Abstract
We provide a map of the projections of taste neurons in the CNS of Drosophila. Using a collection of 67 GAL4 drivers representing the entire repertoire of Gr taste receptors, we systematically map the projections of neurons expressing these drivers in the thoracico-abdominal ganglion and the suboesophageal ganglion (SOG). We define 9 categories of projections in the thoracico-abdominal ganglia and 10 categories in the SOG. The projection patterns are modular, and can be interpreted as combinations of discrete pattern elements. The elements can be interpreted in terms of the taste organ from which the projections originate, the structures from which they originate, and the quality of taste information that they represent. The extensive diversity in projection patterns provides an anatomical basis for functional diversity in responses elicited by different taste stimuli.
Keywords: Drosophila, taste, taste receptors
1. Introduction
The use of Drosophila as a model system for the study of taste was pioneered largely by Obaid Siddiqi. In a seminal paper of 1978, Rodrigues and Siddiqi isolated a collection of taste mutants in addition to a set of olfactory mutants (Rodrigues and Siddiqi 1978). Their analysis of gustatory physiology and behaviour in the wild type and in these mutants laid a foundation for the next 35 years of research on Drosophila taste.
Taste allows the fly to evaluate potential food sources. The presence of nutritious sugars, which taste sweet, and toxins, which taste bitter, is detected by gustatory organs. Their input is integrated in the CNS and provides a basis for feeding decisions. Drosophila is a valuable system in which to investigate taste (Montell 2009), in part because it allows incisive analysis of basic principles of sensory function, but also because it provides a model for studying how feeding decisions are made by insects that devour massive quantities of the world’s food supply (van der Goes van Naters and Carlson 2006).
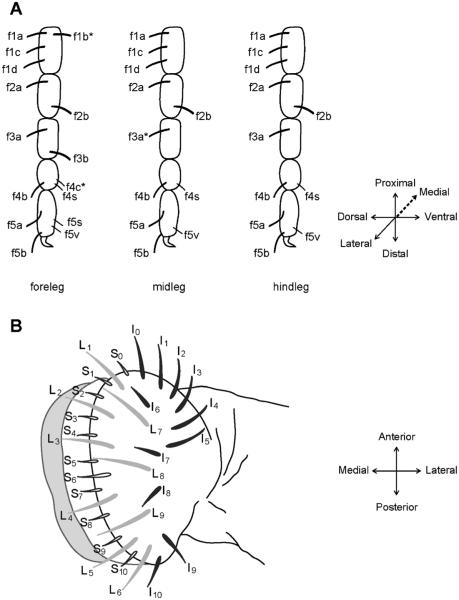
The first contact between a fly and a food source is made by the legs (figure 1A). The tarsal segments of the legs contain taste sensilla. The foreleg, midleg and hindleg contain ~28, 21, and 22 taste sensilla, respectively (Meunier et al. 2000; Ling et al. 2014). On the fly head is another taste organ called the labellum, which is covered with ~60 sensilla (figure 1B). The labellar sensilla divide into morphological classes: ‘L’ for large, ‘I’ for intermediate, and ‘S’ for small (Shanbhag et al. 2001; Hiroi et al. 2002; Weiss et al. 2011). The labellum also contains smaller sensory structures called taste pegs (Falk et al. 1976; Ray et al. 1993; Shanbhag et al. 2001). The sensilla of the legs and labellum are stereotyped in structure and position, and each contains a pore at the tip.
Figure 1.
Legs and labellum of Drosophila melanogaster. (A) Map of female tarsal sensilla. Each sensillum has a symmetric counterpart on the opposite side of the leg, except those marked by an asterisk. (From Ling et al. 2014.) (B) A typical Drosophila labellum, which consists of two palps, each with 31 sensilla identified by virtue of morphology and position. Large (L), intermediate (I), and small (S) sensilla are shaded differently for clarity. (From Weiss et al. 2011).
When taste sensilla make contact with a food source, molecules from the food source enter via the pore and activate neurons inside. Most sensilla contain four chemosensory neurons. Classical electrophysiological studies by Siddiqi and others revealed that one neuron is excited by sugars, one by bitter compounds and high concentrations of salt, another by low salt concentrations, and one by water or low osmolarity (Dethier 1976; Falk et al. 1976; Rodrigues and Siddiqi 1978; Nayak and Singh 1983; Fujishiro et al. 1984; Arora et al. 1987; Hiroi et al. 2004).
