 and 34.78 ± 0.44 ºC, respectively). Medical infrared imaging indicated a mean difference of 1.4 ºC between the masseter and anterior temporalis regions. Analysis of the comparison between the absolute and normalized mean temperatures was performed using the pairwise comparison of receiver operating characteristic curves, and no statistically significant difference was observed (p > 0.05). The sensitivity and specificity of the thermographic assessment for the masseter region was of 70% and 73%, respectively and for the anterior temporalis region was of 80% and 62%, respectively.
Conclusions:
This method of evaluating masticatory muscle regions of this preliminary study seems to indicate that it can be used as an aid in complimentary diagnosing of TMDs.
Keywords: dentistry, masticatory muscles, temporomandibular joint disorders, infrared thermography
Introduction
Temporomandibular disorders (TMDs) are characterized by orofacial pain and mobility dysfunction of the masticatory structures.1 The aetiology and pathogenesis of TMD have been suggested as indicative of a psychophysiological disorder involving changes in regulatory pain pathways, resulting in maladaptive emotional, physiological and neuroendocrine responses to physical and psychological stressors.2
Numerous complementary diagnostic methods have been proposed for TMDs. The research diagnostic criteria for TMDs (RDC/TMD)3 is the only available TMD diagnostic system. It uses operationally defined measurement criteria to generate computer-derived diagnostic algorithms for the most common TMD forms3 and provide specifications for conducting a standardized clinical physical examination.4
The TMD can be evaluated morphologically and dynamically through medical imaging techniques; however, conventional medical imaging (X-ray, CT and MRI) presently lacks information in analysing physiological functional aspects such as microcirculation and autonomous nervous system. This information would be of major importance for clinical conditions such as trigeminal neuralgia and facial musculoskeletal pain.
Medical infrared thermography is a non-invasive and non-ionizing imaging technique that maps the distribution of thermal radiation of the body surface into images, thus providing a real-time microcirculatory and autonomic nervous dynamic physiological assessment of the cutaneous surface.5–9 Skin temperature is a function of blood flow, which is controlled by the autonomic nervous system and affects both sides of the body simultaneously and uniformly. Most biochemical processes generate heat that must be dissipated, and the skin is the major route for heat dissipation using blood as the heat exchange fluid.10 The literature clearly documents that, in a normal situation, blood flow through the skin of most body parts produces a symmetrical thermal pattern. Qualitative and quantitative changes in symmetrical thermal patterns have been reported as indicators of changes in metabolism, haemodynamics or neuronal thermoregulatory processes in the regions of interest (ROIs).5,7,9,11–13
This preliminary study was designed to evaluate thermographic images of masticatory muscle regions of volunteers with and without orofacial pain. It aims to verify the feasibility of using the temperature outcome as complementary diagnostic information for the myogenous TMD.
Methods and materials
This diagnostic study was approved by the Research Ethics Committee, School of Dentistry, University of São Paulo, São Paulo, Brazil, under protocol no. 210/2010; CAAE 0036.0.017.000-10. The standards for the reporting of diagnostic accuracy studies (STARD) guidelines were followed in this investigation. All volunteers gave their signed informed consent.
Volunteers
The volunteers were recruited at the clinic of Dentistry Faculty, University of São Paulo, São Paulo, Brazil. Patients with and without TMD were recruited during a period of 2 months (n = 34).
Clinical examination
All participants underwent anamnesis and oral clinical examination. The inclusion criteria were a negative history of systemic conditions (e.g. diabetes, hypothyroidism, fibromyalgia or hypertension), headaches, facial scars or wheals, dental pain, parafunctional habits, pre-menopausal females in the second phase of the menstrual cycle and menopausal females absent of hot flushes14 (n = 26). Eight participants were excluded because they did not fulfil the criteria.
Thermal images have been taken from 26 volunteers. The participant was instructed in the use of a visual analogue scale. The RDC/TMD3 was applied to detect the presence of TMD and for all volunteers photographic digital images were taken. The thermal images were taken by an expert physician, followed by the RDC/TMD3 assessment, the photographic images were taken by a trained specialist in TMD. The study was double blinded, and the exclusion criterion was articular TMDs diagnosed by the RDC/TMD.3 After all examinations, they were divided into two groups according to the RDC/TMD:3 asymptomatic (control group of females without myogenous TMD, n = 13) and symptomatic participants (bilateral myogenous TMD group, n = 10). We excluded three volunteers who were symptomatic for articular TMD.
