See Mayes (doi:10.1093/brain/awu284) for a scientific commentary on this article.
The hippocampus is thought to support only conscious memory, while neocortex supports both conscious and unconscious memory. Duss et al. show that amnesic patients with damage to the hippocampal–anterior thalamic axis exhibit a diminished form of unconscious encoding and retrieval, suggesting that certain forms of unconscious memory are hippocampus-dependent.
Keywords: flexible, implicit, nondeclarative, relational, subliminal
Abstract
Textbooks divide between human memory systems based on consciousness. Hippocampus is thought to support only conscious encoding, while neocortex supports both conscious and unconscious encoding. We tested whether processing modes, not consciousness, divide between memory systems in three neuroimaging experiments with 11 amnesic patients (mean age = 45.55 years, standard deviation = 8.74, range = 23–60) and 11 matched healthy control subjects. Examined processing modes were single item versus relational encoding with only relational encoding hypothesized to depend on hippocampus. Participants encoded and later retrieved either single words or new relations between words. Consciousness of encoding was excluded by subliminal (invisible) word presentation. Amnesic patients and controls performed equally well on the single item task activating prefrontal cortex. But only the controls succeeded on the relational task activating the hippocampus, while amnesic patients failed as a group. Hence, unconscious relational encoding, but not unconscious single item encoding, depended on hippocampus. Yet, three patients performed normally on unconscious relational encoding in spite of amnesia capitalizing on spared hippocampal tissue and connections to language cortex. This pattern of results suggests that processing modes divide between memory systems, while consciousness divides between levels of function within a memory system.
Introduction
Memory is not a single faculty nor does it serve a single computational goal. Humans have several types of memory that are partially independent, operate within distinct brain circuits, and pursue separate computational goals (Tulving, 1985, 2002; Cohen and Eichenbaum, 1993; Squire and Zola, 1996; O'Reilly and Rudy, 2000; Moscovitch, 2008; Henke, 2010). Evidence for distinct memory systems came from studies in patients with amnesia due to hippocampal damage (Squire and Zola, 1996). Hippocampal patients exhibit impairments of episodic encoding and retrieval, which refers to the conscious encoding and retrieval of personally experienced events (Tulving, 2002). Nevertheless, hippocampal patients have preserved unconscious memory abilities, such as skill acquisition, priming for single items, and conditioning. These preserved memory abilities do not require consciousness of encoding and retrieval and are supported by extra-hippocampal brain regions. Because of this pattern of results, hippocampal processing became firmly associated with consciousness of encoding and retrieval (Squire and Zola, 1996; Tulving, 2002; Moscovitch, 2008).
The different types of memory divide not only on consciousness but also on processing modes (Cohen and Eichenbaum, 1993; Squire and Zola, 1996; O'Reilly and Rudy, 2000; Henke, 2010). The typical processing mode of the hippocampus is the rapid encoding of new and flexible associations between items within and across events (Cohen and Eichenbaum, 1993; O'Reilly and Rudy, 2000; Henke, 2010). Due to their flexible representation, which owes to the hippocampus, the reactivation of associations can be triggered by remotely related retrieval cues in situations that are different from the encoding situation. Inflexible memories, on the other hand, are thought to depend on the neocortex alone. Inflexible memories contain unitized (non-relational) information, whose reactivation is bound to retrieval cues that correspond closely to items present in the encoding situation. Items can be flexibly stored in the context of an event, if they are constituents of a network of interrelated items, or they can be stored out-of-context as isolated, distinct, non-relational and rather inflexible representations (Mayes et al., 2002; Giovanello et al., 2003; Kan et al., 2007). Flexible representations are thought to depend on the hippocampus and its connections with neocortex, while inflexible representations rely on neocortex alone, as exemplified in priming or recognition by familiarity.
Experimental designs often confounded processing modes (flexible relational versus rigid non-relational) with levels of consciousness, which made it difficult to pin down the variable that distinguishes between memory systems—consciousness or processing modes? Flexible relational encoding/retrieval was often assessed with tests of episodic memory that require consciousness of retrieval, while rigid non-relational encoding/retrieval was often assessed with priming tests that do not require consciousness of retrieval. To find out whether processing modes, i.e. flexible relational versus rigid non-relational, rather than consciousness, would distinguish between memory systems, we excluded consciousness of encoding/retrieval in this study by presenting all encoding material subliminally, i.e. invisibly. Using a stringent masking paradigm, we studied unconscious flexible relational versus unconscious rigid non-relational encoding in 11 patients with amnesia due to damage in the hippocampal-anterior thalamic axis and 11 matched control subjects (Table 1). To our knowledge, amnesic patients have not been tested before with subliminal protocols.
Table 1.
Basic data on amnesic patients
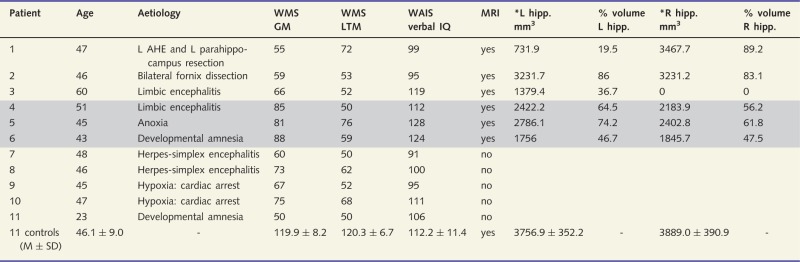 |
*Manually traced hippocampal volumes corrected for total intracranial volume by covariance.
% volume = relative to the controls' mean volume.
Well performing patients are grey-shaded.
AHE = amygdalohippocampectomy; L = left; R = right; WMS = Wechsler Memory Scale; WAIS = Wechsler Adult Intelligence Scale; GM = general memory; LTM = long-term memory; hipp = hippocampus; M = mean; SD = standard deviation.
Based on our notion (Henke, 2010) that processing modes divide between memory systems with flexible relational encoding depending on the hippocampus, we hypothesized that damage in the hippocampal-anterior thalamic axis would impede unconscious flexible relational but not rigid non-relational encoding and retrieval. According to traditional views (Squire and Zola, 1996; Tulving, 2002; Moscovitch, 2008), unconscious encoding of any kind of information should remain unaffected by damage in the hippocampal-anterior thalamic axis. Our second hypothesis postulates a dissociation between conscious and unconscious flexible relational encoding within the hippocampal memory system. Although we assume that flexible relational encoding always depends on the hippocampal memory system, our second hypothesis predicts that a certain amount of preserved hippocampal tissue and preserved hippocampal connections to neocortex may suffice unconscious but not conscious flexible relational encoding. We had hypothesized earlier (Henke, 2010) that unconscious relational encoding might be impaired following large but not small hippocampal lesions based on neuroimaging data showing that neural networks in the medial temporal lobe (and neocortex) tend to be more sparsely recruited during unconscious versus conscious relational encoding and retrieval (Henke et al., 2003a; Degonda et al., 2005; Reber et al., 2012, 2014). Accordingly, a larger level of functionality in the hippocampal-anterior thalamic axis and its connections to neocortex might be required for conscious versus unconscious relational encoding and retrieval.
To test these hypotheses, we collected both behavioural data and functional and structural brain data in patients and controls using MRI to estimate the location and extent of structural brain damage as well as the degree of preserved resting-state functional connectivity between hippocampus and neocortex (Fig. 1). Due to the neocortical deafferentiation secondary to hippocampal neuronal loss, we expected reduced functional connectivity between hippocampus and neocortex in amnesic patients. Any residual viable tissue in the hippocampus must dispose of preserved connections with neocortex to support encoding and retrieval. Our neuroimaging data confirmed medical diagnoses and revealed preserved hippocampal tissue and functional connectivity in certain amnesic patients. We performed also functional MRI during all memory tasks to image the task-underlying neural network in controls as well as residual hippocampal memory functions and functional compensation in amnesic patients. Unfortunately, 5 of 11 patients could not be examined with MRI because of ferrous implants or claustrophobia; but behavioural data were obtained in all patients.
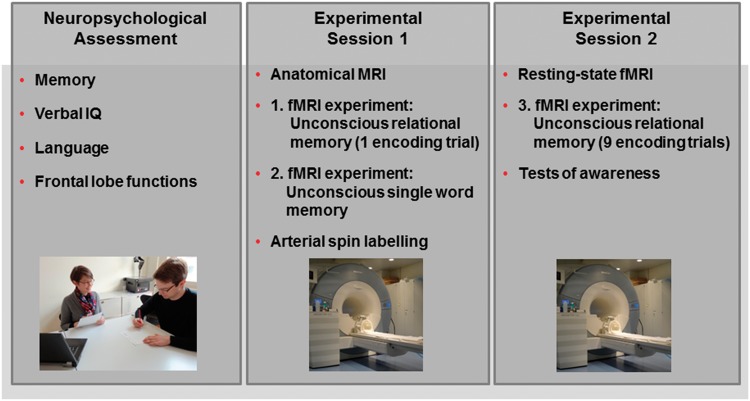
Figure 1.
Study overview. There were three sessions that took place on three half-days. In the first session, we examined patients and controls neuropsychologically. The second and third sessions were devoted to experimentation. Depending on a participant’s MRI compatibility, the two experimental sessions were conducted in the magnetic resonance scanner or in a behavioural laboratory. The second session started with anatomical MRI and ended with arterial spin labelling. In-between these scans, participants performed the functional MRI (fMRI) experiment on unconscious relational memory with one encoding trial and the functional MRI experiment on unconscious single word memory (semantic word priming). The third session started with the resting state functional MRI scan followed by the functional MRI experiment on unconscious relational memory with nine encoding trials. This session ended with the objective tests of stimulus awareness (Supplementary material).
