Abstract
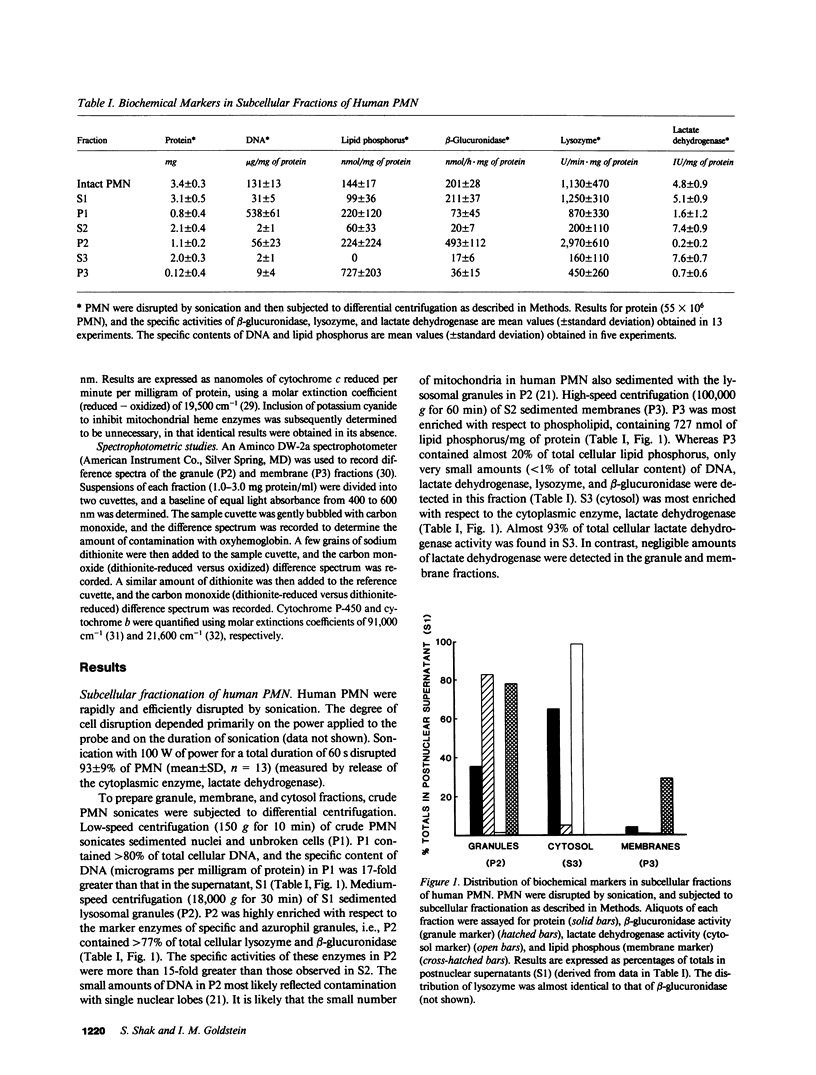
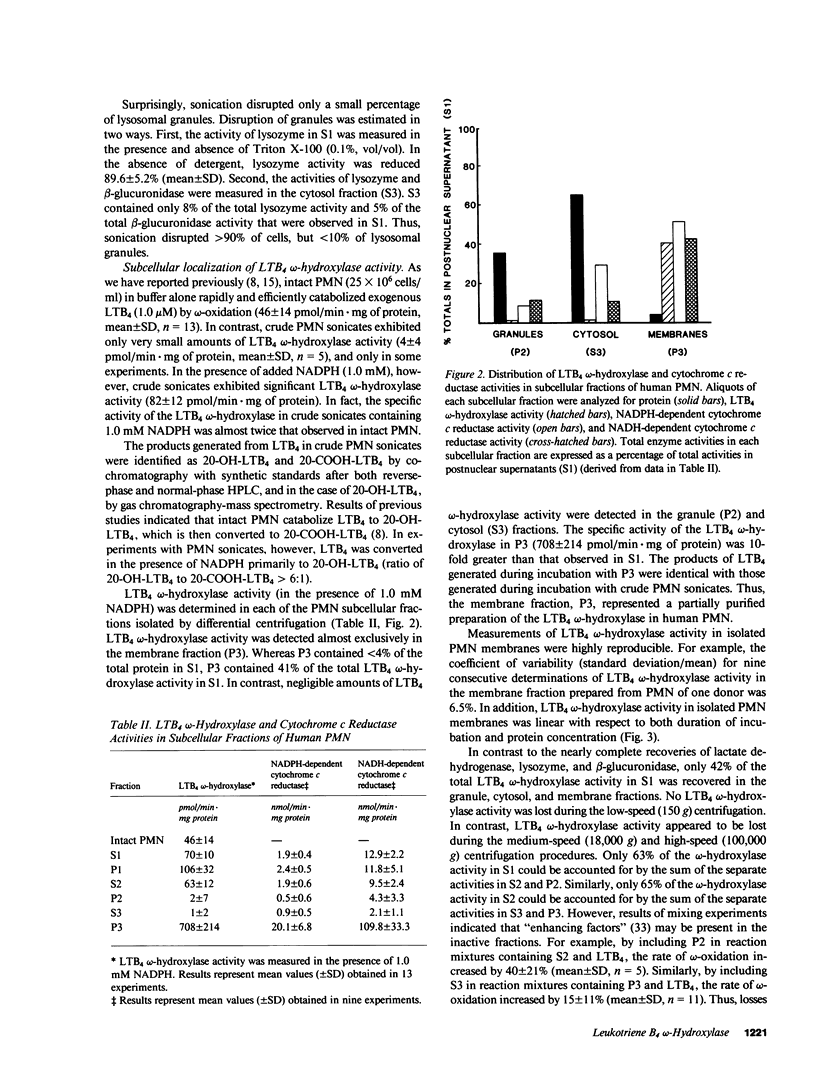
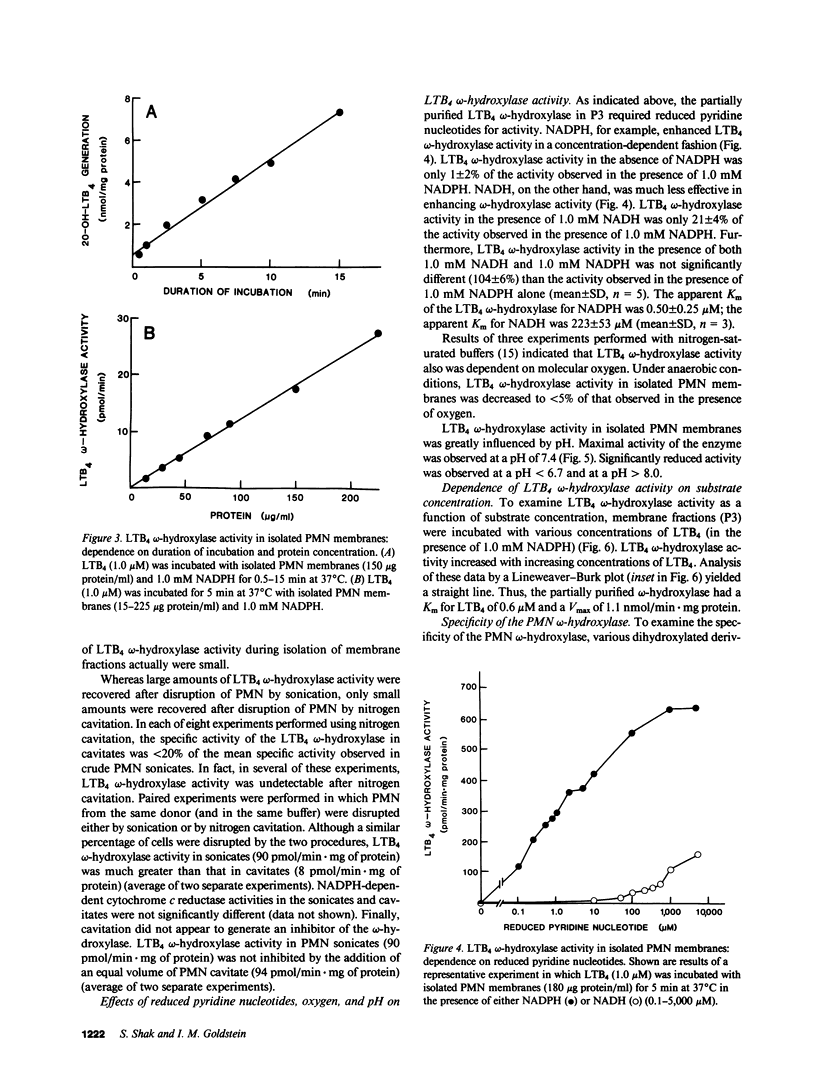
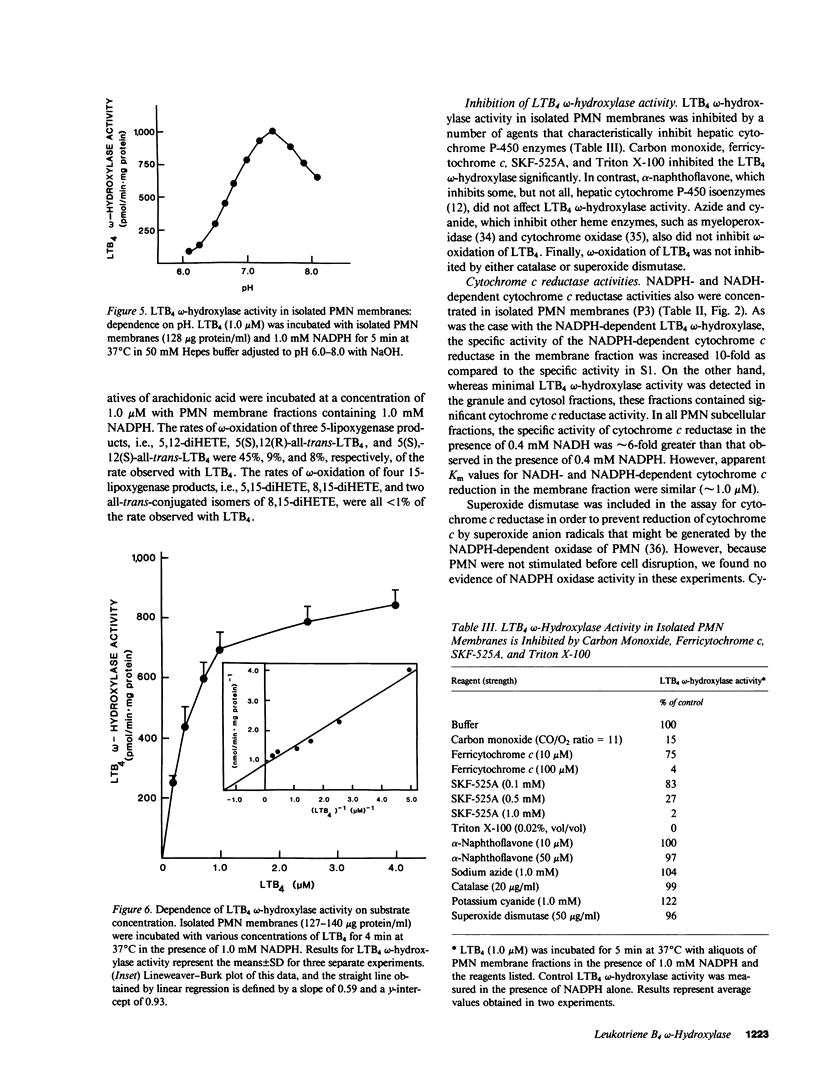
Human polymorphonuclear leukocytes (PMN) not only synthesize and respond to leukotriene B4 (LTB4), but also catabolize this mediator of inflammation rapidly and specifically by omega-oxidation. To characterize the enzyme(s) responsible for omega-oxidation of LTB4, human PMN were disrupted by sonication and subjected to differential centrifugation to yield membrane, granule, and cytosol fractions (identified by biochemical markers). LTB4 omega-hydroxylase activity was concentrated (together with NADPH cytochrome c reductase activity) only in the membrane fraction (specific activity increased 10-fold as compared to whole sonicates, 41% recovery). Negligible activity was detected in granule or cytosol fractions. LTB4 omega-hydroxylase activity in isolated PMN membranes was linear with respect to duration of incubation and protein concentration, was maximal at pH 7.4, had a Km for LTB4 of 0.6 microM, and was dependent on oxygen and on reduced pyridine nucleotides (apparent Km for NADPH = 0.5 microM; apparent Km for NADH = 223 microM). The LTB4 omega-hydroxylase was inhibited significantly by carbon monoxide, ferricytochrome c, SKF-525A, and Triton X-100, but was not affected by alpha-naphthoflavone, azide, cyanide, catalase, and superoxide dismutase. Finally, isolated PMN membranes exhibited a carbon monoxide difference spectrum with a peak at 452 nm. Thus, we have partially purified the LTB4 omega-hydroxylase in human PMN and identified the enzyme as a membrane-associated, NADPH-dependent cytochrome P-450.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrein P. C., Stossel T. P. Prevention of degradation of human polymorphonuclear leukocyte proteins by diisopropylfluorophosphate. Blood. 1980 Sep;56(3):442–447. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Tauber A. I., Karnovsky M. L. Properties of NADH-cytochrome-b5 reductase from human neutrophils. Blood. 