Measurement procedure
Thermographic images were obtained following the standard protocol, recommended by the Academy of Neuromuscular Thermography.15 The subjects were instructed not to apply any powder, make-up or lotion to their facial skin and also not to use a hair dryer or hair straightener.
Participants were acclimatized in a room with a mean temperature of 23 ºC, relative humidity of 60% and air flow inferior to 0.2 m s−1 for 15 min. Their hair was held in place with a headband and a disposable head cover to facilitate image acquisition. Participants were instructed not to palpate, rub, scratch or press their skin at any time until completion of the entire thermographic examination.
The examiner asked the participant to sit on the examination chair and relax their facial muscles, keeping their teeth apart. The camera was fixed on a tripod, and two perpendicular lateral series of thermal images were taken by a thermal specialist, one image of each side, with high definition and thermal sensibility. The distance between the camera and the lateral face being measured was adjusted to 0.80 m, at an angle of 90°, with the lens of the camera parallel to the region being assessed.
Skin-emitted thermal radiation of the masseter and anterior temporalis regions was recorded using a computer-assisted infrared camera (ThermaCAM® T400; FLIR® Systems, Inc., Wilsonville, OR). Thermal sensitivity of the apparatus was 0.05 at 30 ºC, the spectral range was 7.5–12 µm and the spatial resolution 320 × 240 pixels.
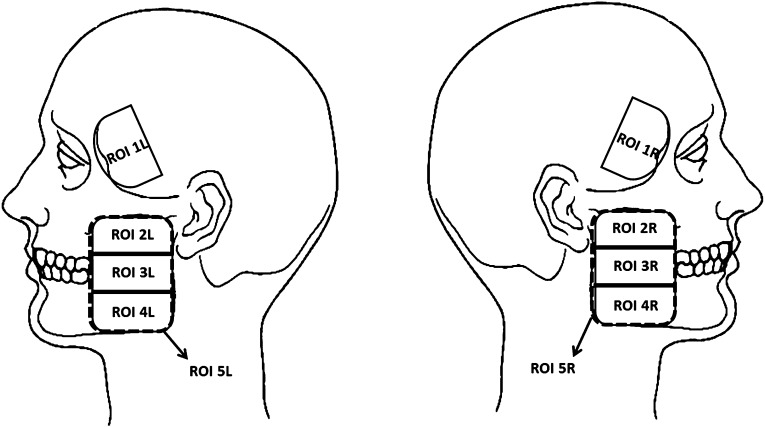
The RDC/TMD is an instrument widely used in research and clinical practice for identifying TMDs and was applied to detect the presence of TMD in this study.3 After that, a trained specialist in TMD management divided the facial surface area over the anterior temporalis and masseter muscles into four regions to facilitate the palpation (Figure 1). The temporalis muscle region was defined as the anterior portion, not covered by hair, whereas the reference points used to define the masseter muscle region were the zygomatic arch (proximal insertion) and the lateral surface of the mandibular angle (distal insertion) (Figure 1). The ROIs were marked with a dermatographic pencil on the patient's skin after the thermal images and before the palpation examination.
Figure 1.
Regions of interest (ROIs), restricted to the masseter and anterior part of the temporal muscles, on left (L) and right (R) sides. ROI 1, anterior temporalis; ROI 2, superior masseter; ROI 3, middle masseter; ROI 4, inferior masseter; ROI 5, masseter.
Digital photographs (Coolpix S51®, 8.1 megapixels; Nikon, Sendai, Japan) of the lateral face were taken after the thermal images in the same position as that used for the infrared thermographic examination. The positioning of the volunteer during the thermal and photographic images was standardized by cephalostat.16 The Frankfurt plane parallel to the horizontal plane was used to assist in proper placement.