Participants underwent three functional MRI experiments that tested the subliminal processing and later retrieval of single nouns (one experiment) and pairs of unrelated nouns (two experiments). All encoding words were presented subliminally with our established masking technique (Degonda et al., 2005; Reber and Henke, 2011; Reber et al., 2012, 2014). Because the masked encoding words were invisible, the later presented supraliminal test words could only invoke unconscious retrieval processes. Compared to controls, we expected patients to exhibit a diminished performance on the relational task that required the unconscious formation and later retrieval of semantic associations between two nouns. An equal performance was expected between groups regarding the processing of single nouns because a rigid, non-relational single word representation should suffice this task.
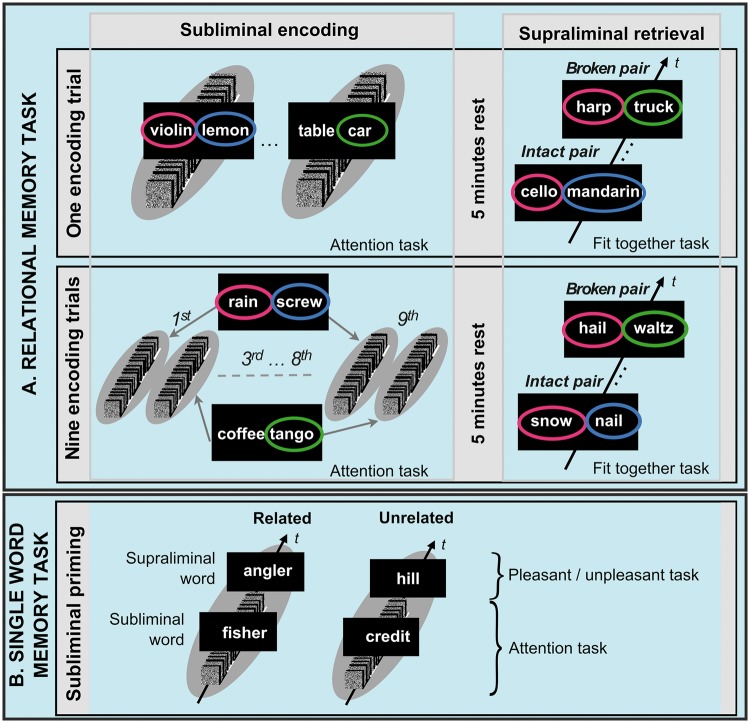
While paradigms were otherwise identical between the two relational experiments, one relational experiment contained a single encoding trial, while the other contained nine encoding trials (Fig. 2 and Supplementary Fig. 1). We presented pattern-masked pairs of unrelated nouns, such as ‘violin – lemon’, for semantic relational encoding. Following a 5 min study-test interval, we presented pairs of new test nouns supraliminally. Because test nouns were semantic neighbours of encoding nouns, the combination of the superordinate conceptual categories was retained from study to test in so-called intact test pairs (encoding ‘violin – lemon’→ retrieval ‘cello – mandarin’) but recombined in so-called broken test pairs. The use of distinct encoding and retrieval nouns required a flexible representation of associations. At test, participants were required to decide whether the two nouns in a retrieval pair would fit together semantically or not. Based on previous experiments (Reber and Henke, 2011; Reber et al., 2014), we expected a successful relational encoding and retrieval to reflect in a larger number of fit responses to intact versus broken test pairs.
Figure 2.
Designs of the three memory functional MRI experiments. (A) Relational memory. Participants performed two functional MRI experiments on unconscious relational encoding and retrieval. These experiments had similar designs but one experiment had a single encoding trial and the other nine. For subliminal encoding, we presented masked pairs of unrelated nouns, while participants performed an attention task. Encoding and retrieval were separated by 5 min of quiet rest. For unconscious retrieval, we presented participants with supraliminal (visible) word pairs. Nouns in these pairs were semantic neighbours of subliminal encoding nouns. Combinations of superordinate concepts and hence semantic relations were retained from study to test in ‘intact pairs’, while superordinate concepts were recombined and semantic relations broken in ‘broken pairs’. Because the study and test format differed in this task, flexibility of memory representation was required. The indirect retrieval task was to decide whether the two nouns in a pair fit together semantically or not. (B) Single word memory. One trial comprised the subliminal (masked) presentation of a noun that was immediately followed by a supraliminal (visible) noun. Subliminal and supraliminal nouns were either related semantically or unrelated. Participants engaged in an attention task during subliminal presentations and gave subjective pleasant-unpleasant judgments to supraliminal nouns. Due to the repetition of superordinate concepts (but not stimuli), semantic priming can be expected in the ‘related’ condition. t = time.
To test the rigid and non-relational encoding and retrieval of single nouns, we used a subliminal semantic priming task (Fig. 2). Semantic priming is a form of memory thought to rely on prefrontal and temporal neocortices, but not hippocampus (Henson, 2003). The semantic priming task required participants to process subliminal nouns and to judge whether subsequently presented supraliminal (visible) nouns were subjectively pleasant or unpleasant in meaning. A supraliminal noun was either preceded by a semantically related (fisher → angler) or unrelated (credit → hill) subliminal noun. Semantic priming was expected to reflect in judgement reaction times, which should distinguish between test nouns that were related versus unrelated to subliminal encoding nouns. Following all functional MRI experiments, participants took objective tests of stimulus awareness (Supplementary Fig. 2), which confirmed that all subliminal material was presented outside participants’ conscious awareness (Supplementary material).
Materials and methods
Participants
We examined 11 right-handed patients [mean age = 45.55 years, standard deviation (SD) = 8.74, range = 23–60] with amnesia due to encephalitis, hypoxia/anoxia, developmental amnesia, amygdalohippocampectomy, and bilateral fornix dissection (Table 1). We also examined 11 right-handed healthy control participants that were matched to individual patients regarding gender, age, and education. All participants (patients and controls) underwent a full neuropsychological examination before experimentation (Supplementary Table 1). Participants’ performance on tests of verbal and visual short- and long-term memory was assessed using Wechsler Memory Scale–Revised. Controls outperformed patients on tests of long-term memory. Compared to their IQ, patients’ memory performance was reduced on average by 2.55 SD (min. reduction: 1.80 SD; max. reduction: 3.37 SD), which indicates severe anterograde amnesia. Patients’ verbal IQ and their lexical-semantic access were normative and equal to that of controls. Although scores on tests of figural and semantic fluency were significantly better in controls than patients, the patients’ scores were still average to low-average with respect to norm values. Patients performed statistically equal to controls on tests of short-term memory, phonemic fluency, interference control (Victoria Stroop), working speed, mental flexibility (Trail Making), and concept finding and shifting (Kramer).
Six of 11 patients fulfilled inclusion criteria for MRI and underwent structural MRI, arterial spin labelling, and functional MRI. The other five patients were MRI-incompatible because of ferrous implants or claustrophobia. Patients, who were not eligible to MRI, performed the memory experiments behaviourally. Although 6 of 11 patients underwent MRI measurements, the memory functional MRI data of only three patients (‘well performing’ patients, see the ‘Results’ section) met quality criteria for analyses. In the resting state scan, where no task was given and patients could just rest, all patients yielded high-quality data.
All participants were reimbursed for travel expenses and insured during their participation in the study. All participants gave semi-informed written consent. They were naïve of subliminal presentations because they were misinformed that the experiments were about attention (task given during subliminal presentations) and language (indirect retrieval task). At the end of the study, participants were debriefed. This study was approved by the local ethics committee.
Three experiments on unconscious encoding and retrieval
Participants either lay in the magnetic resonance scanner with their heads fixated by foam cushions or—if MRI-incompatible—sat in a behavioural laboratory with their heads positioned on a chin rest. The MRI chamber and behavioural laboratory were completely darkened. A digital light processing (DLP BenQ) projector projected the stimuli on a white screen with a refresh rate of 60 Hz. We used the software Presentation® (http://www.neurobs.com) for stimulus presentations. Subliminal presentations were highly accurate and synchronized to the vertical refresh of the video signal. The stimulated visual field spanned 10° (height) × 13° (width). Participants’ responses were recorded with an MRI-compatible response pad and with a standard computer mouse in the behavioural laboratory.
Subliminal stimulus presentation
One subliminal encoding trial covered a 6 s time-window and included 12 masked presentations of the same word or word pair (W) (Degonda et al., 2005). Each word or word pair was flashed for 17 ms flanked by visual noise masks (M), which were presented for 183 ms. Noise masks consisted of random patterns of black and white pixels (800 × 600). A white central fixation cross or a horizontal or vertical bar (F) appeared for 233 ms on a black background at a rate of once per second, i.e. six times during one subliminal encoding trial. Once in these six presentations and at a random location in the order, the fixation cross was exchanged by a horizontal or vertical line segment. Participants were instructed to fixate on the fixation cross and to indicate the occurrence of a horizontal (left button) and vertical line segment (right button) by a right-hand key press. This attention task ensured that participants focused gaze on the middle of the screen and remained attentive throughout subliminal stimulations. Words in pairs were projected to the left and right visual half-fields. Single words were projected to the right visual half-field to be initially processed by the left cerebral hemisphere. All participants were right-handed.
Unconscious relational encoding and retrieval
Design and procedure
We performed two relational memory experiments (Fig. 2 and Supplementary Fig. 1) using functional MRI. One experiment was designed with a single encoding trial and the other experiment with nine encoding trials to find out whether amnesic patients would profit from repeated encoding. Each of the two relational memory experiments entailed three runs. Each run contained an encoding part and a retrieval part.
