Despite remarkable advances in pharmaceutical drug design, effective chemotherapy options for brain and nervous system cancers that demonstrate selective cytotoxicity remain a challenge in medicinal chemistry. A recent National Cancer Institute report stated, “over the last 30–40 years, the mortality rate for brain and other CNS cancers has remained largely unchanged.”1 Current chemotherapeutic options are limited, and typically only provide 6–24 month survival rates.2 Recently, curcumin has become a pharmaceutical target of interest based on its diverse range of biological activity.1 A bioactive metabolite of the rhizome of Curcuma longa and the major component of the traditional Indian folk medicine turmeric, this diarylheptanoid exhibits anti-cancer, anti-inflammatory, anti-oxidant, and chemopreventative properties.3 This naturally occurring metabolite demonstrated apoptotic induction and cytotoxicity toward a variety of cancer cell lines, while exhibiting a remarkable lack of adverse side effects and no systemic toxicity.4 Recent work has shown that curcumin possesses impressive growth inhibition and induction of apoptosis of glioblastoma and neuroblastoma cells in vitro, and decreased tumorigenesis in vivo.5 Unfortunately, its affinity for a wide range of targets, low potency, and unsatisfactory pharmacokinetics limits the clinical viability of this biologically versatile natural product.6
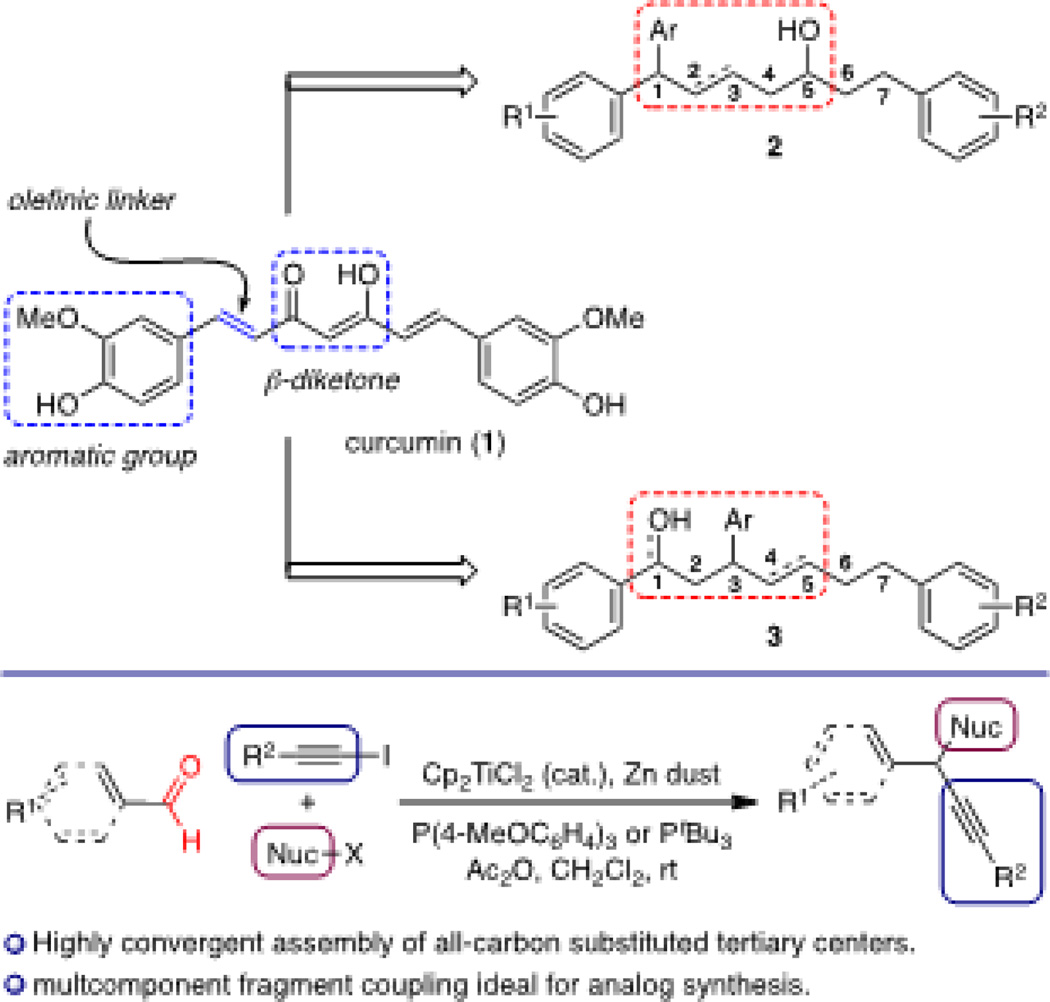
As a result, a number of groups have focused on the design of curcumin structural analogs to optimize specific chemotherapeutic properties. Analog evaluation has primarily fixated on the aryl and β-diketone regions, which are considered crucial for activity.6 In contrast, the olefins at C1 and C6 are commonly believed to serve as linkers for the aromatic and ketone regions, and therefore, significant structural modification of this region has not been studied in detail. We chose to target analogs possessing an aryl-substituted tertiary center at C1 or C3 and evaluate them for brain and nervous system anticancer activity to glean insight into the biological activity of curcumin (Figure 1). Our recently developed titanocene-catalyzed multicomponent coupling reactions7 enables rapid access to C1 and C3 aryl-substituted analogs 2 and 3 of curcumin, thereby allowing us to strategically design, synthesize, and evaluate a diverse array of curcumin-derived arylheptanoid analogs.8
Figure 1.