The body temperatures of volunteers were measured using an infrared, digital thermometer ThermoScan® Instant Ear Thermometer IRT 1020 (Braun®; Braun AG, Frankfurt am Main, Germany). This thermometer measures temperature values of the eardrum and the surrounding tissues, the location most frequently indicated for measuring body temperature owing to its proximity to the hypothalamus and the perfusion of the arterial labyrinth.17
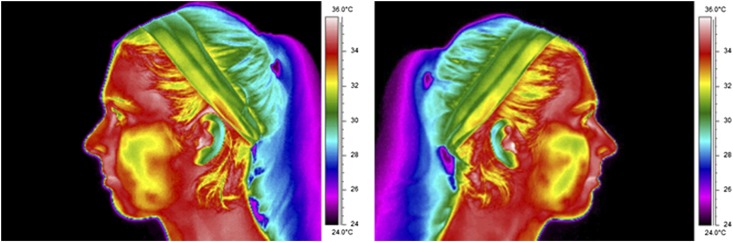
For precise evaluation of the ROIs, the thermographs (Figure 2) were superimposed on the digital photographs using dedicated software (Reporter® v. 8.5—SP3 Professional Edition and QuickReport® v. 1.2; FLIR Systems Inc., Wilsonville, OR). The standardized reference points of the superimposition were midpoint of the median sagittal plane between the chin and the hyoid bone, the lowest portion of the earlobe and the apex of the nasal pyramid. The mean absolute temperatures (T) were averaged according to the landmarks (ROIs) recorded on photographic images, and the normalized temperature (θ)17 of each ROI was calculated individually.
Figure 2.
Examples of lateral facial medical thermal images that were taken during the experiment.
Interpretation of the infrared camera reading was performed using a dimensionless temperature (θ), which combines the measured local temperature with the body (Tb) and ambient temperatures (T∞), according to the following equation:17
This concept is used to produce normalized temperature readings, making any particular skin ROI value independent in terms of measuring units from the body (taken with a tympanic thermometer) and ambient temperatures.17
Data treatment
All images were analysed using the applications FLIR QuickReport® v. 1.2 and FLIR Reporter® v. 8.5, from FLIR Systems, Inc. The emissivity value of the skin of 0.98 was considered for this study.
The data were organized using a Microsoft Excel® (Microsoft Corporation, Redmond, WA) spreadsheet. The analyses were performed using SPSS® v. 19 software (IBM Corporation, Armonk, NY), and the reliability coefficients were based on Pearson's χ2 test.
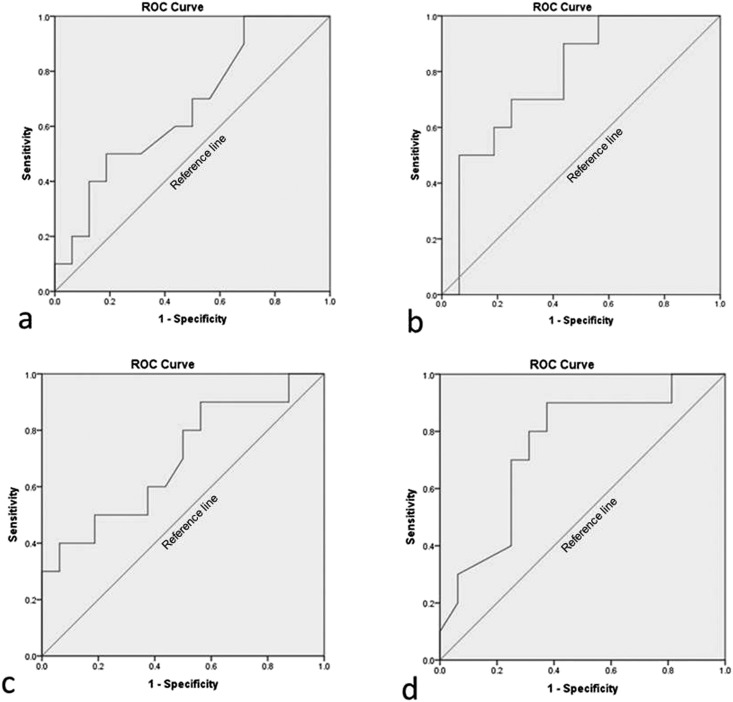
During the data analysis, all volunteers were separated into two groups: symptomatic and asymptomatic for myogenous TMD. For continuous variables, optimal cut-off points were chosen using receiver operating characteristic curve analysis (Figure 3), at the point where sum of specificity and sensitivity is maximum, and equal weight is given to both. The receiver operating characteristic curve is a plot of the true-positive rate (sensitivity) against the false-positive rate (specificity) for the different possible cut-off points of a diagnostic test. It shows the trade-off between sensitivity and specificity. So, the receiver operating characteristic curve tried to establish temperature values that could be used as limits from which we could consider as associated with symptomatic TMD. A level of p < 0.05 was considered statistically significant.