The encoding part encompassed the presentation of 16 subliminal word pairs in the experimental condition and 16 subliminal pairs of consonant strings in the baseline condition. There were 32 pairs of consonant strings in the baseline condition of the encoding part of the experiment with 9-fold encoding (Supplementary Fig. 1). Trials in both experiments were blocked by condition. Condition blocks alternated regularly. One encoding block encompassed two trials, each lasting 6 s. In the experiment with nine subliminal encoding trials, word pairs were not repeated immediately but interleaved by other word pairs and pairs of consonant strings (Supplementary Fig. 1). Before experimentation, participants viewed all encoding words supraliminally to ensure word understanding and allow for familiarization with words (but not word-word combinations).
The retrieval part of each run entailed four conditions. Stimuli of all four conditions were presented supraliminally (visibly). Each condition comprised 16 stimuli, which were presented in blocks of four. Stimuli were presented for 3.5 s with an interstimulus interval of 1 s. Words presented in the intact pair condition and the broken pair condition were semantic neighbours of encoding words. Words in intact pairs retained the categorical semantic relation acquired during subliminal encoding (e.g. encoding: violin – lemon; retrieval: cello – mandarin). In broken pairs, encoded categorical relations were broken by the recombination of concepts (e.g. encoding: violin – lemon … . table – car; retrieval: harp – truck). Both the intact pair and the broken pair condition allowed for semantic word priming. The two other conditions of the retrieval part were baseline conditions; one baseline condition contained pairs of new words and the other pairs of consonant strings. Retrieval was instructed indirectly: participants were required to decide whether the two words in a retrieval pair fit together semantically or not. Because words in a pair were never close semantically, participants were asked to relax their response criterion to aim at an equal number of ‘fit’ and ‘don’t fit’ responses. In baseline blocks where pairs of consonant strings were presented, participants were instructed to decide whether the two strings fit together visually, like two pieces of art. Both instructions fostered a holistic processing of stimuli.
Participants took practice trials before each experiment. In addition, we gave supraliminal relational encoding and retrieval trials to allow participants establishing a task set (Reber and Henke, 2011).
Stimuli
We used 96 triplets of nouns for each of the two relational memory experiments. Nouns in a triplet were members of the same superordinate category (e.g. string instruments: violin, cello, harp; citrus fruit: lemon, mandarin, grapefruit; vehicles: car, bus, truck). The first nouns from the first two triplets were combined for an encoding word pair (e.g. violin – lemon); the second nouns from the first two triplets were combined for an intact retrieval pair (e.g. cello – mandarin); and the third noun of the first triplet plus the third noun of the third triplet was selected to form a broken retrieval pair (e.g. harp – truck) (Fig. 2). Retrieval words were balanced between the intact and broken pair condition. Hence, across participants each retrieval word pair was equally often presented as an intact and as a broken retrieval word pair. Accordingly, encoding word pairs had to be re-arranged for half of participants.
For the baseline condition of the encoding part, we created 48 pairs of consonant strings to be used in the experiment with one encoding trial and 96 pairs of consonant strings to be used in the experiment with nine encoding trials (Supplementary Fig. 1). Each consonant string consisted of the random combination of eight consonants (e.g. bgtmkhwn – nsdplkmr). For the two baseline conditions of the retrieval part of the two relational experiments, we formed two sets of 48 new word pairs and two sets of 48 additional pairs of consonant strings.
Unconscious single word encoding and retrieval
Design and procedure
We conducted a subliminal semantic priming experiment using functional MRI to examine unconscious single word encoding and retrieval. A trial consisted of the subliminal presentation of a noun or a consonant string. This was immediately followed by the supraliminal (3.5 s) presentation of a noun. An inter-stimulus interval of 1 s separated trials. The experiment comprised three conditions. A subliminal noun and the following supraliminal noun were semantically related in the ‘related’ condition and unrelated in the ‘unrelated’ condition. In the baseline condition, we presented a subliminal consonant string that left the semantic processing of the following supraliminal noun uninfluenced. Participants’ task was to decide whether a supraliminal noun was subjectively pleasant or unpleasant in meaning. Before experimentation, we gave practice trials with separate stimuli. We asked participants to establish a classification criterion during the practice trials that allowed them to give an equal number of pleasant and unpleasant responses. Participants were instructed to retain their classification criterion during the experiment. Each experimental condition embraced 56 trials. Each trial represented a block in this functional MRI experiment. To avoid tiring, we split the large number of trials into two functional MRI time-series. We counterbalanced the order of condition blocks across participants.
Stimuli
We selected 224 pairs of concrete German nouns that were strongly related semantically (OpenThesaurus, a database-driven website). Then, we built two stimulus lists for counterbalancing that contained the same 112 supraliminal nouns. These supraliminal nouns were combined with either a semantically related or a semantically unrelated subliminal noun. A supraliminal noun was combined with a semantically related subliminal noun in one list and with a semantically unrelated subliminal noun in the other list. Each of the two stimulus lists was given to half of participants. Another set of 112 nouns was compiled for use in the baseline condition. Every baseline noun was combined with a randomly generated consonant string (e.g. rgplksdn) that was presented as a subliminal prime. Half of nouns were assigned to one list and the other half of nouns to another list. Each list was given to half of participants.
Behavioural data
Reaction times acquired during the retrieval part of each of the three memory experiments were z-transformed per participant. Trials with z-values deviating more than two standard deviations from the individual mean were excluded. In the subliminal semantic priming experiment, the dependent variable was mean reaction time per condition (related, unrelated, baseline). In the two relational memory experiments, the dependent variable was percentage of given fit responses per condition (intact pair, broken pair, new pair, pair of consonant strings).
Neuroimaging
All MRI data were acquired with one single Siemens MAGNETOM Verio whole body magnetic resonance system (3 Tesla, Siemens) equipped with a standard 12-channel head coil.
Structural MRI
We used a 3D T1-weighted magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence for manual hippocampal volumetry and voxel-based morphometry. Imaging parameters were repetition time = 7.92 ms, echo time = 2.48 ms, flip angle θ = 16°, 176 sagittal slices, original voxel size = 1 mm3, 256 × 224 matrix, no interslice gaps.
Voxel-based morphometry
Structural brain images were processed using the VBM8 toolbox (v433) provided by Christian Gaser (http://dbm.neuro.uni-jena.de/vbm.html). This toolbox is based on statistical parametric mapping (SPM8) software (Welcome Department of Imaging Neuroscience Group, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Structural brain images were bias-corrected, segmented into grey and white matter and CSF, and then normalized to a template in MNI space using linear and non-linear parameters. The template is provided by the IXI-database (http://www.brain-development.org/) and is derived from 550 healthy control participants. Normalized grey matter segments were non-linearly modulated; i.e. they were multiplied by the Jacobian determinant of the non-linear deformations derived from the normalization matrix. Hence, we accounted for the fact that some brain regions were expanded and contracted during spatial normalization but still corrected for individual brain size. Finally, the modulated grey matter segments were smoothed with a Gaussian kernel of 8 mm full-width at half-maximum.
Some of our amnesic patients had no grey matter or very low grey matter volumes in temporal or thalamic regions. Therefore, we used the optimal threshold masking approach proposed by Ridgway et al. (2009) to ensure that brain regions with zero or low grey matter volumes were also considered for statistical analysis. Ridgway provided a freely available toolbox (http://www.cs.ucl.ac.uk/staff/g.ridgway/masking/) to calculate an average image of participants’ grey matter segments to create a binary mask that was maximally correlated with the average grey matter image (Ridgway et al., 2009). Statistical analysis was performed with SPM8. We compared maps of regional grey matter volumes between amnesic patients and controls with a voxel-wise independent sample t-test. Equality of variance was assumed, when comparing the map of regional grey matter volumes of an individual amnesic patient to the controls’ maps (http://www.mrc-cbu.cam.ac.uk/people/rik.henson/personal/Henson_Singlecase_06.pdf). Statistical maps were thresholded at a P = 0.001 (uncorrected for multiple comparisons) with a cluster extent of k = 20 voxels. We used thresholds uncorrected for multiple comparisons to test for differences in grey matter volumes between controls and patients because we needed to avoid a type II error or a false negative result. It should be noted that voxel-based morphometry is commonly used to reveal subtle differences in grey matter volume within the normal range (no brain lesions) that may originate from genetic or training differences. Hence, potential differences in grey matter volumes between patients and controls need not reflect neuropathology.
Manual hippocampal volumetry
Manual tracing of hippocampus was performed on the raw DICOM images of patients and controls (n = 17) using the software PMOD V2.7 (PMOD Technologies Ltd, http://www.pmod.com). The structural magnetic resonance images had a voxel size of 1 mm3. Two raters delineated the border of the hippocampus manually on each coronal 1 mm slice. Anatomical criteria were based on Konrad et al. (2009). Left and right hippocampal volumes were highly correlated between raters (rleft = 0.897, rright = 0.929) and did not significantly differ in size [volumeleft: t(16) = 1.002, P = 0.331; volumeright: t(16) = 1.110, P = 0.283]. Rater-averaged left and right hippocampal volumes were corrected for total intracranial volume using the covariance approach (Free et al., 1995; Buckner et al., 2004). Total intracranial volume was computed based on participants’ structural images using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/).
Memory functional MRI
The three memory functional MRI experiments were performed using a single-shot echo-planar blood oxygen level-dependent sequence. We acquired 45 slices in interleaved order parallel to the AC-PC axis covering the whole brain. The measured spatial resolution was 2 × 2 × 2 mm3 with a 1 mm inter slice gap (repetition time = 3 s, echo time = 20 ms, flip angle θ = 90°, 96 × 96 matrix). Participants’ press responses were recorded with a Lumina LP-400 response pad for functional MRI (Cedrus). We used SPM8 for data preprocessing and analysis. Data were slice-time corrected, spatially realigned, and co-registered to participants’ structural reference images. For normalization to the standard MNI space, we applied the deformation fields of participants’ structural MRI images to their functional MRI images. These structural image files were created in the process of voxel-based morphometry, when the tissue segments were normalized to the MNI template. Functional MRI data were then normalized to MNI space and smoothed with a Gaussian kernel of 6 mm full-width at half-maximum.