Physiological analysis of the gust mutants isolated by Rodrigues and Siddiqi showed intriguing, specific defects (Rodrigues and Siddiqi 1981), some of which predicted the expression of taste receptors with ligand specificity. A family of 60 taste receptor genes, the Gr genes, is in fact expressed in neurons of the legs and labellum (Clyne et al. 2000; Dunipace et al. 2001; Scott et al. 2001; Chyb et al. 2003; Thorne et al. 2004; Wang et al. 2004; Marella et al. 2006; Dahanukar et al. 2007; Weiss et al. 2011). This family encodes 68 Gr proteins via differential splicing (Robertson et al. 2003). Some of the Gr genes have been shown to encode receptors sensitive to certain sugars (Dahanukar et al. 2001; Dahanukar et al. 2007; Jiao et al. 2007; Jiao et al. 2008; Freeman et al. 2014), and others encode receptors required for response to certain bitter compounds (Moon et al. 2006; Lee et al. 2009; Moon et al. 2009; Lee et al. 2012).
The Gr genes are expressed in subsets of taste cells. Their expression has been examined using the GAL4-UAS system. Upstream regions of individual Gr genes are coupled to GAL4, which can be used to drive a UAS-GFP construct in cells that express the Gr gene of interest. This analysis has shown that 38 Gr-GAL4 drivers are expressed in labellar sensilla (Weiss et al. 2011), and 28 are expressed in legs (Ling et al. 2014). Different drivers are expressed in different subsets of taste sensilla. Some are expressed in sugar neurons and others in bitter neurons. Some bitter neurons of the labellum coexpress drivers of as many as 29 Gr genes (Weiss et al. 2011).
The physiological defects of gust mutants are associated with behavioral defects (Rodrigues and Siddiqi 1981), which raises one of the most critical problems in contemporary neuroscience: how sensory input is converted into behavioral output. To understand this process it is necessary to know where in the CNS the sensory information is sent. Previous work has shown that taste neurons of the legs project to the neuromeres of the thoracico-abdominal ganglia, with one neuromere corresponding to each of the six legs (Stocker 1994). Some afferents extend via the neck connective to the suboesophageal ganglion (SOG), a taste center that receives input from labellar taste neurons and from the pharynx (Stocker 1994). Gustatory projections from the pharynx, labellum and legs target different areas of the SOG (Thorne et al. 2004; Wang et al. 2004). Analysis with a limited subset of Gr-GAL4 drivers has shown that within the SOG, two patterns of projections are distinguishable: one formed by sugar neurons and one formed by bitter neurons (Marella et al. 2006). However, the vast majority of Gr-GAL4 drivers have not been examined to determine what projection patterns they produce in the CNS.
Here we carry out a systematic analysis of all 68 members of the Gr family to map the projections of taste neurons in the CNS. Using the entire collection of Gr-GAL4 drivers, we define 9 different categories of projections in the thoracico-abdominal ganglia, and 10 categories of projections in the SOG. Analysis of these projection patterns provides new insight into the functional organization of the taste system and provides a foundation for understanding how complex taste information is processed in the CNS.
2. Materials and methods
We used UAS-mCD8-GFP to visualize the general morphology of GAL4-expressing cells. GFP signals were enhanced by immunohistochemistry. Adult brains and thoracico-abdominal ganglia were dissected and immunostained as previously described (Python and Stocker 2002), using an anti-GFP antibody (rabbit polyclonal, Molecular Probes, 1:1000 dilution). To visualize regions with synapses, we used nc82 mouse monoclonal antibody (1:100; a gift of Dr. AloisHofbauer, University of Regensburg). nc82 recognizes the ELKS/CAST/ERC family protein Bruchpilot, which localizes to presynaptic active zones (Laissue et al. 1999; Wagh et al. 2006). As secondary antibodies we used goat anti-mouse and goat anti-rabbit IgG conjugated to either Alexa 568 or Alexa 488 (1:1000; Molecular Probes). All tissues were visualized with a Zeiss LSM 510 or a Biorad 1024 laser-scanning confocal microscope.
