Abstract
Inflammatory stimuli activate ectodomain shedding of TNF-α, L-selectin and other transmembrane proteins. We show that p38 MAP kinase, which is activated in response to inflammatory or stress signals, directly activates TACE, a membrane-associated metalloprotease that effects shedding in response to growth factors and Erk MAP kinase activation. p38α MAP kinase interacts with the cytoplasmic domain of TACE, and phosphorylates it on Thr735, which is required for TACE-mediated ectodomain shedding. Activation of TACE by p38 MAP kinase results in the release of TGF-α family ligands, which activate EGF receptor signaling leading to enhanced cell proliferation. Conversely, depletion of p38α MAP kinase activity suppresses EGF receptor signaling and downstream Erk MAP kinase signaling, as well as autocrine EGF receptor-dependent proliferation. Autocrine EGF receptor activation through TACE-mediated ectodomain shedding intimately links inflammation and cancer progression, and may play a role in stress and conditions that relate to p38 MAP kinase activation.
INTRODUCTION
Ectodomain shedding results in the release of extracellular domains of transmembrane proteins, including cytokines, growth factors and cell surface receptors. This process is mediated by membrane-anchored metalloproteases and often regulates the activities of the substrate proteins. Thus, shedding results in the release of soluble cytokines and growth factors that activate receptors on cells at a distance, regulating cell proliferation and migration. (Blobel, 2005; Seals and Courtneidge, 2003). Ectodomain shedding plays roles in inflammation by controlling the cleavage of inflammatory regulators, such as TNF-α and the adhesion protein L-selectin (Seals and Courtneidge, 2003). Shedding of L-selectin disrupts leukocyte binding to endothelial cells, preventing their recruitment at sites of inflammation, and systemic release of TNF-α greatly contributes to cachexia (Beutler et al., 1986).
Inflammation has also been implicated in cancer. Chronic inflammation due to infection or irritation enhances initiation of tumor development (Mantovani et al., 2008). Conversely, cell-intrinsic changes that contribute to carcinogenesis are often accompanied with inflammatory signaling or production of inflammatory mediators that contribute to tumor promotion and progression (Coussens and Werb, 2002; Greten et al., 2004; Mantovani et al., 2008; Pikarsky et al., 2004). Inflammatory cytokines, pathogenic stimuli and cell stress activate signaling leading to p38 MAP kinase (MAPK), through MKK3, MKK4 or MKK6, which in turn are activated by MAPK kinase kinases (Ono and Han, 2000). p38 MAPK contributes to inflammation, e.g. by activating cytokine expression and modulating their signaling (Dong et al., 2002). Among the four p38 MAPK isoforms, p38α is best characterized and crucial for inflammatory cytokine production. The p38 MAPK pathway can contribute to cancer progression through effects on cell proliferation (Dolado et al., 2007; Ono and Han, 2000; Ventura et al., 2007), and its roles in inflammation, e.g. in inducing VEGF and metalloprotease expression (Coussens and Werb, 2002; Mantovani et al., 2008). Further, inflammatory mediators that induce p38 MAPK activation can stimulate cell proliferation and contribute to malignant progression (Moore et al., 1999; Vidal-Vanaclocha et al., 2000). Yet, the p38 MAPK pathway can also exert a tumor suppressor effect by modulating cell cycle regulation, senescence and DNA damage responses (Han and Sun, 2007), and triggering apoptosis (Dolado et al., 2007).
In carcinomas, EGF receptor (EGFR) activation aids the progression by promoting cell proliferation and cell survival (Yarden and Sliwkowski, 2001), and interference with EGFR activation is the basis for therapies (Zhang et al., 2007). Autocrine EGFR signaling is enhanced by increased receptor and/or TGF-α family ligand expression, as observed in cancers of neuro-ectodermal origin (Hynes and Lane, 2005). Accordingly, increased release of soluble ligands, such as TGF-α and amphiregulin, through ectodomain shedding (Blobel, 2005; Sahin et al., 2004), enhances the EGFR activity and cancer progression (Borrell-Pages et al., 2003; Kenny and Bissell, 2007; Zhou et al., 2006).
Among the metalloproteases, TACE, also known as ADAM17, mediates ectodomain shedding of inflammatory cytokines and TGF-α family ligands (Seals and Courtneidge, 2003). TACE expression is often enhanced in inflammation (Patel et al., 1998) and in carcinomas, especially in breast cancers (Mochizuki and Okada, 2007). The release of TGF-α proteins by TACE stimulates cell proliferation and cancer progression (Borrell-Pages et al., 2003; Kenny and Bissell, 2007; Zhou et al., 2006). How TACE is activated is of great interest. Erk MAPK signaling, often observed in carcinomas, activates TACE and may activate TACE-mediated ectodomain shedding in response to growth factors (Diaz-Rodriguez et al., 2002; Fan and Derynck, 1999; Gechtman et al., 1999). Further, EGFR activation by G protein-coupled receptors involves Src-dependent phosphorylation of TACE resulting in release of amphiregulin (Zhang et al., 2006).
While stimuli that activate p38 MAPK induce ectodomain shedding, the responsible metalloproteases that are activated by this pathway have remained unclear. In fact, it has been proposed that stimuli that activate p38 MAPK do not act through TACE (Diaz-Rodriguez et al., 2002; Hinkle et al., 2003; Takenobu et al., 2003). Using transmembrane TGF-α as model, we show that p38 MAPK directly activates TACE through targeted phosphorylation of its cytoplasmic domain. Activation of TACE by p38 MAPK in breast carcinoma cells leads to a release of TGF-α ligands, resulting in increased EGF receptor activation and proliferation. Our results establish a mechanism for the regulation of EGF receptor and Erk MAPK signaling, and cancer cell proliferation, by the p38 MAPK pathway.
RESULTS
Transmembrane TGF-α as reporter of ectodomain shedding
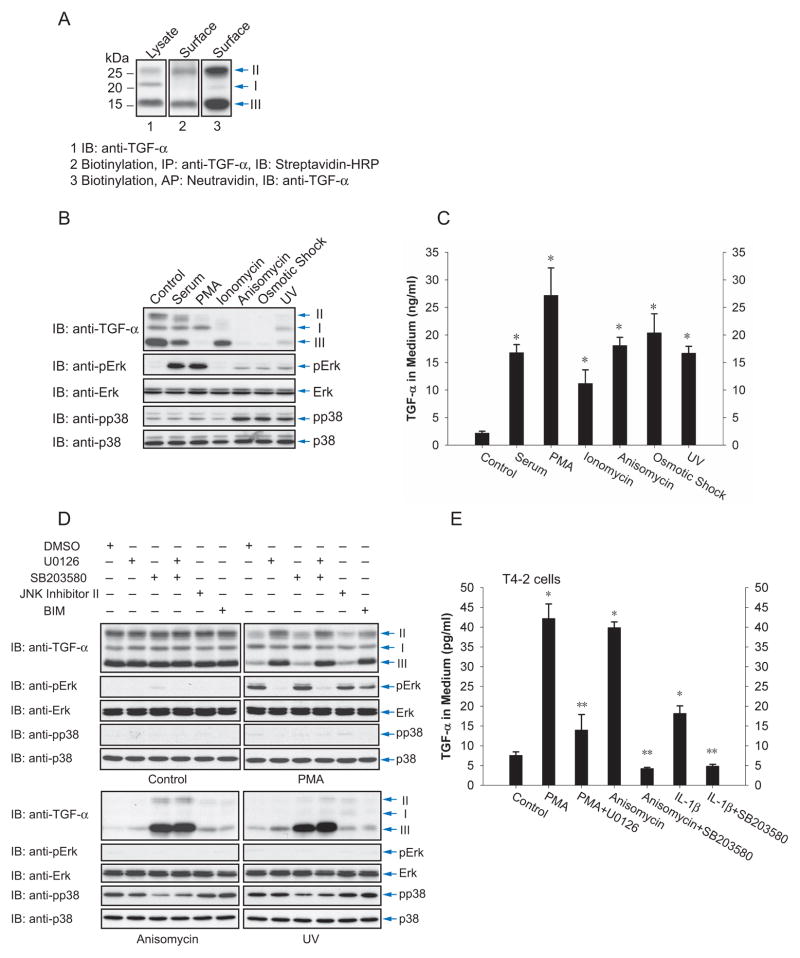
We compared the ectodomain shedding responses to extracellular inducers of Erk or p38 MAPK activation using Cα cells, a CHO cell derivative that expresses TGF-α. TGF-α undergoes N-glycosylation and cleavage of the prodomain, giving rise to three transmembrane forms: (1) a ~22 kd form I that corresponds to the precursor with its N-terminal unglycosylated prodomain and is primarily located in the endoplasmic reticulum; (2) a ~28 kd form II with N-glycosylation of the prodomain, which occurs primarily in the Golgi; and (3) a ~16 kd form III with the prodomain proteolytically removed (Bringman et al., 1987). Ectodomain shedding releases soluble TGF-α and thereby generates a truncated transmembrane protein that is not recognized by anti-TGF-α antibody. Cell surface protein biotinylation revealed that transmembrane TGF-α forms II and III are at the cell surface (Fig. 1A). This scenario of TGF-α maturation allows quantification of ectodomain shedding, based on the decrease of transmembrane TGF-α levels at the cell surface or release of soluble TGF-α in the medium.
Figure 1.