Design and synthesis of curcumin analogs.
We envisioned that our titanocene-catalyzed multicomponent coupling9 would provide a direct route to C1-arylated curcumin analogs through the union of an electron rich arene, benzaldehyde derivative, and an iodoalkyne comprised of the C1–C7 carbon chain oxidized at C5. In an effort to maintain the enol–OH functionality present in curcumin, we targeted secondary alcohol 9 as a mixture of C1 epimers (Scheme 1).
Enantioenriched iodoalkyne 5 was obtained in three steps from known aldehyde 410 using a titanium-catalyzed asymmetric propargylation.11 Treatment of 5, arene 6, and aldehyde 7 with Cp2TiCl2 (5 mol%), PtBu3, Zn, Cs2CO3, and Ac2O provided diarylethynyl methane 8 in 99% yield.12 Treatment with TBAF liberated the secondary hydroxyl group at C5 to give the target alcohol 9 in 83% yield. With alcohol 9 in hand, we sought to reduce the alkyne functionality to the corresponding trans olefin in an effort to provide a comparison to the curcumin carbon backbone. Thus, treatment of 9 with Me2SiHCl followed by a ruthenium-catalyzed alkyne hydrosilylation gave the trans-alkene 10.13 This highly convergent strategy toward constructing the triarylated curcumin C1–C7 carbon framework ultimately permits modular flexibility required for a complete SAR study based on the general structure of 2.
Our synthesis of the C3-arylated curcumin analogs began with construction of the C4–C7 fragment for the titanocene-catalyzed multicomponent coupling (Scheme 2). Esterification of commercially available acid 11 followed by silylation of the phenol and treatment with DIBAL-H gave aldehyde 12 in 64% yield over three steps.14 A Corey-Fuchs alkynylation15 in which the lithium acetylide was quenched with I2 yielded iodoalkyne 13 in 69% yield.16 This establishes a simple and reliable route toward this curcuminoid fragment.