1983 Jul;62(1):152–157. [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: effects of ionophore A23187. Proc Natl Acad Sci U S A. 1979 May;76(5):2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brittinger G., Hirschhorn R., Douglas S. D., Weissmann G. Studies on lysosomes. XI. Characterization of a hydrolase-rich fraction from human lymphocytes. J Cell Biol. 1968 May;37(2):394–411. doi: 10.1083/jcb.37.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bösterling B., Trudell J. R. Leukotriene B4 metabolism by hepatic cytochrome P-450. Biochem Biophys Res Commun. 1983 Jul 29;114(2):850–854. doi: 10.1016/0006-291x(83)90859-8. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Higson F. K., Jones O. T., Harper A. M., Segal A. W. The enzymic reduction and kinetics of oxidation of cytochrome b-245 of neutrophils. Biochem J. 1982 May 15;204(2):479–485. doi: 10.1042/bj2040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T., Harper A. M., Segal A. W. Oxidation-reduction properties of the cytochrome b found in the plasma-membrane fraction of human neutrophils. A possible oxidase in the respiratory burst. Biochem J. 1981 Feb 15;194(2):599–606. doi: 10.1042/bj1940599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Kipnes R. S., Babior B. M. Defect in pyridine nucleotide dependent superoxide production by a particulate fraction from the cranulocytes of patients with chronic granulomatous disease. N Engl J Med. 1975 Sep 25;293(13):628–632. doi: 10.1056/NEJM197509252931303. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., McPhail L. C., Mullikin D., McCall C. E. Reduced nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide phosphate diaphorase activity in human polymorphonuclear leukocytes. Infect Immun. 1974 Sep;10(3):528–534. doi: 10.1128/iai.10.3.528-534.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk H., Schenkman J. B., Bacchin P. G., Hutterer F., Schaffner F., Popper H. Mechanism of cholestasis. 3. Interaction of synthetic detergents with the microsomal cytochrome P-450 dependentbiotransformation system in vitro. A comparison between the effects of detergents, the effects of bile acids, and the findings in bile duct ligated rats. Exp Mol Pathol. 1971 Apr;14(2):263–276. doi: 10.1016/0014-4800(71)90070-0. [DOI] [PubMed] [Google Scholar]