Activation of the p38 MAP kinase pathway induces ectodomain shedding of TGF-α. (A), Cα cells express transmembrane TGF-α forms I, II and III, as shown by immunoblotting of cell lysates. Forms II and III are visualized by cell surface protein biotinylation. (B) and (C), Cα cells were treated as indicated, and ectodomain shedding of TGF-α was examined by immunoblotting of cell lysates for transmembrane TGF-α (B, upper panel), or quantifying TGF-α released into the medium by ELISA (C). In (B) the middle and lower panels show activation of Erk (anti-pErk) or p38 MAPK (anti-pp38) versus total Erk or p38 MAPK. Error bars show the standard deviations based on triplicate values of each data set. *, P < 0.01, compared with control. (D), Effects of pathway inhibitors on TGF-α shedding. Cα cells were treated with PMA, anisomycin or UV light without or with the indicated inhibitor. Immunoblotting of cell lysates shows transmembrane TGF-α, and activated Erk (anti-pErk) or p38 MAPK (anti-pp38) versus total Erk or p38 MAPK. (E), Ectodomain shedding of TGF-α in T4-2 cells treated with PMA, anisomycin or IL-1β without or with U0126 or SB203580. TGF-α released into the medium was quantified by ELISA. *, P < 0.001, compared with control. **, P < 0.01, compared with corresponding PMA, anisomycin or IL-1β stimulation.
Extracellular cues that activate p38 MAP kinase induce ectodomain shedding
Cα cells were treated with PMA, serum, anisomycin, UV light, osmotic shock or ionomycin. Transmembrane TGF-α in cell lysates was visualized by western blotting (Fig. 1B), and soluble TGF-α was measured in the media by ELISA (Fig. 1C). All treatments resulted in a decrease of the TGF-α forms II and III at the cell surface and release of soluble TGF-α, indicating ectodomain shedding. We correlated these results with activation of Erk or p38 MAPK signaling. PMA and serum activated Erk MAPK, and anisomycin, UV light and osmotic shock activated p38 MAPK with only a minimal effect on Erk MAPK (Fig. 1B). These data suggested that p38 MAPK activation, like Erk MAPK signaling, induces TGF-α shedding in Cα cells.
To study the roles of the Erk and p38 MAPK pathways in the induction of ectodomain shedding, we treated Cα cells with the MEK inhibitor U0126, preventing activation of Erk MAPKs, or the p38 MAPK inhibitor SB203580. Since anisomycin, UV light and osmotic shock also activate JNK MAPK, we also tested the effect of a JNK MAPK pathway inhibitor. Further, since PMA activates protein kinase C, we examined the effect of the protein kinase C inhibitor BIM. Fig. 1D shows that TGF-α shedding in response to anisomycin or UV light was unaffected by inhibitors of the Erk or JNK MAPK pathways, or protein kinase C. In contrast, inhibition of p38 MAPK blocked shedding and increased the cell surface levels of TGF-α forms III and II. PMA-induced shedding was blocked by U0126, as reported (Fan and Derynck, 1999; Gechtman et al., 1999), but not by the p38 MAPK inhibitor. These results indicate that p38 MAPK activates ectodomain shedding induced by anisomycin or UV light, and that Erk MAPK or protein kinase C signaling are not involved.
We evaluated whether ectodomain shedding responded to the same signals in Hela cells ectopically expressing TGF-α (Suppl. Fig. S1A, B) and T4-2 breast carcinoma cells (Kenny and Bissell, 2007) (Fig. 1E). PMA, anisomycin and UV light induced TGF-α shedding in Hela cells. We could not detect the low endogenous levels of transmembrane TGF-α in T4-2 cell lysates, but ELISA revealed the release of TGF-α in the medium in response to PMA or anisomycin. As in Cα cells, PMA-induced shedding in Hela or T4-2 cells was inhibited by U0126, and anisomycin-induced shedding was inhibited by SB203580 (Fig. 1E, Suppl. Fig. S1A and B). The cytokine interleukin (IL)-1β, which activates p38 MAPK signaling (Ono and Han, 2000) and is frequently expressed in human breast cancers (Nicolini et al. 2006), also induced TGF-α shedding in T4-2 cells, albeit at a lower level than anisomycin, and this induction was similarly inhibited by SB203580 (Fig. 1E). These data using Cα, Hela and T4-2 cells indicate that p38 MAPK activation results in ectodomain shedding.
p38α MAP kinase activates anisomycin- and UV light-induced ectodomain shedding
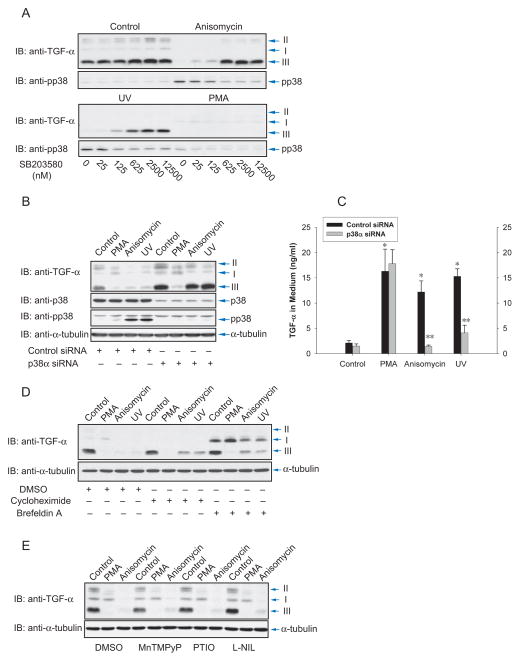
All four p38 MAPKs, p38α, β, γ and δ are activated by MKK3 or MKK6 (Ono and Han, 2000). Two of these, p38α and p38β, are inhibited by SB203580. Activation of p38 MAPK and TGF-α ectodomain shedding in response to anisomycin or UV light were inhibited by SB203580 with similar sensitivities (Fig. 2A). The nearly complete inhibition at 625 nM SB203580 was close to the reported IC50 of 600 nM SB203580 for p38 MAPK inhibition (Cuenda et al., 1995), suggesting that p38α or p38β MAPK mediate anisomycin- and UV light-induced ectodomain shedding.
Figure 2.
p38α MAP kinase mediates anisomycin- and UV light-induced ectodomain shedding. (A), Cα cells were treated with anisomycin, UV light or PMA, and increasing doses of SB203580, and shedding was visualized by immunoblotting for transmembrane TGF-α. The lower panels show p38 MAPK activation using anti-phospho-p38 MAPK immunoblotting. (B) and (C), Cα cells were transfected with p38α MAPK siRNA or control siRNA. Ecdodomain shedding of TGF-α was induced by PMA, anisomycin or UV light, and evaluated by immunoblotting of cell lysates for transmembrane TGF-α (B), or ELISA of TGF-α released in the medium (C). Immunoblotting for p38 MAPK or phospho-p38 MAPK revealed the p38α MAPK activation. *, P < 0.01, compared with control. **, P < 0.01, compared with corresponding control siRNA transfection. (D), Cα cells were treated with PMA, anisomycin or UV light, without or with cycloheximide or brefeldin A. Shedding was assessed by immunoblotting of cell lysates for transmembrane TGF-α. (E), Ectodomain shedding was induced with PMA or anisomycin without or with MNTmPyP, PTIO or L-NIL, and evaluated by immunoblotting of cell lysates for TGF-α.
We isolated p38α MAPK cDNAs from CHO cell mRNA and designed siRNA based on this sequence. Transfection of the siRNA downregulated p38α MAPK expression in Cα cells, and inhibited p38 MAPK activation in response to anisomycin or UV light, as apparent from the minimal p38 MAPK phosphorylation (Fig. 2B). Downregulation of p38α MAPK expression also prevented the induction of ectodomain shedding and release of TGF-α into the medium in response to anisomycin or UV light, assessed in Cα (Fig. 2B, C) and Hela cells (Suppl. Fig. S2A). Downregulation of p38α MAPK expression did not affect shedding in response to PMA, as apparent by western blotting of transmembrane TGF-α (Fig. 2B) and ELISA of TGF-α in the medium (Fig. 2C, Suppl. Fig. S2A). These results indicate that p38α MAPK activates anisomycin- or UV light-induced ectodomain shedding.
p38 MAP kinase-activated shedding does not require protein synthesis or reactive oxygen species
Cycloheximide did not inhibit PMA-induced shedding but slightly inhibited ectodomain shedding in response to anisomycin or UV light (Fig. 2D, lanes 5–8). Brefeldin A, which disrupts transport through the Golgi apparatus, prior to ectodomain shedding of cell surface proteins, enhanced the level of immature TGF-α form I, consistent with the disruption of protein transport through the Golgi. However, it did not affect shedding of the cell surface TGF-α form III in response to PMA, and slightly affected shedding induced by anisomycin or UV light (Fig. 2D, lanes 8–12). These data indicate that new protein synthesis is not required for activation of ectodomain shedding by the Erk or p38 MAPK pathways.
Reactive oxygen species (ROS) and nitrogen oxide (NO) have been proposed to play a role in activating TACE, by inducing dissociation of the inhibitory prodomain from active TACE (Zhang et al., 2001), or activating p38 MAPK signaling (Dolado et al., 2007; Fischer et al., 2004). However, the antioxidant MNTmPyP did not inhibit anisomycin- or PMA-induced ectodomain shedding of TGF-α, nor did the NO scavenger PTIO and iNOS inhibitor L-NIL (Fig. 2E). Our data suggest that ROS and NO are not involved in p38 or Erk MAPK-activated ectodomain shedding.