Figure 3.
Receiver operating characteristic (ROC) curve figures of asymptomatic and symptomatic groups for myogenous temporomandibular disorders. (a) Normalized mean temperature of right temporal muscle (area under the ROC curve = 0.669); (b) normalized mean temperature of left temporal muscle (area under the ROC curve = 0.781); (c) normalized mean temperature of right masseter muscle (area under the ROC curve = 0.697); and (d) normalized mean temperature of left masseter muscle (area under the ROC curve = 0.750).
Results
The sample consisted of 23 female volunteers (mean age, 41 years; range, 33–49 years). They were divided into two groups according to the RDC/TMD: asymptomatic and symptomatic. On the day of the examination, all volunteers had no pain (visual analogue scale = 0).
For all ROIs used, it can be seen that there was no statistical evidence (p > 0.05) of independence between the asymptomatic and symptomatic groups of participants considering the bilateral mean temperature differences (Table 1).
Table 1.
Analysis of bilateral mean temperature differences (°C) for asymptomatic and symptomatic participants
| Muscle ROIs | Asymptomatic (n = 13) |
Symptomatic (n = 10) |
p-value | ||
|---|---|---|---|---|---|
| ΔT (°C) | SD | ΔT (°C) | SD | ||
| Anterior temporalis (ROI 1) | 0.0022 | 0.04534 | 0.0550 | 0.361 | >0.05 |
| Superior masseter (ROI 2) | 0.0857 | 0.07880 | 0.2150 | 0.685 | >0.05 |
| Middle masseter (ROI 3) | 0.0272 | 0.06972 | 0.1710 | 0.461 | >0.05 |
| Inferior masseter (ROI 4) | 0.0143 | 0.12854 | 0.2580 | 0.279 | >0.05 |
| Masseter (ROI 5) | 0.0135 | 0.09407 | 0.2775 | 0.429 | >0.05 |
ΔT, differences of mean temperature by right and left sides; ROI, region of interest; SD, standard deviation.
Absolute mean temperatures (Table 2) and normalized mean temperatures (Table 3) for asymptomatic and symptomatic participants were calculated for all muscle ROIs (p < 0.05).
Table 2.
Analysis of absolute mean temperature (°C) for asymptomatic and symptomatic participants
| Muscle ROIs | Asymptomatic (n = 26) |
Symptomatic (n = 20) |
p-value | ||
|---|---|---|---|---|---|
| xT (°C) | SD | xT (°C) | SD | ||
| Anterior temporalis (ROI 1) | 34.78 | 0.44 | 34.37 | 0.64 | 0.0016 |
| Superior masseter (ROI 2) | 33.49 | 1.00 | 32.81 | 0.83 | 0.0116 |
| Middle masseter (ROI 3) | 33.54 | 0.92 | 32.91 | 0.85 | 0.0093 |
| Inferior masseter (ROI 4) | 33.45 | 0.89 | 32.82 | 0.94 | 0.0050 |
| Masseter (ROI 5) | 33.49 | 0.92 | 32.85 | 0.86 | 0.0067 |
xT, absolute mean temperature; ROI, region of interest; SD, standard deviation.
Table 3.
Analysis of normalized mean temperature (θ) for asymptomatic and symptomatic participants
| Muscle ROIs | Asymptomatic (n = 26) |
Symptomatic (n = 20) |
p-value | ||
|---|---|---|---|---|---|
| xθ | SD | xθ | SD | ||
| Anterior temporalis (ROI 1) | 0.877 | 0.04 | 0.831 | 0.04 | 0.0001 |
| Superior masseter (ROI 2) | 0.795 | 0.08 | 0.732 | 0.05 | 0.0014 |
| Middle masseter (ROI 3) | 0.798 | 0.07 | 0.738 | 0.05 | 0.0008 |
| Inferior masseter (ROI 4) | 0.791 | 0.06 | 0.733 | 0.06 | 0.0004 |
| Masseter (ROI 5) | 0.795 | 0.07 | 0.734 | 0.05 | 0.0006 |
xθ, normalized mean temperature; ROI, region of interest; SD, standard deviation.