Three first-level models were estimated using the data of the controls: one model for the encoding part of the two relational memory experiments, a further model for the retrieval part of the two relational memory experiments, and a third model for the experiment on subliminal semantic word priming. The three models included regressors that were created by convolving a canonical haemodynamic response function and its temporal and spatial derivatives with the box-car functions of the on- and off-sets of the experimental and baseline conditions. The three models also included the six movement regressors that were estimated during spatial realignment. First level contrast images of control participants were analysed by means of random-effects ANOVAs including participants’ mean global activation levels as covariate. For subliminal relational encoding, we computed first-level contrast images of relational word encoding versus encoding pairs of consonant strings (baseline). For unconscious relational retrieval, we computed first-level contrast images comparing brain activity in response to intact word pairs, broken word pairs, new word pairs (baseline condition) and pairs of consonant strings (second baseline condition). First-level contrasts for subliminal semantic word priming compared brain activity to pairs of related words with brain activity to unrelated words. Because one functional imaging block comprised the presentation of a subliminal word and its corresponding supraliminal word, the contrast images contained both encoding- and retrieval-related brain activity. However, since the processing of subliminal words must have been equal between the ‘related’ and ‘unrelated’ condition, subliminal word encoding was subtracted out leaving signal differences due to the processing of the supraliminal retrieval word.
The functional MRI data of the three amnesic patients were analysed in the same way, however using fixed-effects models due to the small sample size. We report t-test contrasts. Height threshold was P = 0.001 (uncorrected for multiple comparisons) and the cluster extent 10 consecutive voxels. A height threshold of P = 0.005 (uncorrected for multiple comparisons) and cluster extent of 5 was applied to the medial temporal lobe because it was our region of interest. The reason why we did not correct for multiple comparisons is that functional MRI signals associated with subliminal stimulus processing are sparse and weak throughout the brain because the processing is carried out by small neural assemblies with neurons exhibiting only short and variable spiking activity (during ∼30 ms) in response to subliminal stimuli (Rolls and Tovee, 1994; Kovács et al., 1995; Rolls et al., 1999).
Resting state functional MRI
The resting state scan took 6 min. We acquired 240 functional images using the following parameters: repetition time = 1.5 s, echo time = 30 ms, flip angle = 90°, 64 × 64 matrix, in-plane resolution = 4 × 4 mm2, slice thickness = 4 mm, inter-slice gap = 1 mm. Hence, voxel size was 4 × 4 × 5 mm3. Participants were instructed to close their eyes in order to relax without falling asleep and to let their thoughts come and go. The preprocessing of the data was identical to the preprocessing of the memory functional MRI data except for smoothing, which was done with a 10 mm3 full-width at half-maximum Gaussian kernel. Independent component analysis was performed with the data of each individual participant using the GIFT toolbox (http://icatb.sourceforge.net/). The number of components to be extracted was estimated by the minimum description length criteria (Li et al., 2007) as implemented in the GIFT toolbox. We identified the component that was most prominent in the remaining tissue of the hippocampus in a fully automated data-driven manner. A subject-specific template image was derived from a bilateral hippocampal region of interest image (Tzourio-Mazoyer et al., 2002). To exclude damaged hippocampal tissue in patients, we masked the hippocampal region of interest image with the grey matter mask, which was created from each person’s map of regional grey matter volumes, which we had gained during the preprocessing stages of voxel-based morphometry. We correlated the z-images of the extracted components with the hippocampal template image per person. The component of interest was the component that correlated the strongest with this template. The selected component was masked again by each person’s grey matter mask. Images were thresholded at z-values greater than 1.5 and lower than −1.5.
Excluding the impact of brain damage on functional MRI signal
Memory functional MRI data of only three patients passed the quality check and hence underwent statistical analyses. Hippocampal and thalamic volumes were merely reduced in these patients and reductions were visually imperceptible. Hence, there were no apparent lesion spaces filled with CSF. Memory-related hippocampal blood oxygen level-dependent responses did not overlap with areas of reduced tissue volume as assessed by voxel-based morphometry in each patient.
For the independent component analysis of the resting-state functional MRI data, individual masks excluded damaged tissue in patients. Based on each patient’s grey matter volumes, which were gained at preprocessing stages of voxel-based morphometry, we created individual hippocampal templates that included only viable hippocampal tissue. These templates were used to identify the component that was most prominent in the residual hippocampal tissue. The selected component was then masked by each patient’s grey matter mask of reduced tissue volume.
Results
Unconscious relational encoding and retrieval
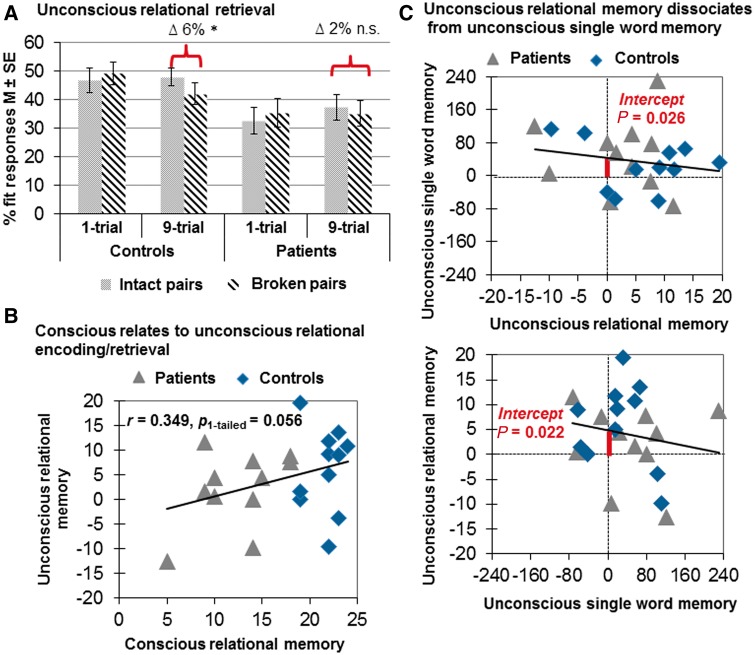
We computed a repeated-measures analysis of variance (ANOVA) with the within-subjects factors Retrieval Condition (intact versus broken retrieval word pairs) and Encoding Intensity (one versus nine encoding trials). The between-subjects factor was Group (controls versus patients). The dependent variable was percentage of fit responses. The ANOVA revealed a significant effect of Group [F(1, 20) = 4.831, P = 0.040, = 0.194] with patients giving generally less fit responses than controls because of a stringent response criterion (patients, M ± SE = 35.1 ± 3.6%; controls, 46.6 ± 3.6%). More importantly, the ANOVA yielded a significant two-way interaction of Retrieval Condition × Encoding Intensity [F(1, 20) = 4.675, P = 0.043, = 0.189]. Participants gave more fit responses to intact (42.7 ± 2.9%) than broken pairs (38.6 ± 3.0%) after having gone through nine instead of just one encoding trial (intact pairs, 39.7 ± 3.5%; broken pairs, 42.3 ± 3.5%). Post hoc tests clarified that this interaction was driven by controls (Fig. 3A). Following nine encoding trials, controls gave on average 6.0 ± 2.5% more fit responses to intact (48.0 ± 3.2%) than broken pairs (42.0 ± 3.8%); this difference was >0 [t(10) = 2.369, P = 0.039, r2 = 0.359]. In contrast, amnesic patients gave only 2.2 ± 2.3% more fit responses to intact (37.4 ± 4.5%) than broken pairs (35.2 ± 4.5%); this difference was not >0 [t(10) = 0.956, P = 0.362]. Hence, only controls established and retrieved new semantic relations between words. The ANOVA yielded no further significant results.
Figure 3.
Behavioural results. (A) Unconscious relational retrieval. Displayed is the percentage of fit responses given to intact and broken word pairs at test. Controls (n = 11), but not amnesic patients (n = 11), gave significantly more fit responses to intact than broken word pairs; they needed nine encoding trials for this result. *P < 0.05. (B) Conscious and unconscious verbal relational encoding and retrieval were marginally correlated, which is consistent with the view that the hippocampal memory system serves both conscious and unconscious encoding/retrieval. This was true for the entire sample consisting of controls and patients (n = 22; r = 0.349, Pone-tailed = 0.056) and for the patients alone (n = 11; r = 0.442, Pone-tailed = 0.087). (C) Unconscious relational memory dissociates from unconscious single word memory. We tested for the stochastic independence of unconscious single word memory (Δ mean RTs in ms to related versus unrelated supraliminal words) and unconscious relational memory (Δ % of fit responses to intact versus broken pairs following 9-trial encoding) by computing two regression analyses using each type of retrieval performance to predict the other. Single word memory was not correlated with relational memory (all participants, n = 22, r = −0.176, P = 0.432). Intercepts in both regression analyses were significant, which indicates that performance on each type of memory was still significantly above chance if performance on the other type of memory was at chance level. n.s. = not significant. RT = reaction time.
Although the difference in performance between patients and controls suggests a significant three-way interaction of Retrieval Condition, Encoding Intensity, and Group, this interaction was not significant [F(1, 20) = 0.308, P = 0.585, = 0.015]. The reason for this lack of significance was the good performance of the ad hoc sample of patients (Table 1, Patients 4–6), who were MRI-compatible and yielded motion-artefact-free MRI images. This was the group of patients whose memory functional MRI data were analysed and reported in this article. These patients are hereafter referred to as ‘well performing’ patients. They gave on average 8.0 ± 0.4% more fit responses to intact (42.7 ± 6.0%) than broken pairs (34.7 ± 6.2%); this difference was >0 [t(2) = 20.184, P = 0.002, r2 = 0.995]. The other eight patients formed an ad hoc group, of which we cannot report memory functional MRI data, because these patients were MRI-incompatible or yielded motion-distorted memory functional MRI images. These patients are hereafter referred to as ‘poorly performing’ patients. Poorly performing patients could not differentiate between intact (35.4 ± 5.9%) and broken pairs (35.5 ± 6.0%) [Δ − 0.01 ± 2.8%; t(7) = −0.004, P = 0.997].