TACE mediates TGF-α release in response to activation of p38 MAP kinase
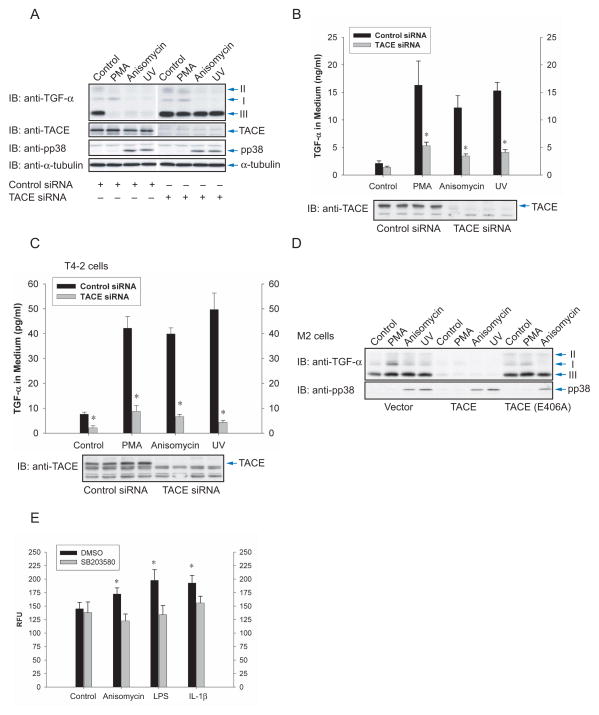
To address the role of TACE, we transfected siRNAs, based on the Chinese hamster sequence of the catalytic domain of TACE, in Cα cells. Silencing TACE expression blocked shedding of transmembrane TGF-α (Fig. 3A), and decreased the release of soluble TGF-α (Fig. 3B), in response to anisomycin or UV light, similarly to its effect on PMA-induced shedding. A similar inhibition of TGF-α release was seen in Hela cells expressing TGF-α (Suppl. Fig. S2B) or T4-2 cells expressing endogenous TGF-α (Fig. 3C), using siRNA to the human TACE sequence.
Figure 3.
TACE effects TGF-α release in response to p38 MAP kinase activation. (A) and (B), Cα cells were transfected with TACE siRNA or control siRNA, stimulated with PMA, anisomycin or UV light, and shedding of TGF-α was evaluated by immunoblotting cell lysates for transmembrane TGF-α (A), or by ELISA of TGF-α in the medium (B). Immunoblotting cell lysates with anti-TACE or anti-phospho-p38 MAPK antibodies showed silencing of TACE expression, or activation of p38 MAPK in response to anisomycin or UV light. *, P < 0.05, compared with corresponding transfection with control siRNA. (C), T4-2 cells, transfected with TACE siRNA or control siRNA, were treated with PMA, anisomycin or UV light, and TGF-α released in the medium was quantified by ELISA. Silencing of TACE expression was apparent by immunoblotting of cell lysates for TACE (lower panel). *, P < 0.05, compared with corresponding transfection with control siRNA. (D), M2 cells, lacking active TACE, were transfected to express TGF-α and TACE or inactive E406A TACE mutant. Shedding of TGF-α was visualized by immunoblotting of cell lysates for transmembrane TGF-α. The lower panel shows p38 MAPK activation, assessed by immunoblotting for phospho-p38 MAPK. (E), In vitro proteolytic activity of TACE immunoprecipitated from T4-2 cells that were treated with anisomycin, LPS or IL-1β, in the absence or presence of SB203580. *, P < 0.05, compared with DMSO control.
CHO-derived M2 cells lack induction of ectodomain shedding in response to PMA or serum, due to an inactivating Cys600 mutation in TACE (Li and Fan, 2004). Anisomycin and UV light were, similarly to PMA or serum, unable to induce shedding. However, introduction of TACE conferred TGF-α shedding in response to PMA, as well as anisomycin or UV light, and this rescue was not seen with the catalytically inactive Glu406Ala TACE (Fig. 3D). These results indicate that TACE mediates shedding in response to p38 MAPK activation.
We also incubated T4-2 cells with anisomycin, IL-1β or lipopolysaccharide (LPS), which is also known to activate p38 MAPK (Ono and Han, 2000), immunoprecipitated TACE and assessed its activity in vitro by cleavage of a substrate peptide spanning a known TACE cleavage site. As shown in Fig. 3E, anisomycin, LPS and IL-1β increased the activity of TACE, and those increases were abolished by incubating the cells with p38 MAPK inhibitor SB203580 prior to isolation of TACE. We conclude that p38 MAPK activation results in increased enzymatic activity of TACE. In these assays, all TACE was immunoprecipitated, and not only the TACE at the cell surface that accounts for only a small fraction of TACE in the cell lysate and is likely the population that is activated by p38 MAP kinase.
TACE is directly phosphorylated by p38α MAP kinase
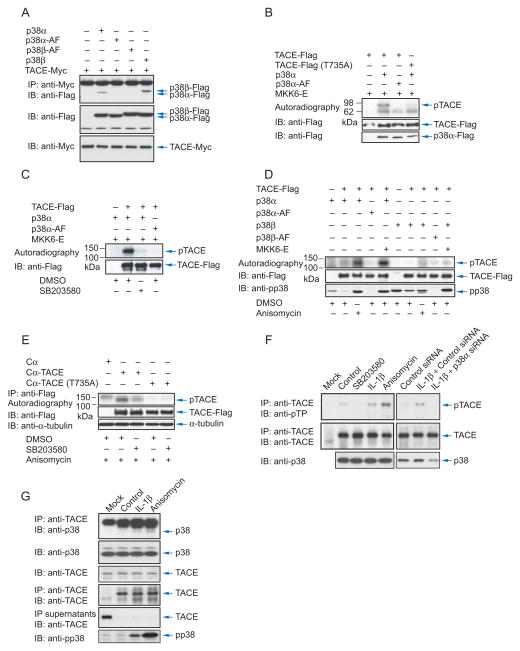
Since activation of p38α MAPK resulted in ectodomain shedding by TACE, we explored whether p38 MAPK interacts with TACE. Using Cα cells expressing tagged TACE and p38α MAPK, we showed that p38α MAPK associated with TACE. In contrast, the kinase-inactive AF mutant of p38α MAPK did not associate. Also p38β MAP kinase, but not its kinase-inactive mutant, associated with TACE (Fig. 4A).
Figure 4.
p38α MAP kinase interacts with and phosphorylates TACE. (A), Cα cells were transfected to expresss Myc-tagged TACE with Flag-tagged p38α or p38β MAPK or inactive p38α-AF or p38β-AF. Cell lysates were subjected to anti-Myc immunoprecipitation, and anti-Flag immunoblotting. TACE and p38 MAPK expression were shown by immunoblotting of cell lysates. (B), TACE, TACE (T735A) mutant, p38α MAPK, p38α-AF and activated MKK6 (MKK6-E) were generated by in vitro transcription and translation. p38α MAPK was then activated through phosphorylation by MKK6-E. Phosphorylated p38α MAPK and TACE were purified using anti-Flag beads, and incubated with 32P-γATP in a kinase assay. The lower panels show immunoblotting of the proteins in the kinase assays. TACE is smaller than in vivo expressed TACE due to lack of glycosylation. (C), In vitro kinase assays as in (B) were performed with purified proteins from transfected Cα cells, with SB203580 added to inhibit p38α MAPK. (D), Cα cells were transfected to express TACE with p38α or p38β MAPK, or p38α-AF or p38β-AF, with or without MKK6-E. Cells were treated for 30 min or not with anisomycin, lysed, and TACE and p38 MAPK proteins were purified using anti-Flag beads and incubated with 32P-γATP in a kinase assay. The proteins were separated by SDS-PAGE, and autoradiography detected 32P-labelled TACE. (E), Cα cells and Cα cells expressing TACE or T735A TACE were cultured in 32P-orthophosphate, treated with anisomycin, with or without SB203580, and lysed. Immunoprecipitated, 32P-labelled TACE was visualized by SDS-PAGE and autoradiography. Anti-Flag immunoblotting showed the immunoprecipitated TACE-Flag. (F), T4–2 cells, transfected or not with control siRNA or p38α MAPK siRNA, were treated or not with SB203580, anisomycin or IL-1β for 30 min, lysed and subjected to anti-TACE immunoprecipitation and immunoblotting with anti-phosphoThr-Pro (anti-pTP) antibody to reveal phosphorylated TACE, or with anti-TACE antibody to reveal endogenous TACE. The lower panel shows p38 MAPK levels, assessed by immunoblotting. (G), Untransfected T4-2 cells were treated or not with IL-1β or anisomycin for 15 min, lysed and subjected to anti-TACE or or IgG (Mock) immunoprecipitation, followed by anti-p38 MAPK immunoblotting. Immunoblotting shows endogenous TACE and p38 MAPK in cell lysates. Immunoprecipitated TACE was visualized by immunoblotting, and immunoprecipitate supernatants were adsorbed to Con A-Sepharose followed by immunoblotting using anti-TACE antibody.
Using purified tagged proteins translated in vitro we phosphorylated wild-type and kinase-inactive p38α MAPK with MKK6. These were then immunopurified and incubated with γ32P-ATP and purified tagged TACE, which, due to lack of glycosylation, was smaller than TACE made in mammalian cells. TACE was phosphorylated by activated wild-type p38α MAPK but not its inactive mutant (Fig. 4B). Similar results were obtained using proteins that were expressed in Cα cells. Thus, p38α MAPK or its kinase-inactive mutant were coexpressed with activated MKK6, and immunopurified p38α MAPK was incubated with tagged TACE that was also immunopurified from transfected cells. Fig. 4C shows phosphorylation of TACE by p38α MAPK, which was inhibited by SB203580. These data illustrate that TACE is a kinase substrate for p38α MAP kinase.
We evaluated whether p38 MAPK phosphorylates TACE in response to anisomycin using in vitro kinase assays with tagged p38α or p38β MAPK and TACE that were coexpressed in Cα cells and then purified. Anisomycin induced TACE phosphorylation to a level similar to coexpression of activated MKK6 (Fig. 4D). Kinase-inactive p38α MAPK did not phosphorylate coexpressed TACE, and p38β MAPK only weakly phosphorylated TACE, when compared with p38α MAP kinase, either in response to anisomycin or when coexpressed with activated MMK6 (Fig. 4D). Thus, even though p38α and p38β MAPK associated similarly with TACE in vitro (Fig. 4A), TACE was the preferred substrate of p38α MAP kinase.