With our C4–C7 fragment in hand, we turned our attention toward the crucial titanocene-catalyzed multicomponent coupling. Thus, treatment of iodoalkyne 13, aldehyde 14, and silyl enol ether 1518 with Cp2TiCl2 (2 mol %), (4-MeOC6H4)3P, Zn(0), and Ac2O gave a mixture of β-aryl ketones 16a and 16b in which the TMS-phenol protodesilylation proved remarkably facile (Scheme 3). Silylation of the resulting free phenol in 16a gave ketone 16c in 91% yield. Diastereoselective ketone reduction using Corey’s CBS reagent provided alcohol 17 in 77% yield as a 1:1 mixture of diastereomers in 92% ee each.19
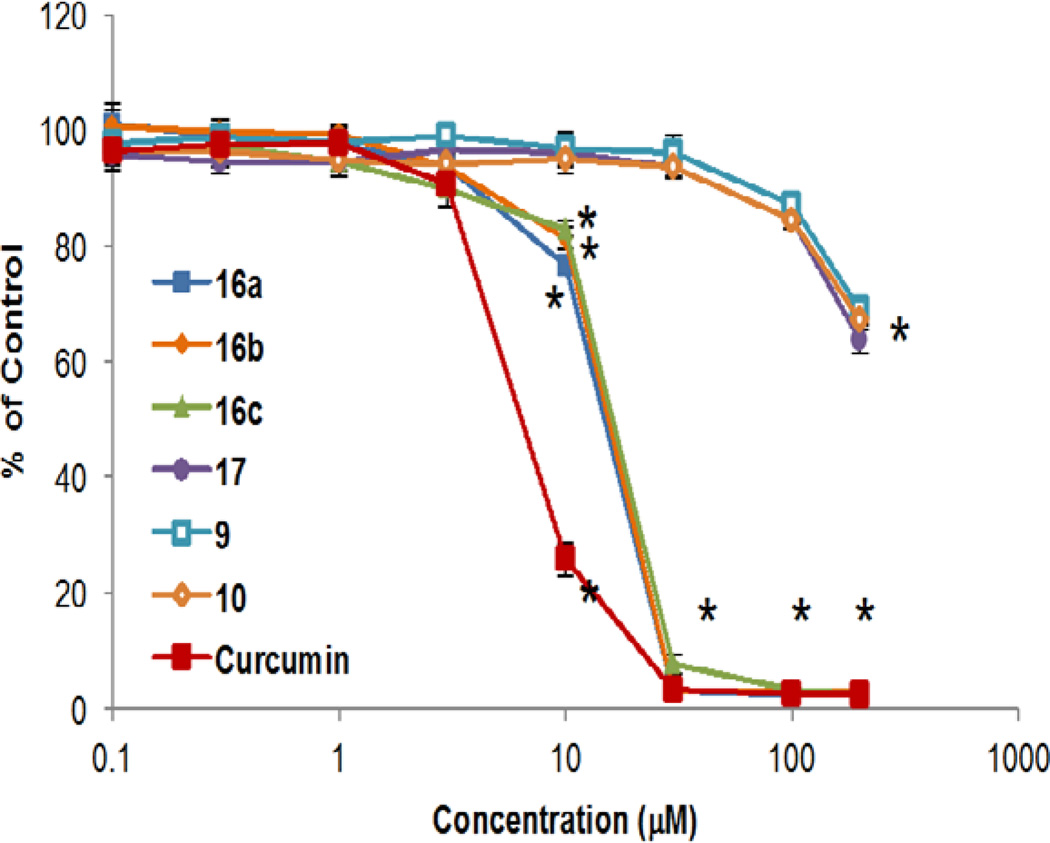
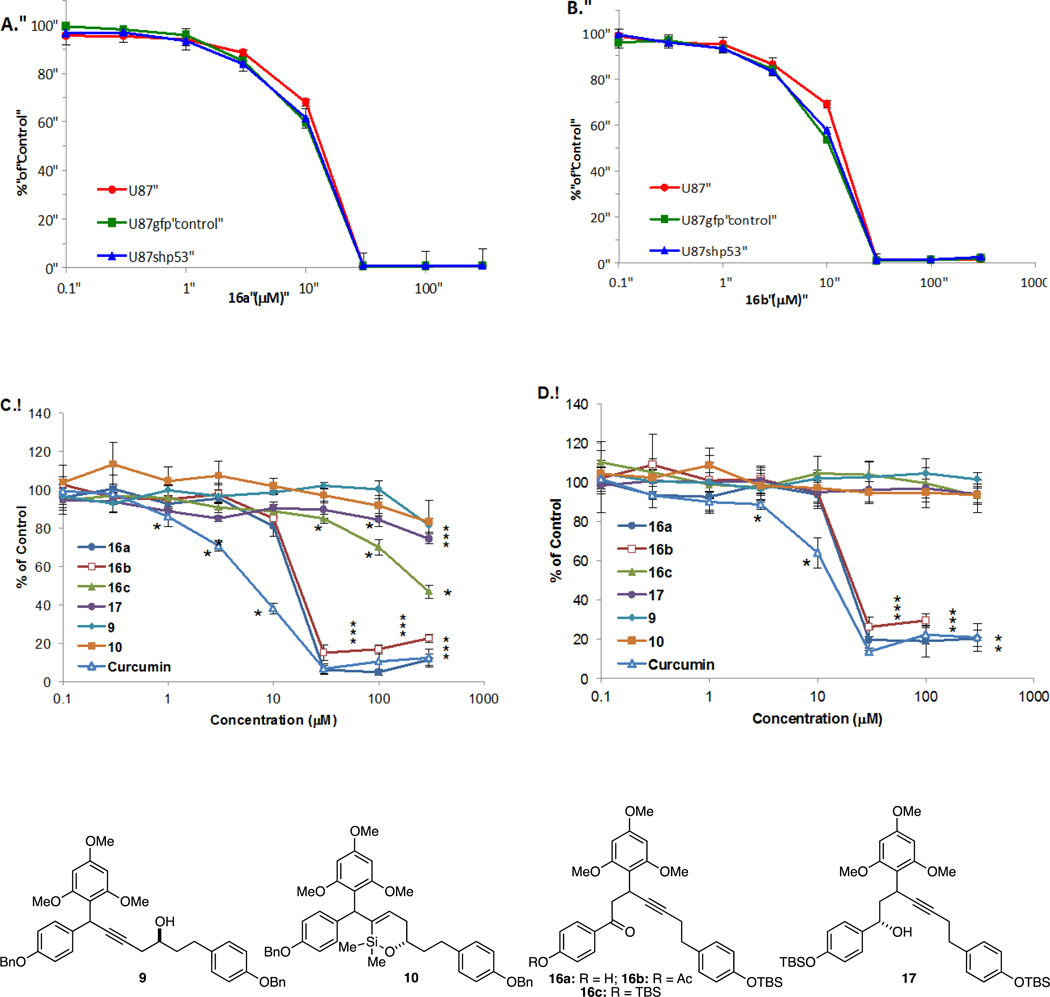
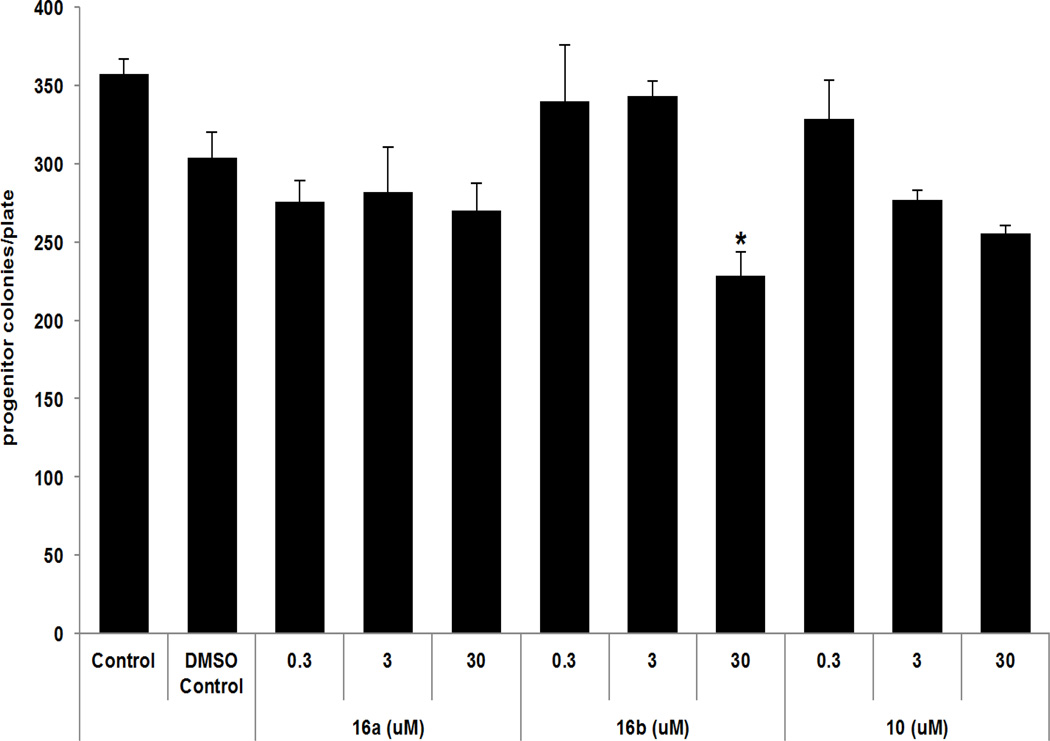
Structural-activity relationships of compounds were evaluated in human U87 MG GBM and neuroblastoma (NB) SK-N-SH and SK-N-FI cells. Cells were exposed to increasing concentrations of compounds 9, 10, 16a–c, or 17. Cell growth was determined using a methylene blue staining assay that measures cell mass and correlates with cell number (Figure 2).20 Both 16a and 16b significantly affected growth in the low µM range in U87 GBM cells as illustrated by the IC50 values of 16a, 16b and curcumin21 (Table 1, entries 1–8). Since p53 mutations can be found in a variety of cancers, including GBM and NB, we evaluated the dependency of compound efficacy on p53 status.22 U87MG cells (parental p53 wild type, vector control, and p53 knockdown) had identical sensitivity profiles, indicating that 16a and 16b can block growth independent of p53 expression (Figure 3, A