The absolute mean temperature (Table 2) and the normalized mean temperature over the temporalis muscle (Table 3) was higher than that over the masseter muscle for all participants (p < 0.05).
The absolute (Table 2) and normalized (Table 3) mean temperatures of the muscle ROIs of asymptomatic and symptomatic subjects were compared. The absolute mean temperature in symptomatic participants was lower than that in the control group (p < 0.05).
In the assessment of the absolute mean temperature of the anterior temporalis ROI, it showed higher sensitivity and lower specificity (respectively, 80% and 62%), when compared with the sensitivity and specificity observed for the masseter ROI (70% and 73%, respectively), as presented in Table 4.
Table 4.
Sensitivity and specificity of the thermographic assessment for masticatory muscle regions
| Muscle regions | Measures | Cut-off point | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Anterior temporalis (ROI 1) | xT (°C) | 34.870 | 80.00 | 61.54 |
| xθ | 0.867 | 80.00 | 57.69 | |
| Superior masseter (ROI 2) | xT (°C) | 33.130 | 75.00 | 65.38 |
| xθ | 0.787 | 90.00 | 50.00 | |
| Middle masseter (ROI 3) | xT (°C) | 33.200 | 75.00 | 69.23 |
| xθ | 0.753 | 75.00 | 69.23 | |
| Inferior masseter (ROI 4) | xT (°C) | 32.630 | 60.00 | 73.08 |
| xθ | 0.730 | 65.00 | 73.08 | |
| Masseter (ROI 5) | xT (°C) | 33.000 | 70.00 | 73.08 |
| xθ | 0.740 | 65.00 | 76.92 |
xθ, normalized mean temperature; xT, absolute mean temperature; ROI, region of interest.
Assessing the normalized muscle mean temperature for the anterior temporalis and the masseter ROI showed almost the same sensitivity and specificity when compared with the absolute mean temperature, as shown in Table 4.
Discussion
Only adult females were investigated because masticatory pain disorders are more prevalent in females than in males.18 Age differences would not have influenced the results since the sample age interval is concentrated within 33 and 49 years of age.
In this research, the sample size calculation was not taken into consideration because it was a preliminary study where the proposed methodology would be tested and the number of participants was obtained by recruitment over 2 months.
Medical infrared thermography is a non-invasive and non-ionizing bidimensional imaging technique that maps the distribution of body surface thermal radiation into images. It is based on the capture and transformation of infrared radiation emitted by the human skin to form images that reflect the local vasomotor response. Although it is not standardized at all, qualitative and quantitative changes in symmetrical thermal patterns have been reported as indicators of changes in metabolism, haemodynamics or neuronal thermoregulatory processes in the ROI.9,11–13,16,17,19,20
Different results about temperature values in skin facial surface were present in the literature. This can be owing to the usage of distinct capture methodologies.6,21,22 The isolated measure of punctual temperature should not be used for myogenous TMD diagnosis as pointed by some authors.23
Despite numerous articles addressing infrared thermography as a promising method in the diagnosis of TMD,5,6,10–13,16,24–26 so far, no study has specifically used superimposition of ROI images, as performed in this study. The facial surface area over the masseter muscle was divided into three regions to evaluate the thermal pattern of the smaller cutaneous areas and to compare this pattern to that of the entire muscle. Only the anterior portion of the temporalis muscle was evaluated.
In this study, infrared imaging revealed a difference of 1.4 ºC between mean anterior temporalis and masseter muscle temperatures and a difference of 0.09 between the corresponding normalized temperatures in asymptomatic subjects, indicating that the temporalis was significantly more hyper-radiant than the masseter (p < 0.05). Similar results were found in the related literature.13,16,24,27,28 This difference in the region of muscle temperature may be explained on the basis of anatomy: the temporalis muscle is thinner than the masseter muscle and is influenced by the superficial path of the temporalis artery, which makes the region more hyper-radiant.16
Several studies report hyper-radiation in regions affected by muscle tension.13,27,29,30 This research did not confirm these findings. In fact, it was found that in the affected muscle region, there was a presence of lower temperature than in a corresponding non-affected area. Additionally, an inverse behaviour of the local vasomotor response was observed in the ROIs, corresponding to the affected masseter and temporalis muscles in our study, whereby temperatures decreased with the severity of myogenous TMD.16 Cutaneous temperature is a function of blood flow, controlled by the autonomic nervous system. A muscle contraction may lead to a reduced blood flow response, thus reducing local muscle oxygenation (hypoxia) and increasing the level of metabolic bioproducts in muscle tissue.16,25,28,29 As a result, local temperature may decrease through vasoconstrictive action.16
The results of sensitivity and specificity of the thermographic assessment for masticatory muscle regions suggest greater diagnostic accuracy when absolute mean temperature (×T) is used, even when translated to dimensionless temperature (×θ). These results suggest that thermal values, if used in conjunction with physical assessment, can serve as a means of screening and improved diagnostic accuracy in myogenous TMDs.
