See Toga and Thompson (doi:10.1093/brain/awu276) for a scientific commentary on this article.
Hyun Kook Lim et al. investigate the relationship between amyloid burden and intrinsic functional connectivity in cognitively normal older adults. Individuals with amyloid deposition show aberrant functional connectivity in the default mode and central executive networks compared to those without detectable amyloid. Changes may reflect early deleterious effects of amyloid deposition.
Keywords: amyloid imaging, normal older adults, functional MRI, large scale intrinsic networks
Abstract
Although previous studies demonstrated decreased functional connectivity in the default mode network in the cognitively normal older adults with amyloid burden, effects of amyloid burden in the other large-scale intrinsic connectivity networks are not yet clear. The aim of this study was to investigate the distinctive association pattern of amyloid-β deposition on the three large-scale intrinsic connectivity networks (the default mode network, salience network and central executive network) in older adults with normal cognition. Fifty-six older adults with normal cognition underwent functional magnetic resonance imaging and were dichotomized using 11C-labelled Pittsburgh compound B positron emission tomography imaging into subjects with (PiB+; n = 27) and without (PiB−; n = 29) detectable amyloid burden. We found that the functional connectivities of (i) the default mode network were greater; (ii) the salience network were not different; and (iii) the central executive network were lower in the Pittsburgh compound B positive group, compared with the Pittsburgh compound B negative group. Anterior cingulate cortex Pittsburgh compound B retention was negatively correlated with the functional connectivities of the posterior default mode network, and positively correlated with fronto-parietal functional connectivity (within the central executive network) in the Pittsburgh compound B positive group. The anti-correlation strength between the default mode network and the central executive network was negatively correlated with the anterior cingulate cortex Pittsburgh compound B levels. Additionally, significant group × episodic memory interactions with functional connectivities in the posterior default mode network, and the frontal default mode network were observed. Our results of aberrant default mode network functional connectivity and distinctive correlation patterns between the Pittsburgh compound B retention in the anterior cingulate cortex and functional connectivities in the default mode network and central executive network in the Pittsburgh compound B positive group might reflect a detrimental effect of amyloid retention on functional changes in the course of Alzheimer’s disease progression.
Introduction
Beta-amyloid (amyloid-β) protein accumulation in the human brain is a characteristic of Alzheimer’s disease and a key component in current theories of the disease’s pathogenesis (Hardy and Selkoe, 2002). The presence of amyloid-β might contribute to the deleterious effects occurring in synaptic processes, leading to impaired memory consolidation and failure to form new memories (Vannini et al., 2012). Fibrillar amyloid-β protein deposition in the brain is measurable in vivo using the PET ligand Pittsburgh compound B (PiB) (Cohen et al., 2013). As amyloid-β protein is known to accumulate for a number of years before the cognitive symptoms of Alzheimer’s disease manifest (Jack et al., 2010), emphasis in many research studies has moved towards detection of the earliest signs of amyloid-β deposition in cognitively normal individuals (Cohen et al., 2013). Although amyloid-β deposition may be one of the earliest biological alterations in the pathophysiological cascade of Alzheimer’s disease, additional events must occur before the clinical symptoms of Alzheimer’s disease appear (Jack et al., 2009). Therefore, identification of the earliest functional brain change in cognitively normal elderly subjects with amyloid burden is considered an important early milestone in progression to Alzheimer’s disease. As compared to task-based functional MRI studies, which require interpretation of the functional connectivity within the context of the experimental paradigm, resting state functional MRI studies interrogate the intrinsic functional connectivity networks (ICN) without an experimentally determined context (Vemuri et al., 2012). Previous studies have shown that ICN change is sensitive to functional brain changes related to Alzheimer’s disease pathology across the clinical spectrum (Damoiseaux, 2012).