In Cα cells, TACE was phophorylated in vivo in response to anisomycin by endogenous p38α MAPK, as apparent by the inhibition by SB203580 (Fig. 4E). We also examined the phosphorylation of endogenous TACE in T4-2 cells. Since p38 MAPK is a proline-directed Ser/Thr kinase, we used anti-phosphoThr-Pro to detect phospho-TACE by immunoblotting (Fig. 4F). SB203580 inhibited the low basal level of phosphorylation, and anisomycin and IL-1β enhanced the immunoreactivity of TACE. The lower level of immunoreactivity of TACE upon IL-1β stimulation, when compared to anisomycin, is consistent with the lower level of p38 MAPK activation (Fig. 4G) and shedding (Fig. 1E). The enhanced phosphorylation of endogenous TACE upon stimulation was abolished by SB203580 (Suppl. Fig. S3) or when cells were transfected with siRNA to p38α MAPK (Fig. 4F). Finally, the ability of p38α MAPK to associate with TACE and increased phosphorylation of TACE in response to anisomycin or IL-1β suggest that p38 MAPK interacts with TACE upon stimulation. Even though kinase-substrate interactions are notoriously difficult to detect by coimmunoprecipitation, we detected a low level interaction of endogenous p38 MAPK and TACE in T4-2 cells, in response to anisomycin or IL-1β; again the effect of anisomycin was stronger than that of IL-1β. These data show that TACE is a substrate for p38α MAP kinase, and that p38α MAPK phosphorylates TACE on Thr in response to anisomycin and IL-1β.
TACE phosphorylation at Thr735 is required for activation of shedding by p38 MAP kinase
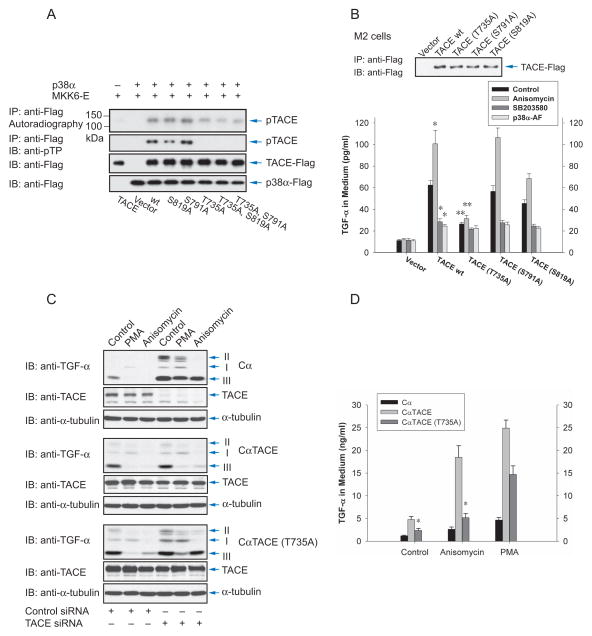
A previous study reported that Erk MAPK phosphorylates Thr735 in the TACE cytoplasmic domain (Diaz-Rodriguez et al., 2002). In addition, Ser819 is phosphorylated, and Ser791 is dephosphorylated, in response to growth factor stimulation (Fan et al., 2003). To assess whether these sites play roles in TACE phosphorylation by p38α MAPK, we expressed wild-type TACE, TACE mutants with either site replaced by alanine, and two double mutants in transfected cells. Immunopurified wild-type and mutant TACE were subjected to in vitro phosphorylation by p38α MAPK that was activated through coexpression with activated MKK6 (Fig. 5A, Suppl. Fig. S4). The 32P-labeling of TACE correlated with phosphoThr-Pro immunoreactivity. Mutation of Thr735 strongly decreased the TACE phosphorylation by p38α MAPK and abolished the immunoreactivity, while mutation of Ser819 mildly decreased its phosphorylation. In contrast, the Ser791 mutation conferred increased phosphorylation. Since only Pro-Gln-Thr735-Pro in the TACE cytoplasmic domain has Thr in a Thr-Pro dimer, and the Thr735Ala mutant is not phosphorylated by p38α MAP kinase, we conclude that the phosphoPro-Thr antibody recognizes phosphoThr735 and that activation of p38 MAPK results in TACE phosphorylation at Thr735. This is consistent with Pro-Gln-Thr735-Pro as a predicted p38 MAPK target sequence (scansite.mit.edu), and the lack of phosphorylation of Thr735Ala TACE in vitro using purified proteins (Fig. 4B), and in Cα cells in response to anisomycin (Fig. 4E), whereas wild-type TACE was phosphorylated.
Figure 5.
Phosphorylation of TACE at Thr735 is required for activation of shedding by p38 MAP kinase. (A), Wild-type or mutant TACE were expressed in Cα cells, purified using anti-Flag beads, incubated with 32P-γATP and p38α MAPK, which was separately co-expressed with MKK6-E and purified using anti-Flag beads. Phosphorylated TACE was visualized by autoradiography (upper panel), or immunoblotting with anti-pTP antibody (middle panel). Anti-Flag immunoblotting revealed the levels of TACE and p38α MAPK. (B), M2 cells, transfected to express TGF-α and low level wild-type or mutant TACE, were treated with anisomycin or SB203580, or additionally transfected to express p38α-AF. Ectodomain shedding was evaluated by measuring TGF-α in the medium. TACE was visualized by anti-Flag immunoblotting of proteins precipitated with anti-Flag M2 beads (upper panel). *, P < 0.01, compared with wild-type TACE control. **, P < 0.01, compared with wild-type TACE or wild-type TACE with anisomycin. (C) and (D), Cα cells and Cα cells expressing human wild-type or T735A TACE were transfected with control or TACE siRNA to silence endogenous TACE expression. Ectodomain shedding was induced with PMA or anisomycin, and evaluated by immunoblotting for transmembrane TGF-α (C) or measuring TGF-α in the medium (D). TACE was visualized by immunoblotting; siRNA-mediated silencing of endogenous TACE could only be revealed in Cα cells that do not express human TACE. *, P < 0.01, compared with cells expressing wild-type TACE.
To examine the role of TACE phosphorylation in response to p38 MAPK activation, we co-expressed each TACE mutant individually with TGF-α in M2 cells that lack active TACE expression, and measured ectodomain shedding by the amount of TGF-α released into the medium (Fig. 5B). Without TACE coexpression, very little TGF-α was detected in the medium, and no increase was observed upon p38 MAPK activation. TACE expression enhanced the level of TGF-α in the medium, consistent with a basal shedding that is apparent when TACE is overexpressed. The TGF-α release increased in response to anisomycin, and decreased when the cells were treated with SB203580 or expressed the dominant negative AF mutant of p38α MAPK, suggesting a role of p38α MAPK in the basal shedding activity. The Thr735Ala mutant of TACE had a much lower level of basal shedding, similar to the level when p38 MAPK was inhibited, and its activity was only marginally enhanced in response to anisomycin. The Ser819Ala mutation conferred a lower overall activity when compared to wild-type TACE, whereas TACE Ser791Ala had an activity profile similar to that of wild-type TACE.
We also generated derivatives of Cα cells expressing wild-type or mutant TACE at levels comparable to endogenous TACE (Fig. 5C). Because of the endogenous TACE activity, anisomycin and PMA stimulated shedding in cells expressing wild-type or Thr735Ala TACE, as apparent by the decrease in the TGF-α forms II and III (Fig. 5C). Silencing endogenous TACE expression with siRNA that did not affect the transfected human TACE expression blocked TGF-α shedding (Fig. 5C). In CαTACE cells that express wild-type human TACE, the endogenous TACE silencing did not affect the TGF-α shedding by human TACE in response to Erk or p38 MAPK activation. In contrast, the shedding in response to anisomycin was severely impaired in Cα cells expressing Thr735Ala TACE. This was most apparent from the minimal decrease in TGF-α form III, which was comparable to the level in Cα cells that had endogenous TACE expression silenced and did not express human TACE. The shedding in response to PMA was much less affected by the Thr735Ala mutation.
The induction of TGF-α shedding in these cells was confirmed by ELISA for TGF-α in the medium (Fig. 5D). When silencing endogenous TACE expression, the strong increase of soluble TGF-α in response to anisomycin was only observed when wild-type human TACE was expressed, whereas its Thr735Ala mutant conferred a limited response to p38 MAPK activation. TGF-α shedding in response to Erk MAPK signaling in these cells was largely unaffected by the Thr735Ala mutation (Fig. 5D).