3. Results
3.1 A map of taste neuron projections in the thoracico-abdominal ganglia
We examined the projections in the thoracico-abdominal ganglia of all 68 individual members of the Gr family. We analysed 67 Gr-GAL4 drivers; one, Gr23a-GAL4, represents both Gr23a.a and Gr23a.b, which are translated from alternative splice forms of the same gene that contain the same 5′ region (Clyne et al. 2000). For each receptor, two or more independent Gr-GAL4 lines were examined. The Gr-GAL4 constructs were used to drive expression of UAS-GFP, which was then visualized by immunohistochemistry. Both males and females were examined, and no gross differences in labelling were observed between the sexes.
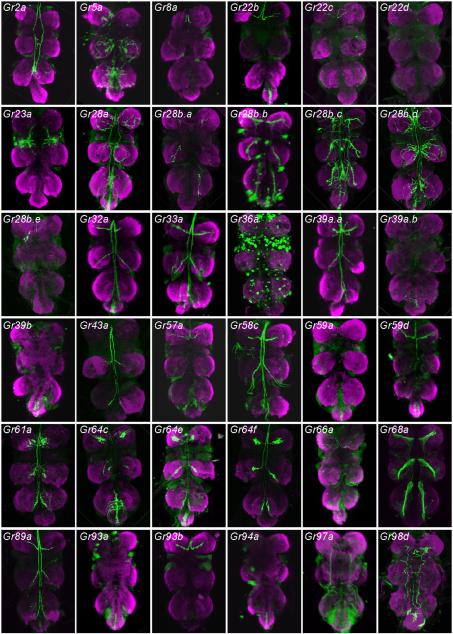
We found that 36 drivers labelled the thoracico-abdominal ganglion (figure 2). These drivers included all of the 28 Gr-GAL4 constructs that had been expected to show expression in these ganglia because they labelled neurons in the tarsi (Ling et al. 2014). Of these 28 drivers, 26 produced patterns that could be classified into several discrete categories (figure 3).
Figure 2.
Axonal projections of neurons labelled by the indicated Gr-GAL4 drivers in thoracico-abdominal ganglia. mCD8-GFP was used as a reporter gene, and GFP was detected with an anti-GFP antibody. Anterior is up.
Figure 3.
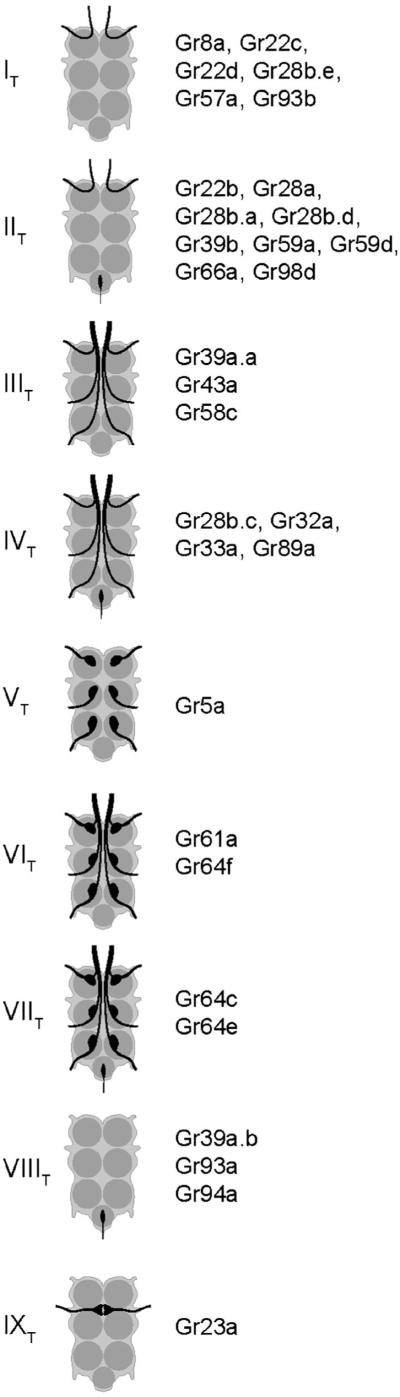
Categories of projection patterns in the thoracico-abdominal ganglia.