In this regard, several previous studies have reported on ICN changes in the brains of subjects with amyloid deposition but no cognitive impairments (Hedden et al., 2009; Sperling et al., 2009; Sheline et al., 2010; Mormino et al., 2011). They demonstrated decreased functional connectivity in the default mode network (DMN) in cognitively normal subjects with amyloid burden and distinctive correlation patterns between PiB retention and the DMN functional connectivity (Hedden et al., 2009; Sperling et al., 2009; Sheline et al., 2010; Mormino et al., 2011). All of these studies limited their connectivity analyses to the DMN, most likely because it is altered early in the pathogenesis of Alzheimer’s disease. Besides DMN, other stable ICNs have been identified in the human brain thus far, including the central executive network (CEN) and the salience network (SN). These ICNs have been found to play crucial roles in information processing, in a well coordinated manner with the DMN (Menon, 2011). The DMN is related with activity in the CEN, which is activated during goal-oriented activity (Uddin et al., 2009; Bressler and Menon, 2010). This reciprocal relationship between these two networks reflects two types of distinct modes of cognitive processing: the DMN, serving untargeted inner thought in one mode; and the CEN, serving focused, stimulus-dependent attention in the second mode (Fox et al., 2005; Buckner et al., 2008). In addition, functional switching between the DMN and CEN is known to be regulated by the salience network, which is responsible for generating appropriate behavioural responses to salient stimuli (Menon and Uddin, 2010). Hence, functional changes in all three of these networks need to be investigated to explore more integrative neurobiological models of the effect of amyloid-β protein deposition on ICNs. In pursuit of this goal, the previous resting state functional MRI studies have demonstrated aberrant functional connectivity of the DMN, SN and CEN, along with loss of anti-correlation between the DMN and CEN in the disease continuum of Alzheimer’s disease (Agosta et al., 2012; Brier et al., 2012). Furthermore, a recent functional MRI study showed that spatial patterns of PiB uptake were positively correlated with ICNs, and that amyloid-β deposition had a negative impact on functional connectivity in subjects with amnestic mild cognitive impairment (MCI) with amyloid burden (Myers et al., 2014). However, despite previous work on prodromal and clinical Alzheimer’s disease, the distinctive pattern between amyloid burden and functional connectivity in the ICNs in cognitively normal older adults remains to be elucidated.
Other questions regarding the effect of amyloid-β on brain ICN also remain incompletely answered. Although Mormino et al. (2011) found a negative association between PiB retention and the DMN functional connectivity in cognitively normal elderly subjects, they did not examine the associations between amyloid load and DMN in PiB− and PiB+ subjects separately. Cognitively normal elderly subjects with evidence of amyloid deposition have been reported to have a greater rate of change in cognitive function (Storandt et al., 2009), and an increased rate of progression to Alzheimer’s disease (Morris et al., 2009). Therefore, it is likely informative to separately analyse amyloid-β-related effects on the ICN of PiB+ and PiB− subjects in order to distinguish effects of normal ageing from effects of preclinical Alzheimer’s disease (Cohen et al., 2013). Moreover, the lack of significant relationships between episodic memory scores and functional connectivity in the study by Mormino et al. (2011), could be attributable to the inclusion of PiB− individuals, in which PiB measures may primarily reflect non-specific retention.
The aim of this study is to investigate functional connectivity differences in the three large scale ICNs (the DMN, SN and CEN) and the association of amyloid-β deposition on these ICNs in older adults with normal cognition. In addition, we explore group differences of inter-ICN functional connectivities between the PiB+ and the PiB− groups, and the relationships between inter-ICN functional connectivities and amyloid-β deposition in the PiB+ group. These are done with amyloid-β load treated as a dichotomous variable (PiB+ and PiB−), and also, within the PiB+ group, amyloid-β load is used as a continuous measure, reflecting the local amyloid burden. In addition, in order to characterize the association of ICN with subtle cognitive change, we relate episodic memory performance to ICNs in the three large scale networks in the PiB+ group versus the PiB− group.
As previous studies have found lower DMN functional connectivity in PiB+ (Hedden et al., 2009; Sheline et al., 2010), we hypothesized that we would observe similar findings. As a corollary, we expected that functional connectivities of the SN and the CEN, which were known to be anti-correlated with the DMN (Buckner et al., 2008; Brier et al., 2012) would be higher in the PiB+ group, as compared to the PiB− group. Furthermore, the anti-correlation strength between the DMN and the SN/CEN would be negatively correlated with PiB levels in the PiB+ group, as suggested in a previous study on Alzheimer’s disease and amnestic MCI (Brier et al., 2012). In addition, we expected the regional PiB retention would have distinctive association patterns with the three large ICNs in the PiB+ group. Finally, we hypothesized significant differences between the PiB+ and the PiB− groups in the associations between episodic memory and functional connectivity in the three ICNs.
Materials and methods
Subjects
Fifty-six elderly subjects with normal cognition were included. They were recruited from advertisements in the community, from a cohort of control volunteers at the University of Pittsburgh Alzheimer’s Disease Research Centre (ADRC), and from the University of Pittsburgh Pepper Registry, which is a registry of studies on mobility, balance, and ageing.