and B). Additionally, effect on cell growth was not dependent on functional p53 in either SK-N-SH (wild type p53) or SK-N-FI (mutant p53) neuroblastoma cells, as both were sensitive to 16a and 16b (Figure 3, C and D; Table 1, entries 9–16). Given that normal tissue toxicity can be a limiting factor in many anticancer therapies, we screened for compound effect on normal hematopoietic progenitor cells using colony-forming unit assays.23 Human CD34+ cells isolated from umbilical cord blood served as a fresh source of human progenitor cells and were treated with doses of the most active compounds, 16a and 16b and 10, for purposes of comparison. The assay was performed at concentrations of these compounds that had minimal effect on U87 MG cell growth (0.3 & 3.0 µM) and doses that significantly affected the growth of U87 MG cells (30 µM). As illustrated in Figure 4, compound-mediated toxicity was minimal over a 0.3–30 µM dose-range of 16a and 16b. At 30 µM, treatment with 16b led to a 20%–25% decrease in the number of colonies. These data indicate that, at least in vitro, compound-mediated toxicity to hematopoietic cells was not pronounced.
Figure 2.
Dose-related decreases in cell survival of U87 MG GBM cells by curcumin analogs. Cells were exposed in triplicate to vehicle or increasing concentrations of compounds 9, 10, 16a–c, 17, or curcumin and survival was measured at 5 days post-exposure.17 *p < 0.05 vs. media control, Dunnett’s multiple comparisons test.
Table 1.
Potency of curcumin derivatives in decreasing glioblastoma cell and neuroblastoma cell survival.a