- Estabrook R. W., Werringloer J. The measurement of difference spectra: application to the cytochromes of microsomes. Methods Enzymol. 1978;52:212–220. doi: 10.1016/s0076-6879(78)52024-7. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Rackham A., Zamboni R., Rokach J., Roy S. Comparative biological activities of synthetic leukotriene B4 and its omega-oxidation products. Prostaglandins. 1983 Jan;25(1):29–37. doi: 10.1016/0090-6980(83)90132-6. [DOI] [PubMed] [Google Scholar]
- Gabig T. G., Schervish E. W., Santinga J. T. Functional relationship of the cytochrome b to the superoxide-generating oxidase of human neutrophils. J Biol Chem. 1982 Apr 25;257(8):4114–4119. [PubMed] [Google Scholar]
- Goetzl E. J., Pickett W. C. The human PMN leukocyte chemotactic activity of complex hydroxy-eicosatetraenoic acids (HETEs). J Immunol. 1980 Oct;125(4):1789–1791. [PubMed] [Google Scholar]
- Goldman D. W., Goetzl E. J. Specific binding of leukotriene B4 to receptors on human polymorphonuclear leukocytes. J Immunol. 1982 Oct;129(4):1600–1604. [PubMed] [Google Scholar]
- Hansson G., Lindgren J. A., Dahlén S. E., Hedqvist P., Samuelsson B. Identification and biological activity of novel omega-oxidized metabolites of leukotriene B4 from human leukocytes. FEBS Lett. 1981 Jul 20;130(1):107–112. doi: 10.1016/0014-5793(81)80676-x. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A., Estabrook R. W. Evidence for the participation of cytochrome b 5 in hepatic microsomal mixed-function oxidation reactions. Arch Biochem Biophys. 1971 Mar;143(1):66–79. doi: 10.1016/0003-9861(71)90186-x. [DOI] [PubMed] [Google Scholar]