The inclusion criteria of the subjects were as follows: (i) subjects aged >60 years; (ii) Mini-Mental Status Examination score >27; and (iii) Clinical Dementia Rating = 0 (Morris, 1993). Subjects with any psychiatric, neurological and unstable medical conditions were excluded.
The cognitive testing battery included the following domains: memory, visuospatial construction, language, attention and executive functions. Detailed names of the tests and reviewing process are described in the Supplementary material.
PET acquisition
[11C]PiB was produced and PiB-PET data were collected and analysed as previously described (Price et al., 2005). The individual participant’s MRI was utilized for co-registration and region of interest definition (Price et al., 2005; Cohen et al., 2009) and for correcting PiB-PET data for the diluting effects of expanding cerebrospinal spaces accompanying cerebral atrophy (Meltzer et al., 1999). Analysis of the PiB PET data utilized a standardized uptake value ratio 50–70 min post-injection, using the cerebellum region of interest as the reference.
The PiB PET data were acquired within 6 weeks of clinical screening and cognitive testing. Cut-offs for PiB-positivity were determined using sparse k-means clustering with re-sampling, as described previously (Cohen et al., 2013). Importantly, a subject was defined as PiB+ if they exceeded the cut-off in any one of the six regions shown in Fig. 1. This method was chosen to increase the sensitivity of detecting the earliest evidence of amyloid-β deposition (Cohen et al., 2013). From sparse k-means clustering regional cut-offs were obtained for brain regions that most commonly show amyloid deposition in Alzheimer’s disease: anterior cingulate cortex (cut-off = 1.78), frontal cortex (cut-off = 1.71), lateral temporal cortex (cut-off = 1.50), medial temporal cortex (cut-off = 1.42), parietal cortex (cut-off = 1.63), and precuneus cortex (cut-off = 1.73). Any subject who had PiB retention values exceeding this cut-off point in any one (or more) of these six brain regions was defined as PiB+.
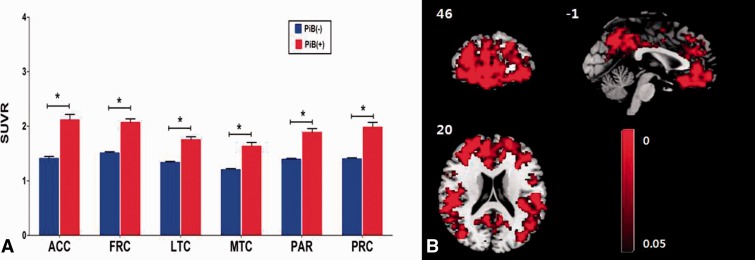
Figure 1.
PiB retention among the cognitively normal subjects without amyloid burden (PiB−) and with amyloid burden (PiB+). Group comparison results between the PiB+ and PiB− groups using (A) region of interest (P < 0.05, Holm-Bonferroni corrected for multiple comparisons) and (B) voxel-wise (P < 0.05, FDR corrected for multiple comparisons) analyses. The standardized uptake value ratio levels in the PiB+ were significantly increased compared with the PiB− group. SUVR = standardized uptake value ratio; ACC = anterior cingulate cortex; FRC = frontal cortex; LTC = lateral temporal cortex; MTC = medial temporal cortex; PAR = parietal cortex; PRC = precuneus cortex. *Significant P < 0.05, Bonferroni corrected for multiple comparisons.
MRI acquisition
Imaging data were collected at the University of Pittsburgh Magnetic Resonance Research Centre (MRRC) using a 3T Siemens Trio machine, and 12-channel Siemens head coil. A standard high-resolution T1-weighted volumetric magnetization prepared rapid gradient echo scan (MPRAGE) sequence was acquired in axial orientation (160 slices, 256 × 240, 1 mm isotropic). For the resting-state scan, T2*-weighted blood oxygen level-dependent acquisition was done using a gradient echo planar imaging sequence: repetition time = 2000 ms, echo time = 34 ms, matrix = 128 × 128 × 29, voxel size = 2 × 2 × 3 mm3, oblique axial acquisition, integrated parallel acquisition techniques = 2. Images were acquired over 5 min (150 volumes). Subjects were instructed to lie still with their eyes open, look at a fixation cross, think of nothing in particular, and not to fall asleep.