Together, these data illustrate that Thr735 phosphorylation is required for TACE activation by p38 MAP kinase, but not by the Erk MAPK pathway.
p38 MAP kinase signaling increases the presentation of TACE at the cell surface
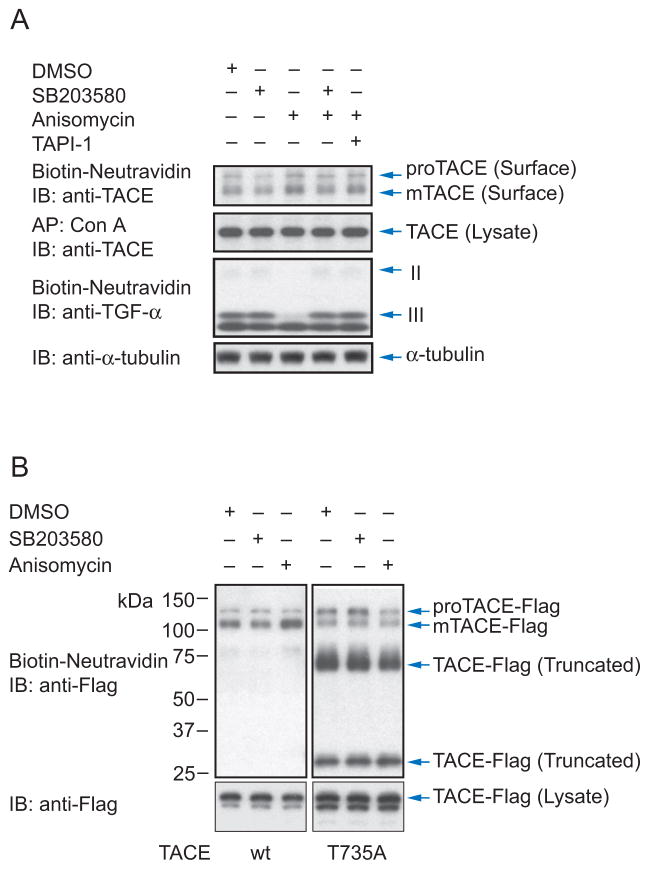
The molecular mechanism of TACE activation has largely remained elusive, but been suggested to affect the secretory transport (Soond et al., 2005). We therefore examined the effect of p38 MAPK activation on the cell surface levels of TACE in Cα cells (Fig. 6A). As reported (Schlondorff et al., 2000), TACE appeared as two forms at the cell surface: proTACE that has an N-terminal prodomain and corresponds to the bulk of TACE that resides inside cells, and active TACE (mTACE) that has this prodomain removed (Fig. 6A). Since cells in culture have basal p38 MAPK signaling, we compared cells in which p38 MAPK signaling was inhibited by SB203580 with cells treated with anisomycin to activate p38 MAPK. p38 MAPK activation resulted in an increased cell surface level of mTACE that was also seen in the presence of the TACE inhibitor TAPI-1 and therefore does not depend on its catalytic activity. The increase in cell surface presentation of mTACE in response to anisomycin correlated with ectodomain shedding of TGF-α, as apparent from the strong decrease in cell surface levels of TGF-α forms II and III (Fig. 6A).
Figure 6.
Activation of p38 MAP kinase enhances TACE levels at the cell surface. (A), Cα cells were treated with anisomycin, without or with SB203580 or TAPI-1. Pro-TACE and active TACE (mTACE) were detected at the cell surface by immunoblotting cell surface biotinylated proteins with anti-TACE antibody, and total glycosylated TACE in the cell lysate was shown by immunoblotting concanavalin A-adsorbed proteins. Cell surface biotinylation detected TGF-α forms II and III at the cell surface, and correlated the increased cell surface mTACE level with increased shedding of TGF-α. (B), Cell surface TACE, detected as in (A), in Cα cells stably expressing wild-type or T735A TACE, and treated with SB203580 or anisomycin. Pro-TACE and mTACE were detected, and truncated TACE forms were at the surface of cells expressing T735A but not wild-type TACE. The lower panel shows the total TACE in cell lysates.
To examine whether TACE phosphorylation at Thr735 by p38 MAPK affects the cell surface presentation of TACE, we stably transfected Cα cells to express a C-terminally Flag-tagged form of human wild-type or T735A mutant TACE (Fig. 6B). Anisomycin induced increased cell surface levels of wild-type mTACE, when compared with cells in the presence of SB203580 (Fig. 6B), consistent with what we observed for endogenous TACE (Fig. 6A). However, anisomycin did not significantly affect the cell surface levels of T735A mTACE. Cells expressing T735A TACE also expressed two smaller Flag-tagged proteins at the cell surface (Fig. 6B). Their size and reactivity with antibody to the C-terminal Flag epitope suggest that they derive through proteolytic removal of the catalytic and disintegrin domains. This process is likely to occur primarily at the cell surface, since total TACE in the cell lysate showed only low levels of these fragments. We conclude that phosphorylation of Thr735 by p38 MAPK regulates the cell surface levels and processing of TACE.
p38 MAP kinase regulates cell proliferation by shedding of EGF receptor ligands by TACE
TACE expression is often increased in carcinomas, especially breast carcinomas, and has been implicated in EGFR-mediated cancer progression by TGF-α family growth factors (Borrell-Pages et al., 2003; Kenny and Bissell, 2007; Zhou et al., 2006). Since p38 MAPK activates TACE and induces the release of TGF-α, we studied the role of p38 MAPK in the proliferation of T4-2 breast carcinoma cells. Like many carcinomas, the proliferation and tumor formation of T4-2 cells depend in part on autocrine EGFR-dependent stimulation, resulting from ectodomain shedding of TGF-α and amphiregulin (Kenny and Bissell, 2007).
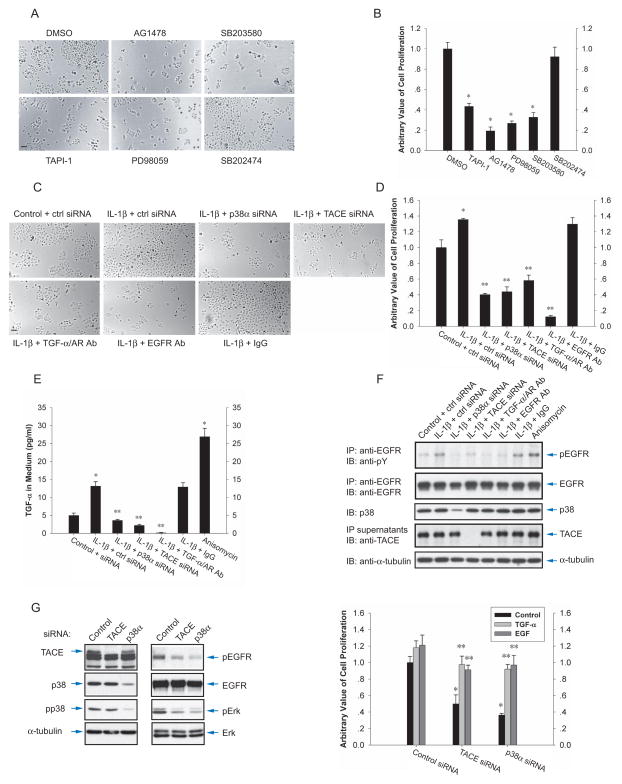
In cell culture, the proliferation of T4-2 cells was inhibited by TAPI-1, an inhibitor of metalloproteases including TACE, the EGFR inhibitor AG1478, or the MEK inhibitor PD98059, which blocks activation of the Erk MAPK pathway downstream from the EGFR (Fig. 7A, B). These results indicate that T4-2 cell proliferation depends on TACE activity and EGFR signaling, as reported (Kenny and Bissell, 2007). In addition, the proliferation was inhibited by SB203580, but not its negative control SB202474, indicating an important role of p38 MAPK.
Figure 7.
p38 MAP kinase signaling stimulates T4-2 cell proliferation by TACE-mediated shedding of EGF receptor ligands. (A) and (B), Effects of TAPI-1, the EGFR inhibitor AG1478, MEK inhibitor PD98059, SB203580, or negative control SB202474 on the proliferation of T4-2 cells. BrdU incorporation was measured by ELISA and expressed relative to untreated control. *, P < 0.01, compared with DMSO control. (C) and (D), Effect of IL-1β on proliferation of cells transfected with p38α MAPK, TACE or control siRNA, or treated with neutralizing antibodies to TGF-α and amphiregulin (AR), or the EGFR, or rabbit IgG. BrdU incorporation was measured by ELISA and expressed relative to untreated control. *, P < 0.01, compared with control. **, P < 0.001, compared with IL-1β-treated cells transfected with control siRNA, or treated with IgG. (E) and (F), T4-2 cells, transfected or not with p38α MAPK, TACE or control siRNA, were treated for 60 (E) or 30 min (F) with IL-1β or anisomycin, without or with neutralizing antibodies to TGF-α and amphiregulin (AR), or the EGFR, or rabbit IgG. TGF-α released in the medium was quantified by ELISA (E). In (F), anti-EGFR immunoprecipitates were blotted with anti-phosphoTyr (anti-PY) to show EGFR activation, or with anti-EGFR for EGFR expression. Endogenous p38 MAPK and TACE were visualized by immunoblotting of cell lysates or Con A-adsorbed proteins. *, P < 0.001, compared with control siRNA. **, P < 0.001, compared with IL-1β stimulation of cells transfected with control siRNA, or treated with IgG. (G), T4-2 cells were transfected with TACE, p38α MAPK or control siRNA. Immunoblotting of cell lysates showed downregulation of endogenous TACE or p38α MAPK expression in siRNA-transfected T4-2 cells. EGF receptor activation and expression are shown as in (F). Erk MAPK activation was shown by immunoblotting for phospho-Erk MAPK (pErk) and total Erk MAPK. The right panel shows the proliferation with or without 5 ng/ml TGF-α or EGF. *, P < 0.01, compared with untreated cells transfected with control siRNA. **, P < 0.01, compared with untreated cells transfected with TACE or p38α MAPK siRNA.
IL-1β enhanced the cell proliferation (Fig. 7C, D), which was inhibited by silencing p38α MAPK or TACE expression. Further, neutralizing antibodies to TGF-α and amphiregulin, or antibodies that prevent ligand binding to the EGFR, inhibited the basal and IL-1-induced proliferation (Fig. 7C, D). Like IL-1β, LPS also enhanced cell proliferation, which was inhibited by SB203580, as well as by TAPI-1 or the EGFR inhibitor (Suppl. Fig. S5). Long-term treatment of cultured cells with anisomycin was toxic and its effect was therefore not examined in these assays. Together, these results indicate that the activation level of p38 MAPK, which is enhanced in response to inflammatory mediators, is a key determinant of the proliferation of T4-2 cells, mediated at least in part by its effect on TACE activity, and acting through the EGFR.