- Jubiz W., Rådmark O., Malmsten C., Hansson G., Lindgren J. A., Palmblad J., Udén A. M., Samuelsson B. A novel leukotriene produced by stimulation of leukocytes with formylmethionylleucylphenylalanine. J Biol Chem. 1982 Jun 10;257(11):6106–6110. [PubMed] [Google Scholar]
- Klass H. J., Hopkins J., Neale G., Peters T. J. The estimation of serum lysozyme: a comparison of four assay methods. Biochem Med. 1977 Aug;18(1):52–57. doi: 10.1016/0006-2944(77)90048-5. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Mikkelsen R. B., Corfman D. H., André-Schwartz J. Neutrophil plasma membranes. I. High-yield purification of human neutrophil plasma membrane vesicles by nitrogen cavitation and differential centrifugation. J Cell Biol. 1980 Jul;86(1):21–28. doi: 10.1083/jcb.86.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer D. Endogenous substrates of monooxygenases: fatty acids and prostaglandins. Pharmacol Ther. 1980;11(3):469–496. doi: 10.1016/0163-7258(80)90038-8. [DOI] [PubMed] [Google Scholar]
- Kupfer D., Miranda G. K., Navarro J., Piccolo D. E., Theoharides A. D. Effect of inducers and inhibitors of monooxygenase on the hydroxylation of prostaglandins in the guinea pig. Evidence for several monooxygenases catalyzing omega- and omega-1-hydroxylation. J Biol Chem. 1979 Oct 25;254(20):10405–10414. [PubMed] [Google Scholar]
- Kupfer D., Navarro J., Piccolo D. E. Hydroxylation of prostaglandins A1 and E1 by liver microsomal monooxygenase. Characteristics of the enzyme system in the guinea pig. J Biol Chem. 1978 Apr 25;253(8):2804–2811. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lee C. W., Lewis R. A., Tauber A. I., Mehrotra M., Corey E. J., Austen K. F. The myeloperoxidase-dependent metabolism of leukotrienes C4, D4, and E4 to 6-trans-leukotriene B4 diastereoisomers and the subclass-specific S-diastereoisomeric sulfoxides. J Biol Chem. 1983 Dec 25;258(24):15004–15010. [PubMed] [Google Scholar]