Data analysis
Independent component analysis
Resting state functional MRI preprocessing was carried out using the Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC) (Beckmann et al., 2005). Individual pre-statistical processing consisted of motion correction, brain extraction, and spatial smoothing using a Gaussian kernel of full-width at half-maximum of 6 mm smoothing kernel. We used affine coregistration to motion-correct the resting state functional MRI data. No excessive head motion was observed (i.e. cumulative translation or rotation >3 mm or 3° and mean point-to-point translation or rotation >0.15 mm or 0.1°). Frame-wise displacement (Power et al., 2012) or the root-mean-square of translational parameters (Van Dijk et al., 2012) were not different between groups (P > 0.05, two-sample t-tests).
A high-pass temporal filtering equivalent to 150 s (0.007 Hz) was also used. Functional MRI volumes were registered to the individual’s structural scan and standard space images using FMRIB’s Nonlinear Image Registration Tool (FNIRT). Preprocessed functional data containing 150 time points for each subject were temporally concatenated across subjects to create a single 4D data set. The between-group analysis of the resting data was carried out using a regression technique (dual regression) that allows for voxel-wise comparisons of resting functional connectivities (Filippini et al., 2009). In this analysis, the data set was decomposed into 29 components in which the model order was automatically estimated using the Laplace approximation to the Bayesian evidence for a probabilistic principal component mode, and ICNs of interest (DMN, SN and CEN) were selected using spatial correlation against a set of previously defined maps (Shirer et al., 2012). The DMN was identified as the spatial map comprising superior frontal, anterior and middle cingulate gyrus, angular gyrus, hippocampus and precuneus (Supplementary Table 4). The SN was identified as the spatial maps comprising anterior cingulate, and the bilateral anterior insular (Supplementary Table 4). The left CEN was identified as the left middle frontal gyrus, left inferior frontal gyrus, left angular gyrus (Supplementary Table 4).
Structural imaging analysis
An optimized voxel-based morphometry was conducted for investigation of the brain atrophy contribution to the changes of functional connectivity. Detailed processes are presented in the Supplementary material.
Statistical analysis
Statistical analyses for demographic data were performed with the Statistical Package for Social Sciences software (SPSS, version 12.0, Chicago, IL). Assumptions for normality were tested for all continuous variables. Normality was tested using the Kolmogorov–Smirnov test. Two-sample independent t-tests were used to assess potential differences between the PiB+ and PiB− groups for all continuous demographic variables and standardized uptake value ratio values, and Chi-square tests for categorical variables. The correlations between the standardized uptake value ratio values and the cognitive function scores were calculated in the PiB+ group using multiple regression tests with age, gender and education controlled. The Holm-Bonferroni method was used for correction for multiple comparisons (Holm, 1979). All statistical analyses used a two-tailed α level of 0.05 for defining statistical significance.
The general linear model was used for measuring the group differences of the independent component analysis maps for each ICN. To examine the relationships between amyloid-β deposition and functional connectivity of the ICNs in the PiB+ group, the global and the regional standardized uptake value ratio values from the six regions of interest used in the PiB analysis (the anterior cingulate cortex, the frontal cortex, the lateral temporal cortex, the medial temporal cortex, the parietal cortex and the precuneus cortex) were correlated with the voxel-wise independent component analysis maps of the DMN, the SN, the CEN functional connectivity using the general linear model. In addition, the general linear model with functional connectivity as the main outcome variable and episodic memory (CERAD Word List Recall) (Morris et al., 1989) scores and group as independent variables were performed as well as their interaction (episodic memory × group). We controlled the effect of age, education and gender from the all general linear model analysis used. The threshold was set at P < 0.05 [false discovery rate (FDR)] to control for multiple comparisons (Genovese et al., 2002).
To calculate the inter-network correlations, we computed the time-course signals of the individual subject for each ICN, which were entered in the partial correlation test between the ICNs with age, education and gender effects controlled. The correlation coefficients between each network were transformed to Z values to ensure the normality of coefficient distribution. To examine the group differences of the inter-ICN functional connectivity, one-way analysis of covariance (ANCOVA) was used with age, gender and education controlled. The relationship between amyloid-β deposition and the inter-ICN functional connectivity was measured with general linear model where age, education and gender effects were used as covariates. The resulting P-values were Holm-Bonferroni corrected for multiple comparisons.