We next studied whether p38 MAPK controls EGFR activation as a consequence of the release of soluble EGFR ligand by TACE. Although T4-2 cells express more amphiregulin than TGF-α (Kenny and Bissell, 2007), we elected to score the TGF-α release using an ELISA, considering that both ligands are released through cleavage by TACE (Sahin et al., 2004). Consistent with previous results (Fig. 1E), IL-1β induced the release of TGF-α, albeit to a lower extent than anisomycin (Fig. 7E). Silencing the expression of p38α MAPK or TACE repressed its release. LPS similarly induced TGF-α release, and the basal and LPS-induced TGF-α release were inhibited by SB203580 or TAPI-1 (Suppl. Fig. S5C).
We examined the autocrine EGFR activation under the same conditions of IL-1 treatment as in Fig. 7E. The EGFR activation, assessed by its level of Tyr phosphorylation (Fig. 7F), correlated with the secreted TGF-α levels (Fig. 7E). IL-1β or anisomycin induced EGFR activation, which was prevented by silencing the p38α MAPK or TACE expression. As expected, neutralizing TGF-α and amphiregulin antibodies or EGFR antibodies prevented the autocrine EGFR activation (Fig. 7F). Consistent with these results, LPS induced EGFR activation, and the p38 MAPK inhibitor SB203580 and TACE inhibitor TAPI-1 inhibited the basal and LPS-induced EGFR activation (Suppl. Fig. S5C, D). Whereas the EGFR kinase inhibitor AG1478 inhibited the autocrine EGFR activation rapidly, SB203580 and TAPI-1 required a longer time to do so (Suppl. Fig. S5C, D), as they act on the release of ligands that activate the EGF receptor rather than on the EGFR itself. These data suggest that the regulation of the ectodomain shedding by TACE through p38 MAPK signaling defines the EGFR activity.
To further define the role of p38α MAPK in EGFR activation, we silenced the TACE or p38α MAPK expression (Fig. 7G, left panel). As in Fig. 7F, silencing TACE or p38α MAPK expression decreased the basal EGFR activation, resulting in decreased Erk MAPK activation (Fig. 7G, middle panel), a downstream effect of EGFR signaling that regulates proliferation (Pages et al., 1993). These results illustrate that TACE and p38α MAPK regulate the activation of Erk MAPK by EGFR signaling.
We next compared the cell proliferation using BrdU uptake (Fig. 7G, right panel). The decrease in TACE or p38α MAPK expression inhibited cell proliferation with 50% and 36%, respectively, consistent with the effects of TAPI-1 or SB203580 on cell proliferation (Fig. 7B and Suppl. Fig. S5E). The stronger inhibition by interfering with p38α MAPK activity, compared with TACE inactivation, may suggest that p38 MAPK signaling involves other aspects of cell proliferation besides the activation of the EGFR through TACE-mediated EGFR ligand shedding. The inhibition of cell proliferation by interfering with p38α MAPK or TACE activity was largely rescued by adding EGFR ligands (Fig. 7G, right panel, Suppl. Fig. S5E).
Together, these results indicate that p38 MAPK activation, e.g. in response to inflammatory mediators or stress, critically regulates the proliferation of these cells. The induction of proliferation by p38 MAPK signaling results at least in part from the activation of TACE, and the release through ectodomain shedding of EGFR ligands and increased EGFR signaling.
DISCUSSION
Activation of shedding in response to p38 MAP kinase signaling is mediated by TACE
We have shown that inducers of p38 MAPK signaling induce ectodomain shedding, and that inhibition of p38 MAPK blocks shedding in response to these inducers. Our results complement reports that p38 MAPK inhibitors prevent shedding in different contexts (Fan and Derynck, 1999; Fischer et al., 2004; Takenobu et al., 2003). Thus, in addition to the Erk MAPK pathway, p38 MAPK activates ectodomain shedding. Among the p38 MAPKs, shedding was specifically activated by p38α MAPK, which has been linked to the activities of inflammatory cytokines (Dong et al., 2002; Ono and Han, 2000). Thus, siRNA specific for p38α MAPK prevented shedding in response to p38MAPK inducers, and no response by other p38 MAPKs was apparent.
TACE was shown to mediate shedding in response to Erk MAPK pathway activation. Which metalloprotease effects shedding in response to p38 MAPK activators has remained unclear, and it has been proposed that shedding in response to such inducers is mediated by other metalloprotease(s) (Diaz-Rodriguez et al., 2002; Hinkle et al., 2003; Takenobu et al., 2003). Our use of siRNA to silence TACE expression, and rescue experiments using wild-type or mutant TACE, show that TACE effects shedding in response to p38 MAPK activation. Further, introduction of wild-type TACE in M2 cells that do not express active TACE (Li and Fan, 2004) rescued ectodomain shedding in response to Erk or p38 MAPK pathway activation. We conclude that TACE is activated by either of these pathways that are in turn activated by diverse stimuli.
Mechanism of TACE activation by the p38 MAP kinase pathway
The activation of TACE-mediated ectodomain shedding by p38 MAPK was rapid and did not require new protein synthesis, suggesting that TACE activation results from a post-translational event. Although ROS was proposed to play roles in activation of p38 MAPK or TACE, the antioxidant MNTmPyP did not affect shedding in response to anisomycin.
Wild-type, but not kinase-defective, p38α MAPK associated with and phosphorylated TACE in response to stimuli that activate p38 MAPK, such as anisomycin and IL-1β. The phosphorylation site, Thr735, is located in a predicted p38 MAPK target sequence, i.e. Pro-Gln-Thr735-Pro, and its mutation prevented TACE phosphorylation by p38 MAPK, and activation of shedding in response to anisomycin or IL-1β. This finding directly links extracellular cues that activate the p38 MAPK pathway with ectodomain shedding. The targeting of a metalloprotease by p38 MAPK adds to our understanding of the role of p38 MAPK, which hitherto is known to primarily regulate transcription and translation (Ono and Han, 2000), and raises the possibility that p38 MAPK targets other ADAM metalloproteases.
The mechanism by which cell signaling activates TACE has remained elusive. It has been proposed that the TACE prodomain contains a cysteine switch involved in TACE activation, that TIMP3 acts as natural inhibitor of TACE, and that TACE maturation and localization are involved in TACE activation. Intriguingly, a phosphomimetic mutation of Thr735 in TACE affects the subcellular localization of TACE toward the secretory pathway (Soond et al., 2005). We now show that p38 MAPK activation enhances the cell surface presentation of active TACE, and that this increase correlates with activation of shedding. Replacing Thr735 with alanine that cannot be phosphorylated resulted in high levels of two truncated TACE forms at the cell surface, which are predicted to be non-functional. Possibly, Thr735 phosphorylation may regulate proteolysis of TACE at the cell surface, a possibility that needs further exploration.
Erk MAPK has been reported to phosphorylate TACE on Thr735 (Diaz-Rodriguez et al., 2002). However, mutation of Thr735 had little effect on the PMA-induced activation of TACE, which occurs through the Erk MAPK pathway. We have shown that growth factors induce Ser819 phosphorylation and Ser791 dephosphorylation. Ser819 phosphorylation depends on Erk MAPK pathway, whereas Ser791 dephosphorylation does not (Fan et al., 2003). We now show that Ser791 mutation, mimicking dephosphorylation, enhances TACE phosphorylation at Thr735 by p38 MAPK, and that mutation of Ser819 decreased Thr735 phosphorylation by p38 MAPK, suggesting a crosstalk between growth factor signaling and p38 MAPK activation of TACE.
Role of regulation of TACE by p38 MAP kinase in EGF receptor function and cancer
Increased autocrine EGFR activation by TGF-α ligands drives carcinoma progression (Hanahan and Weinberg, 2000). Consistent with the notion that soluble TGF-α is more effective than transmembrane TGF-α in carcinoma development (Blobel, 2005; Borrell-Pages et al., 2003), human cancers show increased TACE expression (Mochizuki and Okada, 2007). In addition, increased TACE-mediated release of TGF-α or amphiregulin enhances EGFR-mediated cell proliferation and transformation, as observed in breast carcinoma cells (Kenny and Bissell, 2007; Zhou et al., 2006). We therefore assume that the increased Erk MAPK activity, observed in many carcinomas (Sebolt-Leopold and Herrera, 2004), increases the TACE-mediated release of TGF-α ligands and, consequently, the EGFR activation.
Various studies suggest that the p38 MAPK pathway exerts tumor suppression by modulating aspects of cell behavior. However, inflammatory cytokines that activate p38 MAPK, e.g. IL-1 or TNF-α, have cancer promoting roles (Moore et al., 1999; Vidal-Vanaclocha et al., 2000), and p38 MAPK promotes the proliferation of cancer and immune cells (Dolado et al., 2007; Ono and Han, 2000). Thus, the overall role of p38 MAPK signaling in cancer is likely to depend on the tumor type and cancer stage. Our observations that TACE is activated by p38 MAPK now link inflammatory signals to increased EGFR signaling. Indeed, p38 MAPK activation, due to the inflammatory cytokine release, activates TACE resulting in the release of TGF-α family ligands. These in turn activate the EGFR, leading to increased Erk MAPK signaling and cell proliferation, possibly contributing to carcinoma progression. Further, p38α MAPK activity in autocrine EGFR-dependent cancer cells is required for maintaining self-sufficient growth, a property acquired in cancer progression (Hanahan and Weinberg, 2000). This scenario allows for crosstalk between Erk MAPK and p38 MAPK signaling that may not only contribute to carcinoma progression, but also be of relevance in inflammation that is not associated with cancer.
MATERIALS AND METHODS
Expression plasmids, reagents and antibodies
Plasmids encoding C-terminally Flag-tagged p38α or p38β MAPK, or their kinase-inactive forms p38α-AF and p38β-AF (Huang et al., 1997) or constitutively active MKK6-E (Jiang et al., 1996) were described. Plasmids encoding Flag- or Myc-tagged TACE were made by subcloning the TACE sequence into pRK5 (Graycar et al., 1989). Plasmids encoding the TACE mutants T735A, S791A, S819A, T735A-S791A, T735A-S819A were made by QuikChange site-directed mutagenesis (Stratagene) using pRK5-TACE-Flag. The expression plasmids for TGF-α or TACE E406A mutant have been described (Fan et al., 2003). Coding DNA sequences were verified by DNA sequencing.