In conclusion, within the limitations of this small-scale preliminary study, it may be concluded that the temperature of facial cutaneous areas over the masseter and anterior temporalis muscles decreases in the presence of myogenous TMD. These findings suggest that infrared thermography may prove helpful in evaluating myogenous TMD and may be used as a clinical screening method and for improving diagnostic accuracy. In order to accentuate the findings of this research, the use of a stimulus (thermal, mechanical and chemical) with dynamic thermal imaging is advised. It will provide important and new pathophysiological information characterization for TMD patients and healthy individuals.
Acknowledgments
Acknowledgments
We thank the post-graduate course of School of Dentistry, University of São Paulo, São Paulo, Brazil, and Dr Aida Sabbagh Haddad for their kind encouragement and support.
References
- 1.Dworkin SF, Huggins KH, LeResche L, Von Korff M, Howard J, Truelove E, et al. Epidemiology of signs and symptoms in temporomandibular disorders: clinical signs in cases and controls. J Am Dent Assoc 1990; 120: 273–81. [DOI] [PubMed] [Google Scholar]
- 2.Fillingim RB, Maixner W, Kincaid S, Sigurdsson A, Harris MB. Pain sensitivity in patients with temporomandibular disorders: relationship to clinical and psychosocial factors. Clin J Pain 1996; 12: 260–9. [DOI] [PubMed] [Google Scholar]
- 3.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 1992; 6: 301–55. [PubMed] [Google Scholar]
- 4.John MT, Dworkin SF, Mancl LA. Reliability of clinical temporomandibular disorder diagnoses. Pain 2005; 118: 61–9. doi: 10.1016/j.pain.2005.07.018 [DOI] [PubMed] [Google Scholar]
- 5.Canavan D, Gratt BM. Electronic thermography for the assessment of mild and moderate temporomandibular joint dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995; 79: 778–86. [DOI] [PubMed] [Google Scholar]
- 6.Gratt BM, Anbar M. Thermology and facial telethermography: part II. Current and future clinical applications in dentistry. Dentomaxillofac Radiol 1998; 27: 68–74. doi: 10.1038/sj/dmfr/4600324 [DOI] [PubMed] [Google Scholar]
- 7.Johansson A, Kopp S, Haraldson T. Reproducibility and variation of skin surface temperature over the temporomandibular joint and masseter muscle in normal individuals. Acta Odontol Scand 1985; 43: 309–13. [DOI] [PubMed] [Google Scholar]
- 8.Vardasca R, Simões R. Current issues in medical thermography. In: Tavares JMRS, Natal Jorge RM, eds. Topics in medical image processing and computational vision, lecture notes in computational vision and biomechanics. Vol. 8. Dordrecht, Netherlands: Springer; 2013. pp. 223–37. [Google Scholar]
- 9.Vardasca R, Ring EFJ, Plassmann P, Jones CD. Thermal symmetry of the upper and lower extremities in healthy subjects. Thermol Int 2012; 22: 53–60. [Google Scholar]
- 10.Anbar M, Gratt BM, Hong D. Thermology and facial telethermography. Part I: history and technical review. Dentomaxillofac Radiol 1998; 27: 61–7. doi: 10.1038/sj/dmfr/4600314 [DOI] [PubMed] [Google Scholar]
- 11.Gratt BM, Pullinger A, Sickles EA, Lee JJ. Electronic thermography of normal facial structures: a pilot study. Oral Surg Oral Med Oral Pathol 1989; 68: 346–51. [DOI] [PubMed] [Google Scholar]
- 12.Gratt BM, Sickles EA. Thermographic characterization of the asymptomatic temporomandibular joint. J Orofac Pain 1993; 7: 7–14. [PubMed] [Google Scholar]
- 13.Gratt BM, Sickles EA. Electronic facial thermography: an analysis of asymptomatic adult subjects. J Orofac Pain 1995; 9: 255–65. [PubMed] [Google Scholar]