Neuropsychological profiles differed between the group of poorly performing patients and the group of well performing patients (Supplementary Table 2). The well performing patients yielded better scores than the poorly performing patients on tests of verbal immediate memory; i.e. on the verbal memory index of Wechsler Memory Scale–Revised (WMS-R), t(9) = 3.917, P = 0.004, r2 = 0.630 [subtests Verbal Paired Associates I and Logical Memory I: both t(9) ≥ 2.761, both P = 0.022, both r2 ≥ 0.459]. The well performing patients yielded also better scores than the poorly performing patients on a test of verbal intelligence, namely Wechsler Adult Intelligence Scale–Revised (WAIS-R) [t(9) = 3.114, P = 0.012, r2 = 0.519], and on the Boston Naming Test (BNT), t(7) = 3.055, P ≤ 0.018, r2 = 0.571 (df corrected because equality of variances not assumed). However, the well performing patients’ long-term memory score (WMS-R long-term memory index) was equal to the poorly performing patients’ score [t(9) = 0.640, P = 0.538], which confirms the diagnosis of severe amnesia. The well performing patients’ WMS-R indexes of immediate (general) and long-term memory (delayed recall) were far below the controls’ [both t(12) ≥ 4.346, P ≤ 0.001, r2 ≥ 0.611]. Hence, compared to poorly performing patients, the well performing patients exhibited better abilities in verbal immediate memory (verbal memory index of WMS-R), semantic memory (BNT, WAIS-R), and verbal intelligence (WAIS-R) suggesting superior functions in left temporal and prefrontal cortices.
The Verbal Paired Associates subtest of the WMS-R requires the conscious relational encoding and retrieval of words and hence matches the computational demands of our word pair task that assesses unconscious relational encoding and retrieval. As mentioned, the well performing patients yielded better scores than the poorly performing patients on the immediate recall part of the Verbal Paired Associates subtest [well performing patients: M (SD) = 16.67 (2.31); poorly performing patients: M (SD) = 10.75 (3.37); t(9) = 2.761, P = 0.022, r2 = 0.459]. Nevertheless, the well performing patients still scored lower than the controls on this test [controls: M (SD) = 21.64 (1.80); t(12) = 4.021, P = 0.002, r2 = 0.574]. As expected, conscious and unconscious verbal associative encoding and retrieval were marginally correlated. This was true for the entire sample consisting of controls and patients (n = 22; r = 0.349, Pone-tailed = 0.056; Fig. 3B) and for the patients alone (n = 11; r = 0.442, Pone-tailed = 0.087). In fact, patients were driving this correlation; controls exhibited little variance due to ceiling performance on conscious associative encoding/retrieval. These results conform to the view that the hippocampal memory system serves both conscious and unconscious encoding/retrieval.
Furthermore, the entire sample’s scores of unconscious relational encoding/retrieval correlated also with scores of verbal intelligence (WAIS, verbal IQ) (r = 0.407, Ptwo-tailed P = 0.060, r2 = 0.166) and with scores on the Boston Naming Test (r = 0.663, Ptwo-tailed = 0.002, r2 = 0.440). When only the patients’ data were included in these correlations, solely the correlation with the scores on the Boston Naming Test reached significance (Ptwo-tailed = 0.873, P = 0.0004, r2 = 0.762). None of these scores (Verbal Paired Associates subtest of the WMS-R, WAIS verbal IQ, Boston Naming Test, unconscious relational encoding/retrieval) correlated with unconscious single item encoding/retrieval [controls and patients (n = 22): all r ≤ 0.296, all P ≥ 0.181; patients (n = 11): all r ≤ 0.391, all P ≥ 0.234].
In conclusion, amnesic patients as a group failed on unconscious relational encoding and retrieval. Nevertheless, the ad hoc subgroup of well performing patients exhibited intact unconscious relational encoding and retrieval. The following sections clarify the neural mechanisms underlying the well performing patients’ residual encoding and retrieval capacity.
Hippocampal and neocortical activity supports unconscious relational encoding
Although 6 of 11 patients fulfilled inclusion criteria for MRI, the memory functional MRI data of only three patients—namely, the three well performing patients—fulfilled quality criteria for statistical analyses, i.e. no image artefacts. The resting state functional MRI data were relatively artefact-free in all six patients because patients were not engaged in tasks and could just lay still.
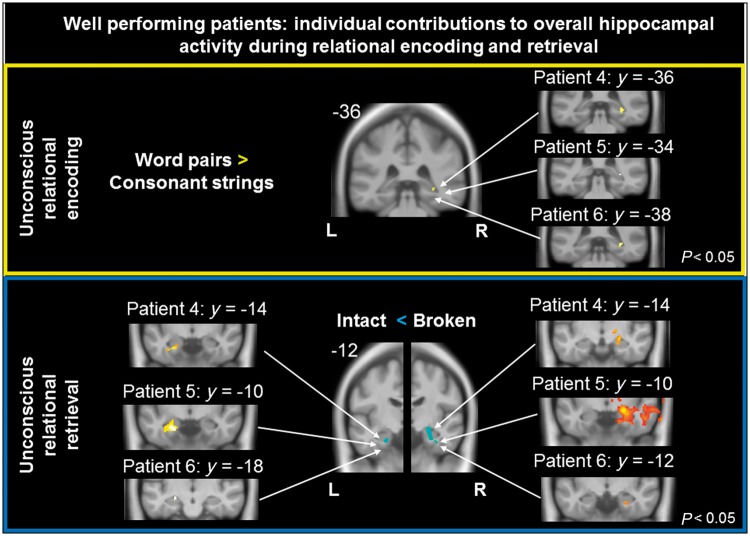
The three well performing patients’ brain activity was compared between relational word encoding (first encoding trial) and the processing of pairs of consonant strings (baseline) using a fixed-effects model suited for small sample sizes. The 11 controls’ first-level contrast images were analysed using a random-effects ANOVA. T-test contrasts showed increased neuronal activity to subliminal word pairs in bilateral hippocampus of controls and in the right hippocampus of patients. Each individual patient exhibited enhanced signal in this right hippocampal region (Fig. 4). That this hippocampal region actually comprises grey matter to support encoding was confirmed in each patient by voxel-based morphometry. Both patients and controls exhibited additional signal enhancement to relational word encoding in bilateral parahippocampal gyrus and areas mediating semantic processing (Patterson et al., 2007; Binder et al., 2009) in the left hemisphere, namely the superior, middle and inferior frontal gyrus, superior and middle temporal gyrus, and cingulate gyrus.
Figure 4.
Brain activity associated with unconscious relational encoding and retrieval. The three well performing patients (Patients 4, 5 and 6) exhibited hippocampal activity changes during unconscious relational encoding and retrieval. Each and every patient contributed to the displayed group effects in hippocampus. For this analysis, we thresholded each patient’s individual contrasts with P = 0.05, uncorrected. Functional MRI data are depicted on coronal slices; the corresponding MNI y-coordinates are indicated.
Hippocampal and neocortical activity supports unconscious relational retrieval
Hippocampal activity distinguished between intact and broken retrieval word pairs in both controls and in the three well performing patients following nine encoding trials (Fig. 4). Interestingly, this signal difference went into opposite directions between patients and controls. Controls increased signal to intact versus broken word pairs in bilateral hippocampus and right thalamus, while patients increased signal to broken versus intact word pairs in bilateral hippocampus and parahippocampal gyrus. We assume that controls focused primarily on detecting preserved semantic relations between words (match detection), while patients focused on the semantic mismatch of words in broken pairs (Kumaran and Maguire, 2007). Both match and mismatch detection operate in the hippocampus, presumably relying on distinct hippocampal subfields, namely CA3 and CA1, respectively (Chen et al., 2011; Duncan et al., 2012). Each individual patient exhibited signal changes in those left and right hippocampal areas that reached significance in group statistics (Fig. 4). Each patient disposed of grey matter in these activated zones.
Controls exhibited further activity increases in response to intact versus broken pairs in bilateral medial prefrontal cortices and the anterior cingulate. These areas support relational reasoning and the retrieval and encoding of schema congruent new information (Krawczyk, 2012; van Kesteren et al., 2012). Hence, medial prefrontal cortex may have mediated the reactivation of subliminally encoded semantic relations (schemas) and the detection of analogous semantic relations in intact retrieval word pairs (van Kesteren et al., 2012). Controls increased activity in response to broken versus intact pairs within left inferior and right middle frontal gyrus, bilateral superior temporal gyri, right parahippocampal gyrus including uncus, and left posterior hippocampus. This left-sided hippocampal deactivation (reverse contrast) was 2.4 cm behind the left-sided activation found in controls (reported above). The controls’ right parahippocampal signal increase to broken pairs conforms to the patients’ bilateral parahippocampal signal increase to broken pairs. These parahippocampal activations in response to recombined concepts, i.e. new combinations of concepts, were located in the rhinal cortex. Together with the hippocampal signal increases displayed by both groups in response to broken versus intact pairs, these rhinal signal increases might underlie the spontaneous relational encoding of the rearranged words in broken pairs. Patients exhibited further activity increases to broken versus intact pairs in left lateral prefrontal cortex, bilateral temporal lobe, angular gyrus, superior parietal lobule, precuneus, bilateral precuneus, and several occipital areas.