| Entry | Tested compounds | IC50 (µM) | |
|---|---|---|---|
| Glioblastoma Cell Lines | |||
| 1 | U87-MG | 16a | 7.5 |
| 2 | 16b | 8.0 | |
| 3 | 16c | 19.1 | |
| 4 | curcumin | 8.1 | |
| 5 | U87-MG sh-gfp | 16a | 7.0 |
| 6 | 16b | 6.9 | |
| 7 | U87-MG sh-p53 | 16a | 7.0 |
| 8 | 16b | 7.0 | |
| Neuroblastoma Cells | |||
| 9 | SK-N-SH | 16a | 18.1 |
| 10 | 16b | 11.7 | |
| 11 | 16c | 48.8 | |
| 12 | curcumin | 6.4 | |
| 13 | SK-N-FI | 16a | 31.1 |
| 14 | 16b | 22.1 | |
| 15 | 16c | >100 | |
| 16 | curcumin | 11.3 | |
IC50 values for each compound were determined using CalcuSyn Software (Biosoft, Cambridge, UK).
Figure 3.
Dose-related decreases in cell survival of glioblastoma and neuroblastoma cells irrespective of p53 status. Cells were exposed in triplicate to vehicle or increasing concentrations of compound and survival was measured at 5 days post-exposure.17 * p < 0.05, 16a and 16b versus vehicle control. (A) Effect of 16a and (B) 16b on U87 parental wild type p53, U87 vector control (U87gfpcontrol), and cells with knock down of p53 (U87 shp53) were determined as described above. (C and D) Dose response curves of wild type 53 NB (SK-N-SH) and mutant p53 NB (SK-N-FI) cells to 16a and 16b. *p < 0.05 vs. media control, Dunnett’s multiple comparisons test.
Figure 4.
Effect of select compounds on human progenitor cell growth. Human CD34+ cells were exposed in triplicate to 0.3–30 µM of compounds 16a, 16b, or 10. Progenitor cell frequency was determined after 14 days. *p < 0.05 vs. media control, Dunnett’s multiple comparisons test (16b at 30 µM).