Category IT, a designation in which the subscript ‘T’ refers to the thoracico-abdominal ganglia, includes six drivers. Of these drivers, five (Gr8a-GAL4, Gr22c-GAL4, Gr22d-GAL4, Gr28b.e-GAL4 and Gr93b-GAL4), show expression in a single neuron of the legs, the bitter neuron of the 5s sensillum in the foreleg (Ling et al. 2014). The specificity of the CNS labelling agrees well with the specificity of the tarsal labelling: the only neuromeres that label are those corresponding to the forelegs. Category IT includes one additional driver, Gr57a-GAL4, which did not show labelling in the legs. We note that labelling in the legs was examined using GFP fluorescence, whereas labelling in the CNS was observed with anti-GFP immunohistochemistry, which is more sensitive. The simplest interpretation of these results, then, is that Gr57a-GAL4 is also expressed in the bitter neuron of the foreleg 5s sensillum, but at lower levels than the other drivers of this category.
Category IIT includes nine drivers, all of which label the same neuromere as Category IT, and no other leg neuromeres, but which also show labelling of the abdominal ganglion. Consistent with these results, all nine of these drivers label the bitter neuron of the 5s sensillum of the foreleg, and no other leg neurons (Ling et al. 2014). The abdominal ganglion receives projections from sensory neurons in abdominal tissues such as the genitalia and intestine, and likely reflect additional expression of Category IIT genes in such tissues. We note that some of these drivers, such as those of the Gr28b subfamily, in fact show some additional labelling (Thorne and Amrein 2008). Such expression could represent a function for these genes in other cells; alternatively, the expression pattern of some drivers may not represent the expression of the endogenous genes with complete fidelity.
Three drivers, Gr39a.a-GAL4, Gr43a-GAL4, and Gr58c-GAL4, show labelling of all six thoracic neuromeres, which agrees with the observation that these drivers labelled sensilla of all three pairs of legs (Ling et al. 2014). These drivers, designated as Category IIIT, differ in the number of tarsal sensilla that they label. Gr43a-GAL4 labels only one sensillum per leg, while the others labelled between two and four. These differences may in part account for differences in the intensity of CNS labelling among drivers of this category.
The four drivers of Category IVT also show labelling of all six thoracic neuromeres, but in addition show labelling of the abdominal ganglion. Gr28b.c-GAL4 provides another example of additional labelling associated with genes of the Gr28b subfamily.
Gr5a-GAL4, which labels neurons that respond to sugars, is the sole member of Category VT. It labels all six thoracic neuromeres, consistent with its labelling of sensilla in all legs. However, the projections are more elaborate than in categories I–IV, with numerous endings over a part of each neuromere. This driver is also distinct in that the labelled neurons do not send projections in the anterior direction from the thoracico-abdominal ganglia. These results agree with those of Dahanukar et al.(2007).
Category VIT contains two other markers of sugar-sensitive neurons, Gr61a-GAL4 and Gr64f-GAL4. These two drivers labelled precisely the same subset of sensilla in the legs. Unlike the case of Gr5a-GAL4, these labelled neurons send projections in the anterior direction from the ganglia. We note that these drivers labelled all the tarsal sensilla labelled by Gr5a-GAL4 neurons and in addition several additional sensilla, which may explain the additional projections.
Two additional markers of sugar-sensitive neurons, Gr64c-GAL4 and Gr64e-GAL4, fall into Category VIIT. They produce patterns similar to those Category VI except that the abdominal ganglion is also labelled.
Three other drivers label the thoracico-abdominal ganglion despite not having labelled tarsal sensilla (Ling et al. 2014). Three, Gr39a.b-GAL4, Gr93a-GAL4, and Gr94a-GAL4 (Category VIIIT) show labelling only in the abdominal ganglion. The absence of labelling in the thoracic neuromeres is consistent with the absence of labelling in tarsal sensilla.
Another driver, Gr23a-GAL4, (Category IXT) labels afferents that have been described as originating in the wing (Stocker 1994). Although we did not observe any GFP fluorescence in the taste sensilla of the anterior wing margin, it is possible that expression levels are below our detection threshold; we have not examined the wings using immunohistochemistry.
Finally, we note five drivers that label the thoracico-abdominal ganglia but that we have not categorized. Gr36a-GAL4 labelled one sensillum, the 5v sensillum, in each leg, but its labelling pattern in the thoracico-abdominal ganglia was punctate and distinct from all others. Gr68a-GAL4, which labelled both neuronal and non-neuronal cells in the legs (Bray and Amrein 2003; Ejima and Griffith 2008), also produced a distinct labelling pattern in the thoracico-abdominal ganglia. Gr2a-GAL4, Gr28b.b and Gr97a-GAL4 did not label tarsal sensilla but produced thoracico-abdominal labelling patterns, each distinct from each other and from all other patterns. Some or all of these drivers may be expressed in peripheral sensory structures that we have not examined, possibly serving other modalities; they raise intriguing possibilities for future investigation.