- Levin W., Lu A. Y., Jacobson M., Kuntzman R., Poyer J. L., McCay P. B. Lipid peroxidation and the degradation of cytochrome P-450 heme. Arch Biochem Biophys. 1973 Oct;158(2):842–852. doi: 10.1016/0003-9861(73)90580-8. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984 Apr;73(4):889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. Y., Junk K. W., Coon M. J. Resolution of the cytochrome P-450-containing omega-hydroxylation system of liver microsomes into three components. J Biol Chem. 1969 Jul 10;244(13):3714–3721. [PubMed] [Google Scholar]
- Lu A. Y., West S. B., Vore M., Ryan D., Levin W. Role of cytochrome b5 in hydroxylation by a reconstituted cytochrome P-450-containing system. J Biol Chem. 1974 Nov 10;249(21):6701–6709. [PubMed] [Google Scholar]
- Mason H. S., North J. C., Vanneste M. Microsomal mixed-function oxidations: the metabolism of xenobiotics. Fed Proc. 1965 Sep-Oct;24(5):1172–1180. [PubMed] [Google Scholar]
- Matsuura S., Fujii-Kuriyama Y., Tashiro Y. Immunoelectron microscope localization of cytochrome P-450 on microsomes and other membrane structures of rat hepatocytes. J Cell Biol. 1978 Aug;78(2):503–519. doi: 10.1083/jcb.78.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Morel F., Vignais P. V. Examination of the oxidase function of the b-type cytochrome in human polymorphonuclear leucocytes. Biochim Biophys Acta. 1984 Feb 27;764(2):213–225. doi: 10.1016/0005-2728(84)90030-6. [DOI] [PubMed] [Google Scholar]
- Navarro J., Piccolo D. E., Kupfer D. Hydroxylation of prostaglandin E1 by kidney cortex microsomal monooxygenase in the guinea pig. Arch Biochem Biophys. 1978 Nov;191(1):125–133. doi: 10.1016/0003-9861(78)90074-7. [DOI] [PubMed] [Google Scholar]
- Nichols B. A., Setzer P. Y., Bainton D. F. Glucose-6-phosphatase as a cytochemical marker of endoplasmic reticulum in human leukocytes and platelets. J Histochem Cytochem. 1984 Feb;32(2):165–171. doi: 10.1177/32.2.6319482. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Oritz de Montellano P. R., Correia M. A. Suicidal destruction of cytochrome P-450 during oxidative drug metabolism. Annu Rev Pharmacol Toxicol. 1983;23:481–503. doi: 10.1146/annurev.pa.23.040183.002405. [DOI] [PubMed] [Google Scholar]