PMA, anisomycin, IL-1β, TAPI-1, SB203580, SB202474, U0126, PD98059, JNK inhibitor II, AG1478, Bisindolylmaleimide I (BIM), brefeldin A, MnTMPyP, PTIO, L-NIL, EGF and TGF-α were from Calbiochem. Cycloheximide, LPS, and anti-Flag (M2) agarose were from Sigma. Protein-A Sepharose was from Amersham, ConA-Sepharose was from GE Healthcare, EZ-link Sulfo-NHS-LC-Biotin and Neutravidin beads were from Pierce. γ-32P ATP and 32P-orthophosphate were from Perkin Elmer.
Goat anti-TGF-α polyclonal antibody (R&D) was used for immunoprecipitation and neutralization, and the monoclonal anti-TGF-α ab-1 antibody (Calbiochem) was used for immunoblotting. The EGFR was immunoprecipitated or neutralized using EGFR antibody clone 528 (Calbiochem), its Tyr phosphorylation was detected by immunoblotting using anti-pY antibody (Zymed Laboratories), and EGFR levels were dtected by immunoblotting with anti-EGFR antibody (Santa Cruz Biotechnology). Anti-amphiregulin goat polyclonal antibody (R&D) was used for neutralization. Anti-Erk, anti-pErk, anti-pp38 MAPK, and anti-pTP antibodies were from Cell Signaling, rabbit anti-p38 MAPK polyclonal antibody and anti-p38α/β MAPK monoclonal antibody were from Santa Cruz Biotechnology. Rabbit anti-TACE polyclonal antibody was from QED Bioscience. Anti-α-tubulin, streptavidin-HRP, anti-Flag (M2), anti-FLAG (rabbit) and anti-Myc (9E10) antibodies were from Sigma.
Cell culture and transfections
CHO cells and derivative Cα (Fan and Derynck, 1999) and M2 cells (Li and Fan, 2004) were cultured in F-12 medium with 10% FBS. Cα cells were also supplemented with 200 μg/ml Geneticin (Roche) to maintain TGF-α expression. T4-2 cells were provided by Dr. M. Bissell (Lawrence Berkeley National Laboratory), and cultured as described (Kenny and Bissell, 2007). Hela cells expressing TGF-α (Imhof et al., 2008) were maintained in DMEM, supplemented with 10% FBS and 2 μg/ml puromycin. Transfections of CHO cells were done using Fugene 6 (Roche). Cα cells were stably transfected with plasmids for wild-type TACE or T735A TACE in the presence of pRK7-Hyg, allowing selection with 600 μg/ml hygromycin B (Roche). Colonies were obtained by serial dilution, and immunoblot analysis for TACE expression was performed using anti-Flag M2 antibody.
Ectodomain shedding assay
Cells at 80% confluence were starved overnight, washed and treated for 60 min with anisomycin (1 μM), LPS (2.5 μg/ml), IL-1β (60 ng/ml), UV light (60 mJ), PMA (25 nM), 20% FBS in serum-free medium, or medium containing 200 mM NaCl (osmotic shock). Effects of an inhibitor or antibody were evaluated by incubating the cells for 30 min prior to and during treatment. T4-2 cells were also incubated with 0.5 μg/ml anti-EGFR neutralizing antibody for 15 min before stimulation to prevent ligand binding to the EGFR. Inhibitors and neutralizing antibodies include the p38 MAPK inhibitor SB203580 (10 μM), its negative control SB202474 (10 μM), MEK1/2 inhibitor U0126 (10 μM), JNK inhibitor II (50 μM), protein kinase C inhibitor Bisindolylmaleimide I (BIM, 1 μM), metalloprotease inhibitor TAPI-1 (10 μM), EGFR kinase inhibitor AG1478 (1 μM), NO scavenger PTIO (100 μM), iNOS inhibitor L-NIL (50 μM), anti-oxidant MnTMPyP (50 μM), brefeldin A (10 μM), cycloheximide (10 μM), anti-TGF-α (1 μg/ml) and anti-amphiregulin (5 μg/ml) neutralizing antibodies, anti-EGFR neutralizing antibody (1 μg/ml), and rabbit IgG (6 μg/ml). After treatment, cells were lysed in MLB (20 mM Tris-Cl, 200 mM NaCl, 10 mM NaF, 1 mM NaV2O4, 1% NP-40, 20 mM β-glycerophosphate, 2 mM Lefabloc, 0.5 mM leupeptin, 1 ug/ml aprotinin, pH 7.5), and lysates were analyzed by 12.5% SDS-PAGE and immunoblotting with anti-TGF-α antibody to visualize transmembrane TGF-α. Released TGF-α was quantified by ELISA (Quantikine Immuno Assay kit; R&D Systems) of medium that was cooled on ice and then cleared at 5000 rpm for 10 min to remove debris.
Coimmunoprecipitations and western blotting
Cα cells were transfected with 0.2 μg plasmids for Flag-tagged p38α or p38β MAPK, or their mutants p38α-AF or p38β-AF, or Myc-tagged TACE. 48 h after transfection, cells were lysed using MLB, and lysates were subjected to immunoprecipitation using anti-Myc monoclonal antibody 9E10 and protein-A Sepharose beads for 2 h. The beads were washed with MLB, and immunoprecipitated proteins were analyzed by 4–20% SDS-PAGE and immunoblotting using anti-Flag antibody M2.
In Fig. 4F–G, endogenous TACE and p38 MAPK were co-immunoprecipitated from T4-2 cells that were incubated for 30 min with SB203580, or transfected with p38α MAPK siRNA or control siRNA for 48 h, and treated with IL-1β or anisomycin for 15 min. Cells were then lysed using MLB with 150 mM NaCl, and lysates were pre-incubated with rabbit IgG and Protein A Sepharose, and subjected to immunoprecipitation using anti-TACE polyclonal antibody (QED) and protein-A Sepharose for 2 h. Immunoprecipitated proteins were analyzed by 4–20% SDS-PAGE and immunoblotting using anti-p38α/β MAPK mIgG1 antibody. Immunoprecipitation supernatants were subjected to Con A Sepharose and the remaining TACE was visualized by immunoblotting using anti-TACE antibody, to ensure effective immunoprecipitation. Fractions of the cell lysates were subjected to SDS-PAGE and immunoblotting to control for protein expression.
Cell surface protein biotinylation
Cells were washed with ice-cold PBS, and incubated with EZ-link Sulfo-NHS-LC-Biotin (0.5 mg/ml in PBS) at 4°C for 60 min. Biotinylation reactions were stopped with glycine (100 mM in PBS), and cells were lysed in MLB. Cell lysates were incubated 2 h with Neutravidin beads (Pierce). The beads were washed with MLB, and the adsorbed proteins were analyzed by SDS-PAGE and blotting with Streptavidin (Sigma) or anti-TGF-α (ab-1), anti-TACE (QED), or anti-Flag antibody (Sigma).
RNA interference
siRNAs (Qiagen) to silence TACE expression targeted the Chinese hamster TACE mRNA sequence 5′-GAGGAUUUAAAGGUUAUGGAA-3′ (nucleotides 900–920; ref: AY313173Z) or the human TACE sequence 5′-AAGAAACAGAGUGCUAAUUUA-3 (Hs_ADAM17_8) (nucleotides 2642–2662; ref: NM_003183.4) in CHO or T4-2 cells, respectively. siRNA to silence p38α MAPK expression in CHO cells targeted the sequence 5′-UCGUGAAGUGUCAGAAGCUUA-3′ (Rn_Mapk14_4), derived from the CHO cell p38α cDNA (Ref: GU371300) that we generated by RT-PCR using primers for nested PCR. siRNA for human p38α MAPK targeted the sequence 5′-CUCAGUGAUACGUACAGCCAA-3′ (Hs_MAPK14_7) (nucleotides 1933–1953; ref: NM_001315). Control siRNA was used to control for possible nonspecific effects of RNA interference. Transfections of siRNA were done using Lipofectamine RNAiMAX (Invitrogen). Reverse transfection was used for Hela and T4-2 cells to reach optimal efficiency. Cells were used at 48 h after transfection.
TACE proteolytic activity assay
TACE activity was measured using the InnoZyme TACE activity kit (CalBiochem). T4-2 cells were seeded in a 12-well plate and treated for 1 h with anisomycin (1 μM), LPS (2.5 μg/ml), or IL-1β (60 ng/ml), without or with SB203580 (10 μM). Cells were lysed with MLB and a portion of cell lysates was subjected to the TACE activity assay. Briefly, TACE in cell lysates was captured by anti-TACE monoclonal antibody and its activity was measured using an internally quenched fluorescent substrate. Cleavage of amide bond of substrate released the fluorophore, resulting in increased fluorescence, which is directly related to the activity of TACE.
In vitro kinase assay
In vitro kinase assays used proteins generated by in vitro translation or in transfected Cα cells. Plasmids encoding MKK-6E, Flag-tagged p38α MAPK or Flag-tagged TACE were subjected to in vitro transcription from the T7 or SP6 promoter, and translation using the TNT Quick-Coupled Transcription/Translation kit (Promega). p38α MAPK, generated in vitro, was activated by incubation with in vitro translated MKK-6E in the presence of 20 μM ATP at 30°C for 30 min. To obtain proteins expressed in transfected cells, Cα cells were transfected with 0.2 μg of plasmid expressing p38α or p38β MAPK, or their mutants, MKK-6E, or wild-type or mutant TACE. TACE and p38α MAPK from in vitro translation or cell lysates were immunoprecipitated using anti-Flag M2 agarose beads and washed with MLB before in vitro kinase assay. In vitro kinase assays were done with γ-32P ATP (Perkin Elmer) in 20 mM HEPES, 10 mM MgCl2, 1 mM DTT, 50 mM NaCl for 20 min at 30°C, and analyzed by 4–20% SDS-PAGE, transferred to PVDF membrane and subjected to autoradiography or western blotting.