Structural differences between patients and controls: voxel-based morphometry
We compared maps of regional grey matter volumes between amnesic patients and controls as well as between well performing patients and poorly performing patients using voxel-wise independent sample t-tests. The voxel-based morphometry analysis revealed areas of larger grey matter volume in controls versus the six magnetic resonance-scanned patients, but not reversed (Table 2). Controls exhibited extended areas of larger grey matter volume in the left hippocampus, left parahippocampal gyrus, left amygdala, bilateral thalamus, left putamen and pallidum as well as left lingual/fusiform gyrus and left middle/inferior temporal gyrus. Hence, besides the expected medial temporal and diencephalic regions, there were areas of reduced neocortical volume in patients versus controls within the left lingual/fusiform gyrus and left middle/inferior temporal gyrus. The inspection of each patient’s voxel-based morphometry contrast (versus controls) revealed no neocortical overlap of reduced tissue volume between all of the six patients. At the contrary, the obtained statistical differences in neocortical volume were driven by certain patients. The reduced volume in the left lingual/fusiform gyrus was due to two well performing patients (Patients 5 and 6; see Table 1) and two poorly performing patients (Patients 1 and 3). The reduced volume in the left middle/inferior temporal gyrus was due to one well performing patient (Patient 6). Consequently, volume reductions in these neocortical regions were not associated with poor unconscious relational encoding/retrieval. This conclusion was corroborated by the direct comparison of grey matter volumes between well performing patients and poorly performing patients. This comparison yielded no significant result indicating that well performing patients exhibited no area of increased tissue volume, not even in the hippocampus. The lack of a hippocampal difference between patient groups owed to the fact that Patient 2 of the group of poorly performing patients had a bilateral fornix dissection but relatively preserved hippocampal volumes. The fornix patients’ large hippocampal volumes compensated for the other poor performers’ small residual hippocampal volumes eliminating the effect of larger hippocampal volumes in well versus poorly performing patients. The reversed contrast revealed that well performing patients had smaller tissue volumes than poorly performing patients in the right posterior cingulate gyrus, right cuneus, left middle occipital gyrus, and cerebellum. Given that the well performing patients excelled in unconscious relational encoding/retrieval, these areas of reduced volume were probably not relevant to relational encoding/retrieval. Next, we report the resting-state functional connectivity of the hippocampus with the rest of the brain in patients and controls.
Table 2.
Differences in grey matter volume (voxel-based morphometry)
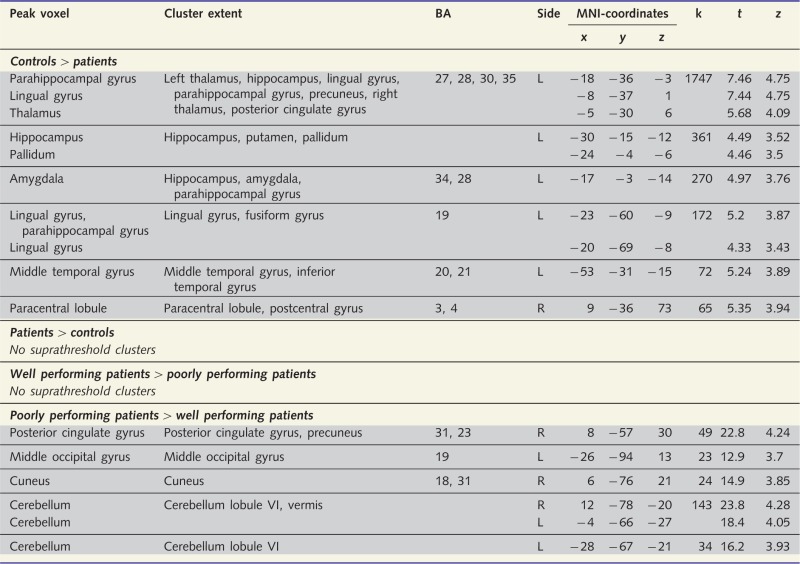 |
Height threshold P = 0.001; extent threshold = 20 voxels.
Local maxima are >8 mm apart. MNI-coordinates stand for the peak voxel within the cluster of voxels exhibiting differential probabilistic grey matter volume.
k = extent of significant cluster (number of voxels); BA = Brodmann area; L = left; R = right.
Hippocampal functional connectivity is associated with memory performance
Although voxel-based morphometry analyses revealed spared hippocampal tissue in several patients, this tissue needs intact functional connections to lateral temporal cortex, which harbours lexical semantic information (Patterson et al., 2007), to enable semantic relational word encoding. We performed a resting-state functional MRI connectivity analysis using independent component analysis, which is data-driven and model-free. We looked at hippocampal functional connectivity with the rest of the brain in controls, in the group of well performing patients, and in those three patients of the group of poorly performing patients that were MRI-compatible (Fig. 5). The controls’ left and right hippocampus was functionally connected with each other as well as with bilateral parahippocampal gyri, amygdalae, and anterior temporal lobes. Remnant hippocampus in well performing patients was similarly connected but this functional connectivity was weaker. Remnant hippocampus in the three poorly performing patients lacked functional connections to lateral temporal cortices. Hence, some access—even though reduced—to lexical-semantic storage sites (Patterson et al., 2007) seems necessary for unconscious encoding and retrieval of semantic word-word relations.
Figure 5.
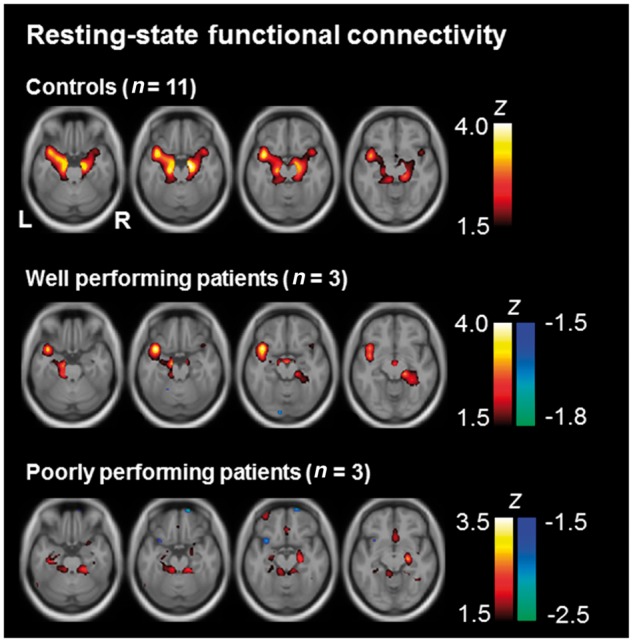
Resting state functional connectivity. Results of the independent component analyses performed on the resting state functional MRI data are depicted in axial slices at MNI z = −24 to −12 in steps of 4 mm. Independent component analyses were performed separately for each participant. The resulting images reflect the degree to which the component that is most prominent in the hippocampus contributes to the signal in other parts of the brain. Z-transformed images were averaged across groups of interest (controls, n = 11; well performing patients, n = 3; MRI-compatible subgroup of the group of poorly performing patients, n = 3).
Intact semantic word priming in patients and controls
For semantic word priming (Fig. 2), participants processed a subliminal noun and then made a pleasant/unpleasant decision on a subsequently presented supraliminal (visible) noun. Supraliminal nouns were either preceded by a semantically related (fisher → angler) or unrelated (credit → hill) subliminal noun, or by a string of consonants (no meaning; baseline condition). The dependent variable was reaction time. We computed a repeated measures ANOVA with the between-subjects factor Group (controls, n = 11; well performing patients, n = 3; poorly performing patients, n = 8) and the within-subjects factor Semantic Relatedness (related versus unrelated). The ANOVA yielded a main effect of Semantic Relatedness [F(1,19) = 7.990, P = 0.011, = 0.296], which reflects successful semantic priming. There was neither a main effect of Group [F(2,19) = 0.389, P = 0.683, = 0.039] nor an interaction of Semantic Relatedness with Group [F(2,19) = 1.254, P = 0.308, = 0.117]. Post hoc tests confirmed equal priming between groups (all t ≤ 1.555, all P ≥ 0.146). Reactions to related supraliminal words took 1689 ± 68 ms (M ± SE) and to unrelated words 1638 ± 91 ms.
Semantic word priming is mediated by prefrontal cortex in patients and controls
We compared the functional MRI signal to related, unrelated, and baseline supraliminal words (targets) using a random-effects one-way ANOVA in controls and fixed-effects pairwise comparisons in the group of well performing patients. Both the group of controls and the group of patients enhanced activity in response to related versus unrelated supraliminal words within left inferior, middle, and superior frontal gyri and the anterior cingulate. Hence, the semantic proximity of supraliminal words to previously flashed subliminal words increased computational demands in prefrontal language areas (Henson, 2003; Segaert et al., 2013). Particularly the left inferior frontal gyrus is known to subserve the detection of semantic relatedness between words. Only the controls exhibited repetition suppression of the functional MRI signal when processing related supraliminal words within temporal and parietal areas. This repetition suppression may not have been essential to task performance because patients manifested no repetition suppression but performed equally well.
Relational retrieval and single word retrieval are uncorrelated
We tested for the stochastic independence of unconscious relational and single word encoding/retrieval by computing two regression analyses. We used the experimental measures of word priming and relational retrieval to predict each other. The intercept of the regression reveals whether performance on the predicted task (y-axis) is greater than zero if performance on the predictor task (x-axis) is at zero (Greenwald et al., 1995) (Fig. 3C). Word priming was not correlated with relational retrieval (all participants, n = 22, r = −0.176, P = 0.432). Intercepts in both regression analyses were significant [predicted: word priming, y-axis intercept = 43.23, SE = 17.93, t(20) = 2.411, P = 0.026, r2 = 0.225] [predicted: relational retrieval, y-axis intercept = 4.81, SE = 1.90, t(20) = 2.474, P = 0.022, r2 = 0.234].