Results
Baseline demographic and clinical data
Table 1 shows the baseline demographic data in the two subject groups. All variables were normally distributed. There was no significant difference in gender, age, and education between the PiB+ and the PiB− group. In addition, there were no significant differences between the PiB+ and PiB− groups on the neuropsychological tests. The standardized uptake value ratio values of the both groups were significantly different in six regions of interest and global level (Fig. 1 and Supplementary Table 1) and the standardized uptake value ratio values were significantly correlated with each region of interest (Supplementary Table 3). In addition, there were no significant correlations between the standardized uptake value ratio values and the cognitive functions (Supplementary Table 9).
Table 1.
Demographic and clinical characteristics of study participants
| PiB− group (n = 29) | PiB+ group (n = 27) | P-value | |
|---|---|---|---|
| Age (years ± SD) | 75.3 ± 6.4 | 76.3 ± 5.8 | NS |
| Education (years ± SD) | 14.8 ± 2.4 | 14.3 ± 2.4 | NS |
| Gender (M:F) | 9:20 | 10:17 | NS |
| Ethnicity | NS | ||
| Caucasian | 24 | 21 | |
| African American | 5 | 3 | |
| Asian | 0 | 2 | |
| American Indian | 0 | 1 | |
| Apo E gene [E4(−): E4(+)] | 26:3 | 24:3 | NS |
| GDS (SD) | 3.1 ± 3.1 | 2.8 ± 2.6 | NS |
| MMSE (SD) | 28.6 ± 1 3 | 28.4 ± 1.5 | NS |
| CEARD-WLRc (SD) | 7.7 ± 1.9 | 7.4 ± 1.3 | NS |
| Mem-WMS-R (SD) | 11.3 ± 2.1 | 9.3 ± 2.4 | NS |
| Rey (SD) | 16.4 ± 3.3 | 15.7 ± 4.3 | NS |
| Block_WAIS-R (SD) | 13.8 ± 3.4 | 14.1 ± 4.8 | NS |
| COFIG (SD) | 19.5 ± 2.0 | 19.3 ± 2.1 | NS |
| CF (SD) | 20.4 ± 6.2 | 19.4 ± 4.8 | NS |
| LF (SD) | 16.0 ± 5.4 | 15.7 ± 4.8 | NS |
| BNT (SD) | 28.9 ± 1.5 | 28.4 ± 2.2 | NS |
| TMT-A (SD) | 28.7 ± 12.1 | 28.3 ± 9.2 | NS |
| TMT-B (SD) | 73.9 ± 27.1 | 85.9 ± 41.9 | NS |
| DSST (SD) | 53.2 ± 11.5 | 50.9 ± 11.9 | NS |
| DSF (SD) | 6.6 ± 1.1 | 6.6 ± 1.2 | NS |
| DSB (SD) | 5.3 ± 1.2 | 5.0 ± 1.1 | NS |
| Stroop Test response time differences (ms ± SD) | 107.5 ± 70.3 | 104.3 ± 60.9 | NS |
| Stroop Test no. of intrusions (SD) | 2.0 ± 1.5 | 2.1 ± 1.4 | NS |
SD = standard deviation; NS = statistically not significant; GDS = Geriatric Depression Rating Scale; MMSE = Mini-Mental Status Examination; CERAD-WLRc = Word List Recall test from the Consortium to Establish A Registry for Alzheimer’s Disease; Mem-WMS-R = Logical Memory Story A from the Wechsler Memory Scale—Revised; Rey = Modified Rey Osterrieth Figure Recalls; Block_WAIS-R = Modified Block Design Subtest from the Wechsler Adult Intelligence Scale–Revised; COFIG = Copying of the Rey Osterrieth Figure; CF = Categorical fluency test; LF = Letter fluency test; BNT = 30-item Boston Naming Test; TMT-A = Trail Making Test-A; TMT-B = Trail Making Test-B; DSST = Digit Symbol Substitution Test; DSF = Digit Span Forward Test; DSB = Digit Span Backward Test.