3.2 A map of taste neuron projections in the SOG
We examined the projections in the SOG labelled by all 67 Gr-GAL4 drivers. Two or more independent Gr-GAL4 lines were examined for each receptor. As in the analysis of the thoracico-abdominal ganglia, projections were visualized using an anti-GFP antibody. Both males and females were examined, and no gross differences in labelling were observed.
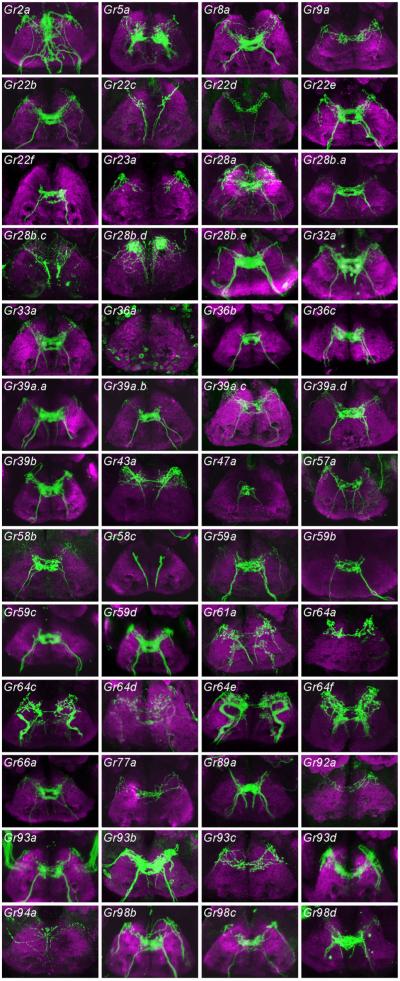
We found that 52 Gr-GAL4 drivers labelled axon termini of the SOG (figure 4).
Figure 4.
Axonal projections of neurons labelled by the indicated Gr-GAL4 drivers in the SOG. mCD8-GFP was used as a reporter gene, and GFP was detected with an anti-GFP antibody. View is from the anterior direction; dorsal is up.
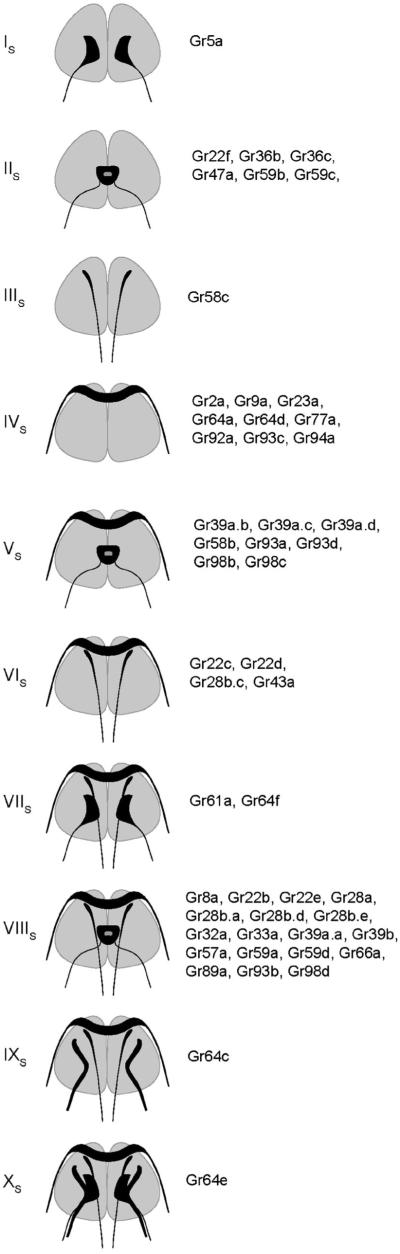
Most drivers fall into one of 10 categories according to the patterns of projections they produce (figure 5).
Figure 5.
Categories of projection patterns in the SOG.