Discussion
We performed three neuroimaging experiments in amnesic patients and controls to find out whether processing modes rather than consciousness would divide between memory systems, namely hippocampal versus neocortical processing. Examined processing modes were flexible relational and rigid non-relational (single item) encoding and retrieval. We hypothesized that only relational encoding would depend on the hippocampal memory system and would therefore be affected in amnesic patients. Consciousness of encoding and retrieval was excluded by the subliminal (invisible) presentation of all encoding material. Unconscious encoding provided for a fair comparison of memory performance between amnesic patients and controls because it eliminated the confounding effect of conscious memory. Subliminal encoding also provided for a powerful demonstration of unconscious memory processing. The addition of functional MRI to behavioural testing allowed for determination of the memory system(s) that supported residual memory functions in amnesic patients. Functional MRI investigations in amnesic patients are rare and mostly include one single patient (Maguire et al., 2001, 2005; Caulo et al., 2005) because of several MRI contraindications—a problem that also affected our study.
Our patients and controls performed equally well on unconscious single word encoding and retrieval. But the relational task separated the two groups. While controls succeeded on unconscious relational encoding and retrieval activating their hippocampus, amnesic patients failed as a group. This is the first demonstration, to our knowledge, that unconscious encoding in the human depends on the hippocampal-anterior thalamic axis and its connections to neocortex. An ad hoc sample of amnesic patients succeeded on unconscious relational encoding capitalizing on residual hippocampal tissue and functional connections to the lateral temporal lobes. Although these well performing patients showed intact unconscious relational encoding, their conscious relational encoding was severely impaired.
Importantly, voxel-based morphometry revealed that our patients exhibited no damage in an overlapping neocortical region that could be the seat of unconscious relational encoding and retrieval. Although group statistics revealed smaller volumes in the left lingual and fusiform gyri as well as the left middle and inferior temporal gyri in patients versus controls, these reductions were due to few patients, who came from both the group of poorly and the group of well performing patients. Hence, volume reductions in these regions were not associated with a poor performance on unconscious relational encoding and retrieval. Moreover, the group of well performing patients exhibited no area of increased neocortical tissue volume relative to the group of poorly performing patients suggesting that poor task performance was not associated with neocortical damage. We therefore assume that damage in the hippocampal-anterior thalamic axis and its disconnection from the neocortex had caused the patients’ performance deficits on conscious and unconscious relational encoding and retrieval.
We draw the following conclusions that we discuss below: (i) separate memory systems mediate relational versus single item memory; (ii) the hippocampal memory system operates with and without consciousness; and (iii) is necessary for unconscious relational encoding and retrieval; and (iv) conscious versus unconscious memory formation may require a greater level of function within the hippocampal-neocortical network.
In the realm of conscious stimulus processing both at the time of encoding and retrieval, there is good evidence in support of a dissociation of memory systems regarding relational versus single item processing in amnesic patients (Mayes et al., 2002; Giovanello et al., 2003; Kan et al., 2007). These studies controlled for differences in memory load and difficulty between tasks. Still, retrieval performance of hippocampal patients was only impaired on tests of relational memory. Brain activation studies in healthy participants point to the same direction reporting hippocampal activity increases during association formation versus single item encoding or retrieval (Henke, 2010). The current results in amnesic patients extend the relational/single item dissociation to the realm of unconscious encoding and retrieval processes.
But not all forms of associative memory are flexible and hippocampal-dependent and not all forms of single item memory are inflexible and hippocampal-independent. In the following, we define the concept of ‘flexibility’ more precisely and explain why our word pair task requires a larger degree of flexibility of memory representation than our single word task. Flexible memory representations can be activated through many, even remotely related retrieval cues in situations that have little or no overlap with the encoding situation. On the other hand, inflexible, unitized memories can only be reactivated by a cue that was present at encoding such as ‘face …’ for the word Facebook; the cue ‘nose …’ would not trigger reactivation. Flexible memories can be reactivated intrinsically, i.e. even without any external retrieval cue, through self-cueing. In this process, one can deliberately choose to reactivate all facets of a memory or only certain facets. Each facet of a flexible memory representation is both related to other facets of the same representation and to facets of other memory representations. Hence, the flexibility of a memory reflects in (i) diverse access options; (ii) selective reactivation options; and (iii) relational binding. Accordingly, not all associative memories are flexible (some are fused like Facebook) and not all single item memories are inflexible because some are relationally integrated into a context as part of an episodic memory. The design of our single word task does not require a flexible representation for successful performance because a retrieval cue is provided, which is a semantic neighbour of the encoding word. Hence, semantic priming, which is inflexible, suffices task requirements. An additional relational integration of the encoding word into the encoding context is unnecessary. The subliminal prime word ‘fisher’ may activate this word’s concept node in the temporal neocortex with activation spreading over to the concept node ‘angler’ that gets primed, or the activation of the concept node ‘angler’ at test may trigger the reactivation of its primed semantic neighbour ‘fisher’ through spreading activation. The results in amnesic patients confirm that a purely neocortical route suffices normal task performance on the single word task. Yet, an inflexible memory representation does not suffice normal performance on our word pair task because this task requires that a new relation be established between two concept nodes (and their semantic neighbours).
We have earlier reported successful subliminal relational encoding and a flexible retrieval of face-word and word-word combinations in behavioural (Duss et al., 2011; Reber and Henke, 2011, 2012) and functional MRI studies (Henke et al., 2003a,b; Degonda et al., 2005; Reber et al., 2012, 2014) in normal participants using the same masking paradigm as used in the current study. New semantic word-word associations were established unconsciously even across time points (Reber and Henke, 2012; Reber et al., 2012). The integration of associative memories over time was possible both within (Reber and Henke, 2012; Reber et al., 2012) and across levels of consciousness (Henke et al., 2013). Our neuroimaging data demonstrated that relational encoding and retrieval as well as the relational integration of information across time-points were assisted by hippocampus (Henke et al., 2003a,b; Degonda et al., 2005; Reber et al., 2012, 2014). However, studies in normal participants cannot clarify whether the hippocampus is necessary for unconscious memory formation or merely co-activated but not essential for the task. The present result of impaired unconscious relational processing in amnesic patients speaks to the necessity of the hippocampus for unconscious relational encoding. This new finding questions the current textbook account of memory systems that attributes to the hippocampus only a role in conscious encoding (Squire and Zola, 1996; Tulving, 2002; Moscovitch, 2008). Instead, this new finding supports the relational memory account (Cohen and Eichenbaum, 1993) and the processing account of memory systems (Henke, 2010).
Remarkably, three amnesic patients succeeded on unconscious relational encoding and retrieval relying on residual hippocampal tissue and functional connections to lateral temporal cortex. These patients had retained hippocampal tissue reaching from 46–74% of normal values. Hence, their volume loss was <60%, which was regarded a critical threshold for preserved relational memory capacities in a study of animal spatial encoding (Kleinknecht et al., 2012). Although the well performing patients were severely impaired on all standard tests of long-term memory, they scored higher on tests of immediate verbal memory and verbal intelligence compared to the other patients. Both their rich semantic knowledge and residual hippocampal tissue and hippocampal connections to temporal language storage sites may have enabled their normal performance on the test of unconscious relational encoding and retrieval (Kan et al., 2009).
These findings settle the debate on intact versus impaired unconscious memory for word pairs in amnesic patients (Schacter, 1998). Behavioural studies in amnesic patients suggested that the severity of amnesia was related to the performance of patients on indirect tests of unconscious memory for word pairs. The word pairs had been presented visibly for conscious encoding but could not be consciously remembered due to amnesia (Schacter and Graf, 1986; Shimamura and Squire, 1989). One reason for this association between severity of amnesia and test performance could be the contamination of unconscious with residual conscious memory in patients with lighter forms of amnesia (McKone and Slee, 1997; Smith and Squire, 2008). Another reason for this relation may be spared functionality in the hippocampal-anterior thalamic axis in lighter forms of amnesia that suffices unconscious but not conscious memory processing. The current findings support this second interpretation because our design excluded consciousness as a confounding variable. Our data suggest that the spared functionality in well performing patients concerned both the hippocampal-anterior thalamic axis and its connections to neocortical language areas. The possibility that processing in neocortical language sites alone had generated unconscious relational memories in these well performing patients can be excluded because all amnesic patients, also those that failed on the relational task, exhibited normal cognitive functions and normal semantic priming for single words that require neocortical language sites. Hence, if language cortex alone supported relational word processing, all patients should have yielded normal results. But this was clearly not the case. Furthermore, relational and single word retrieval were not correlated (Fig. 3C), which would be expected if both tasks relied on neocortical language sites alone. In light of these data, the finding (Verfaelllie et al., 2012) of intactperformance of a group of amnesic patients on an implicit semantic word-word priming task may be attributable to a subgroup of patients with speared functionality in the hippocampal-anterior thalamic axis and its connections to neocortical language areas.
The fact that conscious relational memory was impaired while unconscious relational memory was intact in well performing patients suggests that levels of consciousness of memory processing require different resources within a memory system. A larger level of functionality in the hippocampal-anterior thalamic axis and its connections to neocortex seems required for conscious versus unconscious relational encoding and retrieval. This assumption is supported by the functional connectivity of hippocampus with the anterior temporal lobe, which was strong in controls, weaker in the well performing patients and absent in the other patients (Fig. 5). We assume that consciousness of encoding is a correlate of the strength and coherence of activity in the task-relevant memory system. This is a notion, which is in line with quantitative views of consciousness of stimulus processing (Srinivasan et al., 1999; Bar et al., 2001; Moutoussis and Zeki, 2002). Hence, processing modes appear to divide between memory systems, while consciousness might divide between levels of function within a memory system.
Acknowledgements
We thank Pawel Pomes for help with hippocampal volumetry and all participants for their time and efforts.