Group difference in functional connectivity
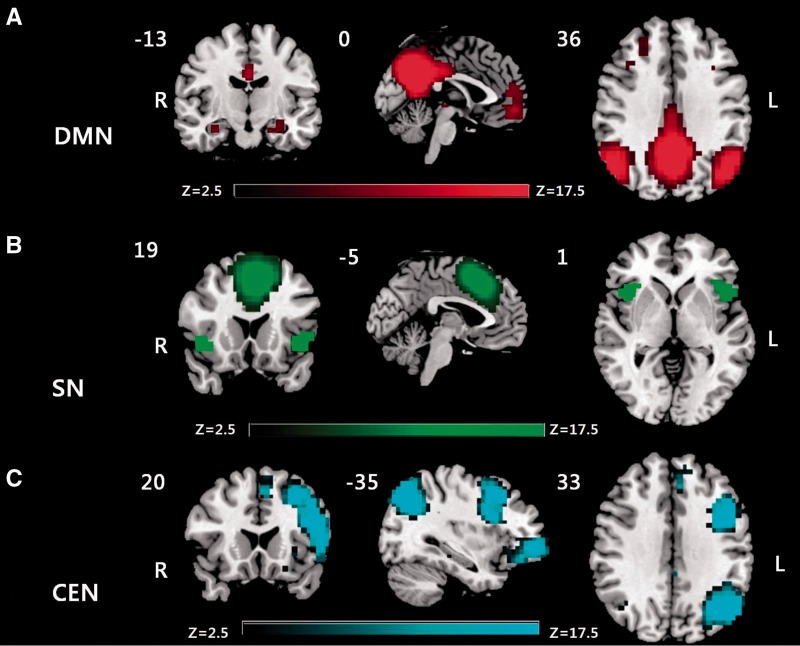
Figure 2 shows the statistical map representing the DMN, the SN, and the CEN determined across all subjects. The coordination tables are shown in Supplementary Table 4.
Figure 2.
Spatial maps of the resting state ICN of interest identified by independent component analysis of all subjects. (A) The DMN; (B) the SN; and (C) the CEN.
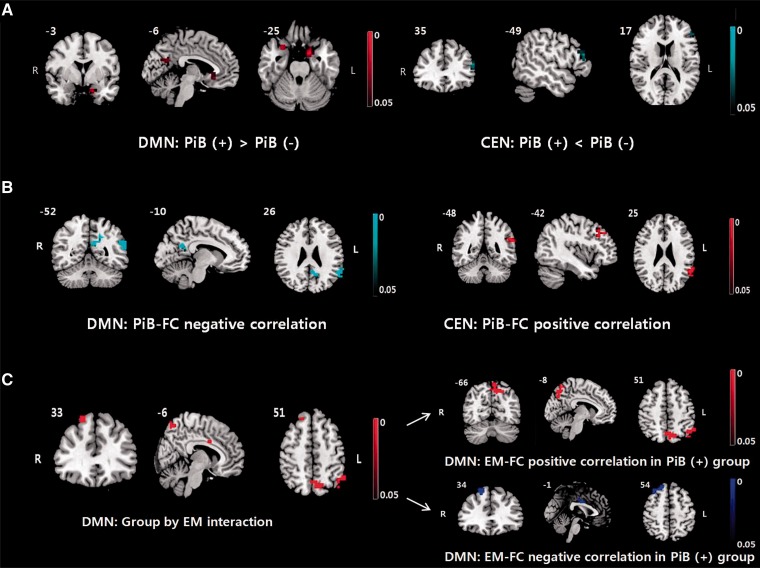
Group independent component analysis shows significantly increased functional connectivity in the DMN in the PiB+ group compared to the PiB− group in the anterior cingulate cortex, precuneus cortex, and bilateral hippocampus (Fig. 3A and Table 2, FDR corrected P < 0.05). In the salience network, there were no significant differences between the PiB+ and the PiB− groups in the functional connectivities. The functional connectivity in the left middle frontal gyrus was significantly decreased within the CEN in the PiB+ group compared to the PiB− group (Fig. 3A and Table 2, FDR corrected P < 0.05).
Figure 3.
Statistical maps of voxel-wise analysis results of independent component analysis. (A) Group differences between the PiB+ and PiB− group. (B) Correlation analysis between the PiB level and functional connectivity in the PiB+ group. (C) Left: Regional DMN functional connectivity showing significant interaction between the group and episodic memory performances. Right: Correlation analysis between episodic memory performances and functional connectivity in the PiB+ group. All P-values were FDR corrected for multiple comparisons (P < 0.05). FC = functional connectivity; EM = episodic memory.
Table 2.