- 14.Keith LE, Thomas WD, Ferganson JL. Effects of activity, alcohol, smoking, and the menstrual cycle on liquid crystal breast thermography. Ohio J Sci 1973; 73: 55–8. [Google Scholar]
- 15.Schwartz RG. The American Academy of Thermology. Guidelines for neuromusculoskeletal thermography. Thermol Int 2006; 16: 5–9. [Google Scholar]
- 16.Haddad DS, Brioschi ML, Arita ES. Thermographic and clinical correlation of myofascial trigger points in the masticatory muscles. Dentomaxillofac Radiol 2012; 41: 621–9. doi: 10.1259/dmfr/98504520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vargas JVC, Brioschi ML, Dias FG, Parolin MB, Mulinari-Brenner FA, Ordonez JC, et al. Normalized methodology for medical infrared imaging. Infrared Phys Tech 2009; 52: 42–7. [Google Scholar]
- 18.de Leeuw R. Orofacial pain: guidelines for assessment, diagnosis and management. The American Academy of Orofacial Pain. 4th edn. Chicago, IL: Quintessence; 2008. [Google Scholar]
- 19.Christensen J, Vaeth M, Wenzel A. Thermographic imaging of facial skin—gender differences and temperature changes over time in healthy subjects. Dentomaxillofac Radiol 2012; 41: 662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawano W, Kawazoe T, Tanaka M, Hikida Y. Deep thermometry of temporomandibular joint and masticatory muscle regions. J Prosthet Dent 1993; 69: 216–21. [DOI] [PubMed] [Google Scholar]
- 21.Gratt BM, Sickles EA, Ross JB, Wexler CE, Gornbein JA. Thermographic assessment of craniomandibular disorders: diagnostic interpretation versus temperature measurement analysis. J Orofac Pain 1994; 8: 278–88. [PubMed] [Google Scholar]
- 22.Leonardi R, Zaborra G, Li Pera AM, Tripi TR. Comparison of telethermography and electromyography in instrumental symptomologic evaluation of the anterior temporalis and the masseter. [In Italian.] Stomatol Mediterr 1991; 11: 5–10. [PubMed] [Google Scholar]
- 23.Dibai Filho AV, Packer AC, Costa AC, Rodrigues-Bigaton D. Accuracy of infrared thermography of the masticatory muscles for the diagnosis of myogenous temporomandibular disorder. J Manipulative Physiol Ther 2013; 36: 245–52. doi: 10.1016/j.jmpt.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 24.Weinstein SA, Weinstein G, Weinstein EL, Gelb M. Facial thermography, basis, protocol, and clinical value. Cranio 1991; 9: 201–11. [DOI] [PubMed] [Google Scholar]
- 25.Pogrel MA, Erbez G, Taylor RC, Dodson TB. Liquid crystal thermography as a diagnostic aid and objective monitor for TMJ dysfunction and myogenic facial pain. J Craniomandib Disord 1989; 3: 65–70. [PubMed] [Google Scholar]
- 26.Biagioni PA, Longmore RB, McGimpsey JG, Lamey PJ. Infrared thermography. Its role in dental research with particular reference to craniomandibular disorders. Dentomaxillofac Radiol 1996; 25: 119–24. doi: 10.1259/dmfr.25.3.9084259 [DOI] [PubMed] [Google Scholar]
- 27.Berry DC, Yemm R. Variations in skin temperature of the face in normal subjects and in patients with mandibular dysfunction. Br J Oral Surg 1971; 8: 242–7. [DOI] [PubMed] [Google Scholar]
- 28.Okeson JP. Orofacial pain: guidelines for assessment, diagnosis, and management. Carol Stream, IL: Quintessence; 1996. [Google Scholar]
- 29.Berry DC, Yemm R. A further study of facial skin temperature in patients with mandibular dysfunction. J Oral Rehabil 1974; 1: 255–64. [DOI] [PubMed] [Google Scholar]
- 30.Fischer AA. Muscle spasm: documentation by thermography and by clinical methods. Thermology 1991; 3: 276. [Google Scholar]