Category IS consists of a sole member, Gr5a-GAL4, which sends bilaterally symmetric projections to the SOG. The pattern is relatively simple and has been observed in earlier studies (Thorne et al. 2004; Wang et al. 2004; Marella et al. 2006; Dahanukar et al. 2007); it is characteristic of labellar neurons that express sugar receptors.
Category IIS contains six members (Gr22f-GAL4 et al.), all of which labelled bitter neurons of the labellum, but none of which labelled neurons of the leg. This pattern has been observed previously and illustrates a brain region receiving bitter input from the labellum (Marella et al. 2006).
A single driver, Gr58c-GAL4, produces the labelling pattern designated IIIS. This driver is expressed in bitter neurons of all legs, and is one of very few drivers expressed in legs but not labellum.
Pattern IVS is produced by nine drivers. This pattern is interpreted as representing input from the pharynx (Wang et al. 2004) (data not shown).
Other patterns can be interpreted in terms of combinations of these basic pattern elements. Pattern VS appears to be a summation of patterns IIS and IVS, and can be interpreted as representing axon terminals from bitter neurons of both the labellum and the pharynx. All drivers of this category labelled bitter neurons of the labellum in a previous study, with the exception of Gr39a.c-GAL4 and Gr93d-GAL4, which could have been expressed in the labellum at a level beneath the threshold for detection of GFP fluorescence (Weiss et al. 2011).
Likewise, Pattern VIS appears to be a synthetic pattern, composed of axon terminals from the legs (Pattern IIIS) and pharynx (Pattern IVS). Consistent with this interpretation, all four drivers that produce this pattern label neurons of tarsal sensilla (Ling et al. 2014). Three of these drivers (Gr22c-GAL4, Gr22d-GAL4, Gr28b.c-GAL4) label bitter neurons, and one (Gr43a-GAL4) labels a sugar neuron in the legs.
Pattern VIIS is also synthetic, but can be interpreted as a summation of three pattern elements, representing input from the legs (Pattern IIIS), sugar neurons of the labellum (Pattern IS), and the pharynx (Pattern IVS). Consistent with this interpretation, both drivers producing this pattern (Gr61a-GAL4 and Gr64f-GAL4) label sugar neurons of the legs and labellum. These results confirm earlier studies (Dahanukar et al. 2007; Slone et al. 2007; Jiao et al. 2008).
The pattern produced by the greatest number of drivers is VIIIS: 18 drivers. This pattern is similar to VIIS in that it includes projections from the legs (pattern IIIS) and pharynx (IVS), but rather than including projections from sugar neurons of the labellum it includes projections from bitter neurons of the labellum. All of these drivers have in fact been observed to label bitter neurons of the labellum and legs, except that Gr22e-GAL4 and Gr57a-GAL4 labelling was not observed in the legs (Ling et al. 2014).
Pattern IXS was produced by a single driver, Gr64c-GAL4. This pattern contains three elements: projections from the legs, from the pharynx, and from taste pegs of the labellum. Pattern XS also is produced by only one driver, Gr64e-GAL4, and can be interpreted as an ensemble of four elements, with input coming from the legs, pharynx, taste pegs and taste sensilla of the labellum.
4. Discussion
We have carried out a comprehensive analysis of the central projection patterns of neurons that express drivers of the Gr receptors. We have identified nine categories of projection patterns in the thoracico-abdominal ganglia and 10 categories in the SOG. The numbers of drivers in each category range broadly, from one to nine in the thoracico-abdominal ganglia and from one to 18 in the SOG.
The projection patterns are modular. The modularity is especially apparent in the SOG. The SOG categories can be interpreted in terms of combinations of five pattern elements. Each pattern element can in turn be interpreted in terms of: (i) the taste organ from which it derives (the labellum, legs or pharynx); (ii) the structures on a particular taste organ from which it derives (labellar sensilla or labellar pegs); (iii) the quality of taste information that it represents (sweet or bitter). It seems entirely plausible that anatomical analysis at higher resolution might distinguish a greater number of pattern elements (Ito et al. 2013). For example, patterns of pharyngeal input that appear similar in the present analysis might at higher resolution be distinguished as different pattern elements, perhaps representing taste information of different quality.