In summary, we have established a universal and direct strategy for the assembly of curcumin analogs using a transition metal-catalyzed multicomponent coupling strategy. This highly convergent approach enables the rapid evaluation of structural analogs based on this biologically active natural product. A series of curcumin-based diarylheptanoid analogs were synthesized and evaluated for anti-glioblastoma and anti-neuroblastoma properties. The potency of the 16a and 16b was only slightly less than that of curcumin and was still in the low micromolar range, These results enabled us to identify a number of lead compounds that provides a foundation toward identifying diarylheptanoid analogs which are cytotoxic against brain and peripheral nervous system cancers and have improved metabolic and pharmacokinetic profiles in vivo. Further investigation of individual diastereomers, the application of our design strategy and subsequent evaluation of curcumin-based analogs is currently underway and will be reported in due course.
Supplementary Material
Acknowledgments
The authors thank the nursing staff and Dr. Arthur Baluyut at the St. Vincent Women's Hospital for donation of their time for collection of umbilical cord blood products and Christophe Marchal for his expert assistance with production of lentiviral vector supernatants. We also acknowledge financial support of this research from the University of Notre Dame, the Walther Cancer Foundation, Riley Children’s Foundation, the Indiana University Simon Cancer Center, the Jeff Gordon Research Foundation (BB and KP), and RO1 CA138798 (BB and KP). This work was also supported by the NIH R01s CA138237 and CA155294 and by the BMBF grant ‘FoneFA’ and the DFG SPP1230 to HH. Indiana University is a Center for Excellence in Molecular Hematology (P30).
References and notes
- 1.(a) Surveillance, Epidemiology, and End Results (SEER) Program and the National Center for Health Statistics. http://seer.cancer.gov/statfacts/html/brain.html. Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Horner MJ, Howlader N, Eisner MP, Reichman M, Edwards BK. SEER Cancer Statistics Review 1975–2004. Bethesda, MD: National Institutes of Health, National Cancer Institute; 2007.
- 2.(a) Balmaceda C, Peereboom D, Pannullo S, Cheung YKK, Fisher PG, Alavi J, Sisti M, Chen J, Fine RL. Cancer. 2008;112:1139. doi: 10.1002/cncr.23167. [DOI] [PubMed] [Google Scholar]; (b) Galanis E, Jaeckle KA, Maurer MJ, Reid JM, Ames MM, Hardwick JS, Reilly JF, Loboda A, Nebozhyn M, Fantin VR, Richon VM, Scheithauer B, Giannini C, Flynn PJ, Jr, D FM, Zwiebel J, Buckner JC. J. Clin. Oncol. 2009;27:2052. doi: 10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Agrawal DK, Mishra PK. Med. Res. Rev. 2010;30:818. doi: 10.1002/med.20188. [DOI] [PubMed] [Google Scholar]; (b) Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biochem. Pharmacol. 2008;76:1590. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]; (c) Goel A, Kunnumakkara AB, Aggarwal BB. Biochem. Pharmacol. 2008;75:787. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]; (d) Ruby AJ, Kuttan G, Dinesh Babu K, Rajasekharan KN, Kuttan R. Cancer Lett. 1995;94:79. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 4.(a) Bush JA, Cheung KJJ, Jr, Li G. Exp. Cell. Res. 2001;271:305. doi: 10.1006/excr.2001.5381. [DOI] [PubMed] [Google Scholar]; (b) Jiang MCM, Yang-Yen HFH, Yen JJJ, Lin JKJ. Nutr. Cancer. 1996;26:111. doi: 10.1080/01635589609514468. [DOI] [PubMed] [Google Scholar]; (c) Nakamura K, Yasunaga Y, Segawa T, Ko D, Moul JW, Srivastava S, Rhim JS. Int. J. Oncol. 