- Potts D. E., Levin D. C., Sahn S. A. Pleural fluid pH in parapneumonic effusions. Chest. 1976 Sep;70(03):328–331. doi: 10.1378/chest.70.3.328. [DOI] [PubMed] [Google Scholar]
- Powell W. S. Properties of leukotriene B4 20-hydroxylase from polymorphonuclear leukocytes. J Biol Chem. 1984 Mar 10;259(5):3082–3089. [PubMed] [Google Scholar]
- Powell W. S., Solomon S. Formation of 20-hydroxyprostaglandins by lungs of pregnant rabbits. J Biol Chem. 1978 Jul 10;253(13):4609–4616. [PubMed] [Google Scholar]
- Shak S., Goldstein I. M. Carbon monoxide inhibits omega-oxidation of leukotriene B4 by human polymorphonuclear leukocytes: evidence that catabolism of leukotriene B4 is mediated by a cytochrome P-450 enzyme. Biochem Biophys Res Commun. 1984 Sep 17;123(2):475–481. doi: 10.1016/0006-291x(84)90255-9. [DOI] [PubMed] [Google Scholar]
- Shak S., Goldstein I. M. Omega-oxidation is the major pathway for the catabolism of leukotriene B4 in human polymorphonuclear leukocytes. J Biol Chem. 1984 Aug 25;259(16):10181–10187. [PubMed] [Google Scholar]
- Shak S., Perez H. D., Goldstein I. M. A novel dioxygenation product of arachidonic acid possesses potent chemotactic activity for human polymorphonuclear leukocytes. J Biol Chem. 1983 Dec 25;258(24):14948–14953. [PubMed] [Google Scholar]
- Sumimoto H., Takeshige K., Sakai H., Minakami S. A cell-free preparation of human neutrophils catalyzing NADPH-dependent conversion of leukotriene B4. Biochem Biophys Res Commun. 1984 Dec 14;125(2):615–621. doi: 10.1016/0006-291x(84)90583-7. [DOI] [PubMed] [Google Scholar]
- ULMER D. D., VALLEE B. L., WACKER W. E. Metalloenzymes and myocardial infarction. II. Malic and lactic dehydrogenase activities and zinc concentrations in serum. N Engl J Med. 1956 Sep 6;255(10):450–456. doi: 10.1056/NEJM195609062551001. [DOI] [PubMed] [Google Scholar]
- Vatsis K. P., Theoharides A. D., Kupfer D., Coon M. J. Hydroxylation of prostaglandins by inducible isozymes of rabbit liver microsomal cytochrome P-450. Participation of cytochrome b5. J Biol Chem. 1982 Oct 10;257(19):11221–11229. [PubMed] [Google Scholar]