There are similarities in the categorization of projections in the two regions of the CNS. All nine drivers of the thoracico-abdominal category IIT fall into the same SOG category, VIIIS. Gr61a-GAL4 and Gr64f-GAL4 are the sole representatives of VIT in the thoracico-abdominal ganglia and of VIIS in the SOG. Gr5a-GAL4 is sui generis in both the thoracico-abdominal ganglia and the SOG.
However, the mapping of drivers in the thoracico-abdominal ganglia is distinct from that in the SOG. For example, six drivers fall into Category IT (Gr8a-GAL4 et al.). These six drivers, however, fall into two categories in the SOG: VIIIS, which includes bitter input from labellar sensilla, and VIS, which does not. Likewise, the three drivers of IIIT (Gr39a.a-GAL4, Gr43a-GAL4, and Gr58c-GAL4) fall into three different SOG categories, IIIS, VIS and VIIIS. Reciprocally, the four drivers that fall into Category VIS fall into three different categories in the thoracico-abdominal ganglion: IT, IIIT and IVT. Taken together, these results suggest that two tastants that elicit activity from the same region of one CNS region may elicit different patterns of input in another.
The diversity of projection patterns documented in this study illustrates that there is an anatomical basis for diversity in behavioural responses to taste stimuli. An outstanding question in the field is the extent to which the fly exploits the opportunity to exhibit different behavioral responses to taste stimuli of different toxicity, nutritive value, or social significance. Siddiqi and colleagues employed a variety of ingenious paradigms for assessing taste behaviour (Rodrigues and Siddiqi 1978, 1981;Arora et al. 1987;Siddiqi et al. 1989; Rodrigues et al. 1991), and their work provides an elegant example for others to follow in investigating the complexity of taste behaviour.
In a more general sense, much remains to be learned about the mechanisms by which taste stimuli drive behavioural responses in Drosophila. The foundations of the problem were laid largely by Siddiqi and colleagues in their classic studies. We have recently built upon this foundation by systematically mapping taste stimuli to neurons of the labellum and legs, and by mapping taste receptors to these neurons. Here we have systematically mapped the projections of these neurons in the CNS. These maps should help guide future exploration of how taste input elicits behavioural output.
Acknowledgements
We thank Zev Wisotsky for determining the pattern of Gr64d-GAL4 labelling in the SOG. This work was supported by NIH grants to JC.
References
- Arora K, Rodrigues V, Joshi S, Shanbhag S, Siddiqi O. A gene affecting the specificity of the chemosensory neurons of Drosophila. Nature. 1987;330:62–63. doi: 10.1038/330062a0. [DOI] [PubMed] [Google Scholar]
- Bray S, Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–1029. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- Chyb S, Dahanukar A, Wickens A, Carlson JR. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc. Natl. Acad. Sci. USA. 2003;100:14526–14530. doi: 10.1073/pnas.2135339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat. Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethier VG. The Hungry Fly. Harvard University Press; Cambridge: 1976. [Google Scholar]
- Dunipace L, Meister S, McNealy C, Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr. Biol. 2001;11:822–835. doi: 10.1016/s0960-9822(01)00258-5. [DOI] [PubMed] [Google Scholar]
- Ejima A, Griffith LC. Courtship initiation is stimulated by acoustic signals in Drosophila melanogaster. PLoS ONE. 2008;3:e3246. doi: 10.1371/journal.pone.0003246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk R, Bleiser-Avivi N, Atidia J. Labellar taste organs of Drosophila melanogaster. J. Morphol. 1976;150:327–342. doi: 10.1002/jmor.1051500206. [DOI] [PubMed] [Google Scholar]
- Freeman EG, Wisotsky Z, Dahanukar A. Detection of sweet tastants by a conserved group of insect gustatory receptors. Proc. Natl. Acad. Sci. USA. 2014;111:1598–1603. doi: 10.1073/pnas.1311724111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishiro N, Kijima H, Morita H. Impulse frequency and action potential amplitude in labellar chemosensory neurons of Drosophila melanogaster. J. Insect. Physiol. 1984;30:317–325. [Google Scholar]