Funding
This work was supported by the Swiss National Science Foundation for Research (Grant number 320000-114012) to K. Henke.
Supplementary material
Supplementary material is available at Brain online.
References
- Bar M, Tootell RB, Schacter DL, Greve DN, Fischl B, Mendola JD, et al. Cortical mechanisms specific to explicit visual object recognition. Neuron. 2001;29:529–35. doi: 10.1016/s0896-6273(01)00224-0. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–96. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–38. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Caulo M, Van Hecke J, Toma L, Ferretti A, Tartaro A, Colosimo C, et al. Functional MRI study of diencephalic amnesia in Wernicke-Korsakoff syndrome. Brain. 2005;128:1584–94. doi: 10.1093/brain/awh496. [DOI] [PubMed] [Google Scholar]
- Chen J, Olsen RK, Preston AR, Glover GH, Wagner AD. Associative retrieval processes in the human medial temporal lobe: Hippocampal retrieval success and CA1 mismatch detection. Learn Mem. 2011;18:523–8. doi: 10.1101/lm.2135211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia and the hippocampal system. Cambridge: MIT Press; 1993. [Google Scholar]
- Degonda N, Mondadori CR, Bosshardt S, Schmidt CF, Boesiger P, Nitsch RM, et al. Implicit associative learning engages the hippocampus and interacts with explicit associative learning. Neuron. 2005;46:505–20. doi: 10.1016/j.neuron.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Duncan K, Ketz N, Inati SJ, Davachi L. Evidence for area CA1 as a match/mismatch detector: a high-resolution fMRI study of the human hippocampus. Hippocampus. 2012;22:389–98. doi: 10.1002/hipo.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duss SB, Oggier S, Reber TP, Henke K. Formation of semantic associations between subliminally presented face-word pairs. Conscious Cogn. 2011;20:928–35. doi: 10.1016/j.concog.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Free SL, Bergin PS, Fish DR, Cook MJ, Shorvon SD, Stevens JM. Methods for normalization of hippocampal volumes measured with MR. Am J Neuroradiol. 1995;16:637–43. [PMC free article] [PubMed] [Google Scholar]
- Giovanello KS, Verfaellie M, Keane MM. Disproportionate deficit in associative recognition relative to item recognition in global amnesia. Cogn Affect Behav Neurosci. 2003;3:186–94. doi: 10.3758/cabn.3.3.186. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Klinger MR, Schuh ES. Activation by marginally perceptible (“subliminal”) stimuli: dissociation of unconscious from conscious cognition. J Exp Psychol Gen. 1995;124:22–42. doi: 10.1037//0096-3445.124.1.22. [DOI] [PubMed] [Google Scholar]
- Henke K. A model for memory systems based on processing modes rather than consciousness. Nat Rev Neurosci. 2010;11:523–32. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- Henke K, Treyer V, Nagy ET, Kneifel S, Dürsteler M, Nitsch RM, et al. Active hippocampus during nonconscious memories. Conscious Cogn. 2003a;12:31–48. doi: 10.1016/s1053-8100(02)00006-5. [DOI] [PubMed] [Google Scholar]
- Henke K, Mondadori CR, Treyer V, Nitsch RM, Buck A, Hock C. Nonconscious formation and reactivation of semantic associations by way of the medial temporal lobe. Neuropsychologia. 2003b;41:863–76. doi: 10.1016/s0028-3932(03)00035-6. [DOI] [PubMed] [Google Scholar]
- Henke K, Reber TP, Duss SB. Integrating events across levels of consciousness. Front Behav Neurosci. 2013;7:68. doi: 10.3389/fnbeh.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA. Neuroimaging studies of priming. Prog Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Kan IP, Alexander MP, Verfaellie M. Contribution of prior semantic knowledge to new episodic learning in amnesia. J Cogn Neurosci. 2009;21:938–44. doi: 10.1162/jocn.2009.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan IP, Giovanello KS, Schnyer DM, Makris N, Verfaellie M. Role of the medial temporal lobes in relational memory: neuropsychological evidence from a cued recognition paradigm. Neuropsychologia. 2007;45:2589–97. doi: 10.1016/j.neuropsychologia.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinknecht KR, Bedenk BT, Kaltwasser SF, Grunecker B, Yen YC, Czisch M, et al. Hippocampus-dependent place learning enables spatial flexibility in C57BL6/N mice. Front Behav Neurosci. 2012;6:87. doi: 10.3389/fnbeh.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad C, Ukas T, Nebel C, Arolt V, Toga AW, Narr KL. Defining the human hippocampus in cerebral magnetic resonance images–an overview of current segmentation protocols. Neuroimage. 2009;47:1185–95. doi: 10.1016/j.neuroimage.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk DC. The cognition and neuroscience of relational reasoning. Brain Res. 2012;1428:13–23. doi: 10.1016/j.brainres.2010.11.080. [DOI] [PubMed] [Google Scholar]
- Kovács G, Vogels R, Orban GA. Cortical correlate of pattern backward masking. Proc Natl Acad Sci USA. 1995;92:5587–91. doi: 10.1073/pnas.92.12.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. Match-mismatch processes underlie human hippocampal responses to associative novelty. J Neurosci. 2007;27:8517–24. doi: 10.1523/JNEUROSCI.1677-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YO, Adali T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp. 2007;28:1251–66. doi: 10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Rudge P, Cipolotti L. The effect of adult-acquired hippocampal damage on memory retrieval: an fMRI study. Neuroimage. 2005;27:146–52. doi: 10.1016/j.neuroimage.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Vargha-Khadem F, Mishkin M. The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain. 2001;124:1156–70. doi: 10.1093/brain/124.6.1156. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Hunkin NM, Roberts N. Relative sparing of item recognition memory in a patient with adult-onset damage limited to the hippocampus. Hippocampus. 2002;12:325–40. doi: 10.1002/hipo.1111. [DOI] [PubMed] [Google Scholar]
- McKone E, Slee JA. Explicit contamination in “implicit” memory for new associations. Mem Cognit. 1997;25:352–66. doi: 10.3758/bf03211291. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. The hippocampus as a “Stupid,” domain-specific module: implications for theories of recent and remote memory, and of imagination. Can J Exp Psychol. 2008;62:62–79. doi: 10.1037/1196-1961.62.1.62. [DOI] [PubMed] [Google Scholar]
- Moutoussis K, Zeki S. The relationship between cortical activation and perception investigated with invisible stimuli. Proc Natl Acad Sci USA. 2002;99:9527–32. doi: 10.1073/pnas.142305699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW. Computational principles of learning in the neocortex and hippocampus. Hippocampus. 2000;10:389–97. doi: 10.1002/1098-1063(2000)10:4<389::AID-HIPO5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–87. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Reber TP, Henke K. Rapid formation and flexible expression of memories of subliminal word pairs. Front Psychol. 2011;2:343. doi: 10.3389/fpsyg.2011.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber TP, Henke K. Integrating unseen events over time. Conscious Cogn. 2012;21:953–60. doi: 10.1016/j.concog.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Reber TP, Luechinger R, Boesiger P, Henke K. Unconscious relational inference recruits the hippocampus. J Neurosci. 2012;32:6138–48. doi: 10.1523/JNEUROSCI.5639-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber TP, Luechinger R, Boesiger P, Henke K. Detecting analogies unconsciously. Front Behav Neurosci. 2014;8:9. doi: 10.3389/fnbeh.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway GR, Omar R, Ourselin S, Hill DL, Warren JD, Fox NC. Issues with threshold masking in voxel-based morphometry of atrophied brains. Neuroimage. 2009;44:99–111. doi: 10.1016/j.neuroimage.2008.08.045. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Tovee MJ. Processing speed in the cerebral cortex and the neurophysiology of visual masking. Proc Biol Sci. 1994;257:9–15. doi: 10.1098/rspb.1994.0087. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Tovee MJ, Panzeri S. The neurophysiology of backward visual masking: information analysis. J Cogn Neurosci. 1999;11:300–11. doi: 10.1162/089892999563409. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Memory and awareness. Science. 1998;280:59–60. doi: 10.1126/science.280.5360.59. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Graf P. Preserved learning in amnesic patients - perspectives from research on direct priming. J Clin Exp Neuropsychol. 1986;8:727–43. doi: 10.1080/01688638608405192. [DOI] [PubMed] [Google Scholar]
- Segaert K, Weber K, de Lange FP, Petersson KM, Hagoort P. The suppression of repetition enhancement: a review of fMRI studies. Neuropsychologia. 2013;51:59–66. doi: 10.1016/j.neuropsychologia.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Squire LR. Impaired priming of new associations in amnesia. J Exp Psychol Learn Mem Cogn. 1989;15:721–8. doi: 10.1037//0278-7393.15.4.721. [DOI] [PubMed] [Google Scholar]
- Smith CN, Squire LR. Experience-dependent eye movements reflect hippocampus-dependent (aware) memory. J Neurosci. 2008;28:12825–33. doi: 10.1523/JNEUROSCI.4542-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci USA. 1996;93:13515–22. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Russell DP, Edelman GM, Tononi G. Increased synchronization of neuromagnetic responses during conscious perception. J Neurosci. 1999;19:5435–48. doi: 10.1523/JNEUROSCI.19-13-05435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. How many memory-systems are there. Am Psychol. 1985;40:385–98. [Google Scholar]
- Tulving E. Episodic memory: From mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Kesteren MTR, Ruiter DJ, Fernández G, Henson RN. How schema and novelty augment memory formation. Trends Neurosci. 2012;35:211–9. doi: 10.1016/j.tins.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Verfaelllie M, LaRocque KF, Keane MM. Intact implicit verbal relational memory in medial temporal lobe amnesia. Neuropsychologia. 2012;50:2100–6. doi: 10.1016/j.neuropsychologia.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]