2002;21:825. [PubMed] [Google Scholar]; (d) Ramachandran C, Rodriguez S, Ramachandran R, Raveendran Nair PK, Fonseca H, Khatib Z, Escalon E, Melnick SJ. Anticancer Res. 2005;25:3293. [PubMed] [Google Scholar]; (e) Sharma RA, Gescher AJ, Steward WP. Eur. J. Cancer. 2005;41:1955. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 5.(a) Dhandapani KM, Mahesh VB, Brann DW. J. Neurochem. 2007;102:522. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]; (b) Freudlsperger C, Greten J, Schumacker U. Anticancer Res. 2008;28:209. [PubMed] [Google Scholar]; (c) Liontas A, Yeger H. Anticancer Res. 2009;24:987. [PubMed] [Google Scholar]; (d) Perry M-C, Demeule M, Régina A, Moumdjian R, Béliveau R. Mol. Nutr. Food Res. 2010;54:1192. doi: 10.1002/mnfr.200900277. [DOI] [PubMed] [Google Scholar]; (e) Purkayastha S, Berliner A, Fernando SS, Ranasinghe B, Ray I, Tariq H, Banerjee P. Brain Res. 2009;1266:130. doi: 10.1016/j.brainres.2009.01.066. [DOI] [PubMed] [Google Scholar]; (f) Senft C, Polacin M, Priester M, Seifert V, Kögel D, Weissenberger J. BMC Cancer. 2010;10:491. doi: 10.1186/1471-2407-10-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For selected references describing modification of the 1,3-dicarbonyl region of curcumin, see: Lin L, Shi Q, Nyarko AK, Bastow KF, Wu C-C, Su C-Y, Shih CCY, Lee K-H. J. Med. Chem. 2006;49:3963. doi: 10.1021/jm051043z. Tan K-L, Koh S-B, Ee RP-L, Khan M, Go M-L. Chem Med Chem. 2012;7:1567. doi: 10.1002/cmdc.201200293. Yamaguchi M, Moore TW, Sun A, Snyder JP, Shoji M. Integr. Biol. 2012;4:905. doi: 10.1039/c2ib20045g. Zuo Y, Huang J, Zhou B, Wang S, Shao W, Zhu C, Lin L, Wen G, Wang H, Du J, Bu X. Eur. J. Med. Chem. 2012;55:346. doi: 10.1016/j.ejmech.2012.07.039. Ohori H, Yamakoshi H, Tomizawa M, Shibuya M, Kakudo Y, Takahashi A, Takahashi S, Kato S, Suzuki T, Ishioka C, Iwabuchi Y, Shibata H. Mol. Cancer Ther. 2006;5:2563. doi: 10.1158/1535-7163.MCT-06-0174. Zhang Q, Zhong Y, Yan L-N, Sun X, Gong T, Zhang Z-R. Biorg. Med. Chem. Lett. 2011;21:1010. doi: 10.1016/j.bmcl.2010.12.020. Batie S, Lee JH, Jama RA, Browder DO, Montano LA, Huynh CC, Marcus LM, Tsosie DG, Mohammed Z, Trang V, Marshall PA, Jurutka PW, Wagner CE. Biorg. Med. Chem. 2013;21:693. doi: 10.1016/j.bmc.2012.11.033.
- 7.(a) Campos CA, Gianino JB, Pinkerton DM, Ashfeld BL. Org. Lett. 2011;13:5680. doi: 10.1021/ol202401n. [DOI] [PubMed] [Google Scholar]; (b) Gianino JB, Ashfeld BL. J. Am. Chem. Soc. 2012;134:18217. doi: 10.1021/ja308891e. [DOI] [PubMed] [Google Scholar]; (c) Campos CA, Gianino JB, Ashfeld BL. Org. Lett. 2013;15:2656. doi: 10.1021/ol400943a. [DOI] [PubMed] [Google Scholar]; (d) Lepore AJ, Pinkerton DM, Ashfeld BL. Adv. Synth. Catal. 2013;355:1500. [Google Scholar]
- 8.(a) Anastas P, Kirchhoff M. Acc. Chem. Res. 2002;35:686. doi: 10.1021/ar010065m. [DOI] [PubMed] [Google Scholar]; (b) Felpin F-X, Fouquet E. Chem Sus Chem. 2008;1:718. doi: 10.1002/cssc.200800110. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Edmonds DJ, Bulger PG. Angew. Chem. Int. Ed. 2006;45:7134. doi: 10.1002/anie.200601872. [DOI] [PubMed] [Google Scholar]; (d) Tietze LF. Chem. Rev. 1996;96:115. doi: 10.1021/cr950027e. [DOI] [PubMed] [Google Scholar]; (e) Tietze LF, Beifuss U. Angew. Chem. Int. Ed. 1993;32:131. [Google Scholar]
- 9.For recent work on titanocene-catalyzed metallations and related processes, see: Fleury LM, Ashfeld BL. Org. Lett. 2009;11:5670. doi: 10.1021/ol902374v. Fleury LM, Ashfeld BL. Tetrahedron Lett. 2010;51:2427. Fleury LM, Gianino JB, Ashfeld BL. Tetrahedron Lett. 2012;53:5376. Fleury LM, Kosal AD, Masters JT, Ashfeld BL. J. Org. Chem. 2012 doi: 10.1021/jo301726v. ASAP Article. Kosal AD, Ashfeld BL. Org. Lett. 2010;12:44. doi: 10.1021/ol9024315. Wilson EE, Oliver AG, Hughes RP, Ashfeld BL. Organometallics. 2011;30:5214.