In vivo 32P-orthophosphate labeling
Cα, CαTACE and CαTACE(T735A) cells at confluence were washed with phosphate-free medium, and incubated in phosphate-free medium for 3 h, then in phosphate-free medium with 0.5 mCi/ml 32P-orthophosphate (Perkin Elmer) for 2 h. Anisomycin (1 μM) was added for the last 30 min, before cell lysis in MLB. Proteins, immunoprecipitated with anti-Flag agarose, were analyzed by 4–20% SDS-PAGE, transferred to PVDF membrane and analyzed by autoradiography or immunoblotting with anti-Flag antibody. Parallel cell lysates without isotope were analyzed by SDS-PAGE and immunoblotting with anti-α-tubulin antibody.
Cell proliferation assay
Cell proliferation was quantified by BrdU incorporation. T4-2 cells, in some cases 24 h after transfection with siRNA to TACE or p38α MAPK, or control siRNA, were seeded in a 48-well plate and treated with SB203580 (10 μM) or its negative control SB202474 (10 μM), TAPI-1 (10 μM), EGFR inhibitor AG1478 (1 μM), MEK inhibitor PD98059 (10 μM), LPS (2.5 μg/ml), or IL-1β (60 ng/ml), anti-TGF-α (1 μg/ml) and anti-amphiregulin (5 μg/ml), or anti-EGFR neutralizing antibody (1 μg/ml), or rabbit IgG (6 μg/ml) for 72 h. Cells were imaged using a LEICA DMI4000B microscope, and cells were incubated with BrdU for 3 h before ELISA. In the rescue analyses, EGF or TGF-α (5 ng/ml) was added. BrdU incorporation assays were done using the BrdU Cell Proliferation Assay kit (Calbiochem).
Supplementary Material
Acknowledgments
This research was sponsored by grants RO1-CA63101 and PO1-HL60231 (Project III) to R.D. We are grateful to J. Han and R. J. Davis for plasmids encoding p38 MAPK, MKK3 or MKK6, and M.J. Bissell for T4-2 cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beutler B, Krochin N, Milsark IW, Luedke C, Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986;232:977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Borrell-Pages M, Rojo F, Albanell J, Baselga J, Arribas J. TACE is required for the activation of the EGFR by TGF-α in tumors. EMBO J. 2003;22:1114–1124. doi: 10.1093/emboj/cdg111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringman TS, Lindquist PB, Derynck R. Different transforming growth factor-α species are derived from a glycosylated and palmitoylated transmembrane precursor. Cell. 1987;48:429–440. doi: 10.1016/0092-8674(87)90194-2. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- Diaz-Rodriguez E, Montero JC, Esparis-Ogando A, Yuste L, Pandiella A. Extracellular signal-regulated kinase phosphorylates tumor necrosis factor α-converting enzyme at threonine 735: a potential role in regulated shedding. Mol Biol Cell. 2002;13:2031–2044. doi: 10.1091/mbc.01-11-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolado I, Swat A, Ajenjo N, De Vita G, Cuadrado A, Nebreda AR. p38α MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell. 2007;11:191–205. doi: 10.1016/j.ccr.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- Fan H, Derynck R. Ectodomain shedding of TGF-α and other transmembrane proteins is induced by receptor tyrosine kinase activation and MAP kinase signaling cascades. EMBO J. 1999;18:6962–6972. doi: 10.1093/emboj/18.24.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Turck CW, Derynck R. Characterization of growth factor-induced serine phosphorylation of tumor necrosis factor-α converting enzyme and of an alternatively translated polypeptide. J Biol Chem. 2003;278:18617–18627. doi: 10.1074/jbc.M300331200. [DOI] [PubMed] [Google Scholar]
- Fischer OM, Hart S, Gschwind A, Prenzel N, Ullrich A. Oxidative and osmotic stress signaling in tumor cells is mediated by ADAM proteases and heparin-binding epidermal growth factor. Mol Cell Biol. 2004;24:5172–5183. doi: 10.1128/MCB.24.12.5172-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gechtman Z, Alonso JL, Raab G, Ingber DE, Klagsbrun M. The shedding of membrane-anchored heparin-binding epidermal-like growth factor is regulated by the Raf/mitogen-activated protein kinase cascade and by cell adhesion and spreading. J Biol Chem. 1999;274:28828–28835. doi: 10.1074/jbc.274.40.28828. [DOI] [PubMed] [Google Scholar]
- Graycar JL, Miller DA, Arrick BA, Lyons RM, Moses HL, Derynck R. Human transforming growth factor-β3: recombinant expression, purification, and biological activities in comparison with transforming growth factors-β1 and -β2. Mol Endocrinol. 1989;3:1977–1986. doi: 10.1210/mend-3-12-1977. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Han J, Sun P. The pathways to tumor suppression via route p38. Trends Biochem Sci. 2007;32:364–371. doi: 10.1016/j.tibs.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hinkle CL, Mohan MJ, Lin P, Yeung N, Rasmussen F, Milla ME, Moss ML. Multiple metalloproteinases process protransforming growth factor-α (proTGF-α) Biochemistry. 2003;42:2127–2136. doi: 10.1021/bi026709v. [DOI] [PubMed] [Google Scholar]
- Huang S, Jiang Y, Li Z, Nishida E, Mathias P, Lin S, Ulevitch RJ, Nemerow GR, Han J. Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity. 1997;6:739–749. doi: 10.1016/s1074-7613(00)80449-5. [DOI] [PubMed] [Google Scholar]
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- Imhof I, Gasper WJ, Derynck R. Association of tetraspanin CD9 with transmembrane TGFα confers alterations in cell-surface presentation of TGF{alpha} and cytoskeletal organization. J Cell Sci. 2008;121:2265–2274. doi: 10.1242/jcs.021717. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Chen C, Li Z, Guo W, Gegner JA, Lin S, Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38β) J Biol Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- Kenny PA, Bissell MJ. Targeting TACE-dependent EGFR ligand shedding in breast cancer. J Clin Invest. 2007;117:337–345. doi: 10.1172/JCI29518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Fan H. Loss of ectodomain shedding due to mutations in the metalloprotease and cysteine-rich/disintegrin domains of the tumor necrosis factor-α converting enzyme (TACE) J Biol Chem. 2004;279:27365–27375. doi: 10.1074/jbc.M401690200. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Okada Y. ADAMs in cancer cell proliferation and progression. Cancer Sci. 2007;98:621–628. doi: 10.1111/j.1349-7006.2007.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, et al. Mice deficient in tumor necrosis factor-α are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006;17:325–337. doi: 10.1016/j.cytogfr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Patel IR, Attur MG, Patel RN, Stuchin SA, Abagyan RA, Abramson SB, Amin AR. TNF-α convertase enzyme from human arthritis-affected cartilage: isolation of cDNA by differential display, expression of the active enzyme, and regulation of TNF-α. J Immunol. 1998;160:4570–4579. [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, Hartmann D, Saftig P, Blobel CP. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlondorff J, Becherer JD, Blobel CP. Intracellular maturation and localization of the tumour necrosis factor α convertase (TACE) Biochem J. 2000;347(Pt 1):131–138. [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 2003;17:7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- Soond SM, Everson B, Riches DW, Murphy G. ERK-mediated phosphorylation of Thr735 in TNFα-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci. 2005;118:2371–2380. doi: 10.1242/jcs.02357. [DOI] [PubMed] [Google Scholar]
- Takenobu H, Yamazaki A, Hirata M, Umata T, Mekada E. The stress- and inflammatory cytokine-induced ectodomain shedding of heparin-binding epidermal growth factor-like growth factor is mediated by p38 MAPK, distinct from the 12-O-tetradecanoylphorbol-13-acetate- and lysophosphatidic acid-induced signaling cascades. J Biol Chem. 2003;278:17255–17262. doi: 10.1074/jbc.M211835200. [DOI] [PubMed] [Google Scholar]
- Ventura JJ, Tenbaum S, Perdiguero E, Huth M, Guerra C, Barbacid M, Pasparakis M, Nebreda AR. p38α MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet. 2007;39:750–758. doi: 10.1038/ng2037. [DOI] [PubMed] [Google Scholar]
- Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, Fuentes AM, Anasagasti MJ, Martin J, Carrascal T, Walsh P, Reznikov LL, Kim SH, et al. IL-18 regulates IL-1β-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc Natl Acad Sci U S A. 2000;97:734–739. doi: 10.1073/pnas.97.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, Greene MI. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest. 2007;117:2051–2058. doi: 10.1172/JCI32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Thomas SM, Lui VW, Xi S, Siegfried JM, Fan H, Smithgall TE, Mills GB, Grandis JR. Phosphorylation of TNF-α converting enzyme by gastrin-releasing peptide induces amphiregulin release and EGF receptor activation. Proc Natl Acad Sci U S A. 2006;103:6901–6906. doi: 10.1073/pnas.0509719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Oliver P, Lancaster JJ, Schwarzenberger PO, Joshi MS, Cork J, Kolls JK. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. FASEB J. 2001;15:303–305. doi: 10.1096/fj.00-0371fje. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Peyton M, He B, Liu C, Girard L, Caudler E, Lo Y, Baribaud F, Mikami I, Reguart N, et al. Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in non-small cell lung cancer. Cancer Cell. 2006;10:39–50. doi: 10.1016/j.ccr.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.