- Hiroi M, Marion-Poll F, Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog. Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- Hiroi M, Meunier N, Marion-Poll F, Tanimura T. Two antagonistic gustatory receptor neurons responding to sweet-salty and bitter taste in Drosophila. J. Neurobiol. 2004;61:333–342. doi: 10.1002/neu.20063. [DOI] [PubMed] [Google Scholar]
- Ito M, Masuda N, Shinomiya K, Endo K, Ito K. Systematic analysis of neural projections reveals clonal composition of the Drosophila brain. Curr. Biol. 2013;23:644–655. doi: 10.1016/j.cub.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc. Natl. Acad. Sci. USA. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Wang X, Ren Q, Montell C. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr. Biol. 2008;18:1797–1801. doi: 10.1016/j.cub.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laissue PP, Reiter C, Hiesinger PR, Halter S, Fischbach KF, Stocker RF. Three-dimensional reconstruction of the antennal lobe in Drosophila melanogaster. J. Comp. Neurol. 1999;405:543–552. [PubMed] [Google Scholar]
- Lee Y, Kang MJ, Shim J, Cheong CU, Moon SJ, Montell C. Gustatory receptors required for avoiding the insecticide L-canavanine. J. Neurosci. 2012;32:1429–1435. doi: 10.1523/JNEUROSCI.4630-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc. Natl. Acad. Sci. USA. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling F, Dahanukar A, Weiss LA, Kwon JY, Carlson JR. The molecular and cellular basis of taste coding in the legs of Drosophila. J. Neurosci. 2014;34:7148–7164. doi: 10.1523/JNEUROSCI.0649-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Meunier N, Ferveur JF, Marion-Poll F. Sex-specific non-pheromonal taste receptors in Drosophila. Curr. Biol. 2000;10:1583–1586. doi: 10.1016/s0960-9822(00)00860-5. [DOI] [PubMed] [Google Scholar]
- Montell C. A taste of the Drosophila gustatory receptors. Curr. Opin. Neurobiol. 2009;19:345–353. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SJ, Kottgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr. Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr. Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak SV, Singh RN. Sensilla on the tarsal segments and mouthparts of adult Drosophila melanogaster meigen (Diptera: Drosophilidae) Int. J. Insect Morphol. and Embryol. 1983;12:273–291. [Google Scholar]
- Python F, Stocker R. Adult-like complexity of the larval antennal lobe of D. melanogaster despite markedly low numbers of odorant receptor neurons. J. Comp. Neurol. 2002;445:374–387. doi: 10.1002/cne.10188. [DOI] [PubMed] [Google Scholar]
- Ray K, Hartenstein V, Rodrigues V. Development of the taste bristles on the labellum of Drosophila melanogaster. Dev. Biol. 1993;155:26–37. doi: 10.1006/dbio.1993.1003. [DOI] [PubMed] [Google Scholar]
- Robertson HM, Warr CG, Carlson JR. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2003;100:14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues V, Sathe S, Pinto L, Balakrishnan R, Siddiqi O. Closely linked lesions in a region of the X chromosome affect central and peripheral steps in gustatory processing in Drosophila. Mol. Gen. Genet. 1991;226:265–276. doi: 10.1007/BF00273612. [DOI] [PubMed] [Google Scholar]
- Rodrigues V, Siddiqi O. Genetic-analysis of chemosensory pathway. Proc. Indian Acad. Sci. [B] 1978;87:147–160. [Google Scholar]
- Rodrigues V, Siddiqi O. A gustatory mutant of Drosophila defective in pyranose receptors. Mol. Gen. Genet. 1981;181:406–408. doi: 10.1007/BF00425621. [DOI] [PubMed] [Google Scholar]
- Scott K, Brady R, Jr., Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Shanbhag SR, Park SK, Pikielny CW, Steinbrecht RA. Gustatory organs of Drosophila melanogaster: fine structure and expression of the putative odorant-binding protein PBPRP2. Cell Tissue Res. 2001;304:423–437. doi: 10.1007/s004410100388. [DOI] [PubMed] [Google Scholar]
- Siddiqi O, Joshi S, Arora K, Rodrigues V. Genetic Investigation of Salt Reception in Drosophila melanogaster. Genome. 1989;31:646–651. [Google Scholar]
- Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr. Biol. 2007;17:1809–1816. doi: 10.1016/j.cub.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Thorne N, Amrein H. Atypical expression of Drosophila gustatory receptor genes in sensory and central neurons. J. Comp. Neurol. 2008;506:548–568. doi: 10.1002/cne.21547. [DOI] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr. Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- van der Goes van Naters W, Carlson JR. Insects as chemosensors of humans and crops. Nature. 2006;444:302–307. doi: 10.1038/nature05403. [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]