- 10.Boulard L, BouzBouz S, Cossy J, Franck X, Figadère B. Tetrahedron Lett. 2004;45:6603. [Google Scholar]
- 11.(a) Hanawa H, Uraguchi D, Konishi S, Hashimoto T, Maruoka K. Chem. Eur. J. 2003;9:4405. doi: 10.1002/chem.200305078. [DOI] [PubMed] [Google Scholar]; (b) Usanov DL, Yamamoto H. J. Am. Chem. Soc. 2011;133:1286. doi: 10.1021/ja1102822. [DOI] [PubMed] [Google Scholar]
- 12.Gianino JB, Ashfeld BL. J. Am. Chem. Soc. 2012;134:18217. doi: 10.1021/ja308891e. [DOI] [PubMed] [Google Scholar]
- 13.(a) Fürstner A, Radkowski K. Chem. Commun. 2002:2182. doi: 10.1039/b207169j. [DOI] [PubMed] [Google Scholar]; (b) Micoine K, Fürstner A. J. Am. Chem. Soc. 2010;132:14064. doi: 10.1021/ja107141p. [DOI] [PubMed] [Google Scholar]; (c) Trost BM, Ball ZT. J. Am. Chem. Soc. 2003;125:30. doi: 10.1021/ja028766h. [DOI] [PubMed] [Google Scholar]; (d) Trost BM, Ball ZT, Jöge T. J. Am. Chem. Soc. 2002;124:7922. doi: 10.1021/ja026457l. [DOI] [PubMed] [Google Scholar]
- 14.Rauniyar V, Hall DG. J. Org. Chem. 2009;74:4236. doi: 10.1021/jo900553f. [DOI] [PubMed] [Google Scholar]
- 15.Corey EJ, Fuchs PL, editors. Tetrahedron Lett. 1972;13:3769. [Google Scholar]
- 16.Yin J, Wang Y, Pei J. Org. Lett. 2008;10:3113. doi: 10.1021/ol801163v. [DOI] [PubMed] [Google Scholar]
- 17.To quantify cell mass, cells were stained with methylene blue, solubilized in 0.1N HCl, and absorbance determined at 610nm. Mean and standard deviation were determined.
- 18.Saitoh T, Oyama T, Sakurai K, Niimura Y, Hinata M, Horiguchi Y, Toda J, Sano T. Chem. Pharm. Bull. 1996;44:956. [Google Scholar]
- 19.(a) Corey EJ, Bakshi RK, Shibata S, Chen CP, Singh VK. J. Am. Chem. Soc. 1987;109:7925. [Google Scholar]; (b) Helal CJ, Magriotis PA, Corey EJ. J. Am. Chem. Soc. 1996;118:10938. [Google Scholar]
- 20.Oliver MH, Harrison NK, Bishop JE, Cole PJ, Laurent GJ. J. Cell. Sci. 1989;92:513. doi: 10.1242/jcs.92.3.513. [DOI] [PubMed] [Google Scholar]
- 21.Curcumin was purchased from a commercial source as a mixture of curcumin, demethoxycurcumin and bisdemthoxycurcumin. Purification via flash column chromatography (1:1 hexanes/ethyl acetate) provided curcumin in ≥97% purity that was used for analysis. In.
- 22.Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, Olivier M. Hum. Mutat. 2007;28:622. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Baluyut AR, Ernstberger A, Juliar B, Sinn AL, Mayo LD, Goebel WS, Pollok KE. Clin. Cancer Res. 2011;8:2195. doi: 10.1158/1078-0432.CCR-10-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.