Results from voxel-wise independent component analysis
| Region | L/R | Cluster | T score | P-value* | MNI (x, y, z) | ||
|---|---|---|---|---|---|---|---|
| Group differences | |||||||
| DEFAULT MODE NETWORK | |||||||
| PiB+ > PiB− | |||||||
| Anterior cingulate | L | 22 | 3.13 | 0.031 | −6 | 30 | −3 |
| Precuneus | L | 26 | 3.75 | 0.012 | −6 | 57 | 28 |
| Hippocampus | L | 16 | 3.75 | 0.012 | −21 | −3 | −25 |
| Hippocampus | R | 15 | 3.72 | 0.013 | 26 | −3 | −25 |
| CENTRAL EXECUTIVE NETWORK | |||||||
| PiB+ < PiB− | |||||||
| Middle frontal gyrus | L | 19 | 3.31 | 0.018 | −49 | 35 | 17 |
| Anterior cingulate PiB-FC correlations | |||||||
| DEFAULT MODE NETWORK | Negative correlation | ||||||
| Angular gyrus | L | 55 | 4.75 | <0.001 | −62 | −54 | 24 |
| Posterior cingulate | L | 50 | 4,42 | <0.001 | −6 | −50 | 20 |
| CENTRAL EXECUTIVE NETWORK | Positive correlation | ||||||
| Middle frontal gyrus | L | 17 | 3.68 | 0.014 | −30 | 22 | 48 |
| Inferior parietal gyrus | L | 26 | 3.77 | 0.011 | −58 | −58 | 28 |
| EM-FC relationship | |||||||
| Group by EM interaction | |||||||
| DEFAULT MODE NETWORK | |||||||
| Precuneus | L | 16 | 3.75 | 0.012 | −6 | −64 | 51 |
| Angular gyrus | L | 23 | 3.75 | 0.012 | −35 | −58 | 51 |
| Middle cingulate gyrus | L | 16 | 3.77 | 0.011 | −4 | −10 | 41 |
| Superior frontal gyrus | R | 14 | 3.77 | 0.011 | 18 | 35 | 57 |
| EM-FC correlation in the PiB + group | |||||||
| DEFAULT MODE NETWORK | |||||||
| Positive correlation | |||||||
| Precunues | L | 37 | 3.77 | 0.011 | −6 | −71 | 47 |
| Angular gyrus | L | 42 | 3.75 | 0.012 | −42 | −58 | 47 |
| Negative correlation | |||||||
| Middle cingulate gyrus | L | 43 | 3.72 | 0.013 | −3 | −1 | 33 |
| Superior frontal gyrus | R | 24 | 3.77 | 0.011 | 24 | 31 | 57 |
*FDR corrected P-values for the multiple comparisons.
EM = episodic memory; FC = functional connectivity; MNI = Montreal Neurological Institute coordinate.
Pittsburgh compound B retention and functional connectivities
Figure 3B and Table 2 show correlation analysis results between the regional PiB retention and the functional connectivities within the DMN in the PiB+ group alone. In the DMN of the PiB+ group, the functional connectivity of the left angular gyrus and the posterior cingulate demonstrated negative correlations with PiB retention in the anterior cingulate cortex (FDR corrected P < 0.05). In the salience network, there was no significant correlation between PiB retention and the functional connectivities in the PiB+ group. In addition, the functional connectivity of the left middle frontal gyrus and the inferior parietal gyrus (within the CEN) showed significant positive correlations with PiB retention in the anterior cingulate cortex, which was not significant when the mean functional connectivity of the angular gyrus and posterior cingulate (within the DMN) were partialled out (FDR corrected P < 0.05).
Effect of amyloid-β on the relationships between episodic memory and functional connectivities
In the DMN, there was a significant group × episodic memory interaction in the functional connectivities of (i) the left angular gyrus and the posterior cingulate; and (ii) the right superior frontal gyrus and the mid-cingulate cortex (Fig. 3C and Table 2, FDR corrected P < 0.05). As the interaction between group and episodic memory was statistically significant, to determine the direction of the association between functional connectivity and episodic memory, we performed separate general linear models for each PiB group (functional connectivity as outcome and episodic memory as the independent variable) with age, gender and education controlled. The episodic memory scores showed positive correlation with the left angular gyrus and the precuneus cortex, and negative correlations in the right superior frontal gyrus and left mid-cingulate cortex (Fig. 3C, FDR corrected P < 0.05) in the PiB+ group. However, no significant correlations between the episodic memory score and the functional connectivity were observed in the PiB− group. In the SN and CEN, no significant interactions or correlations were observed.
Inter-intrinsic connectivity network functional connectivity and relationship with PiB retention
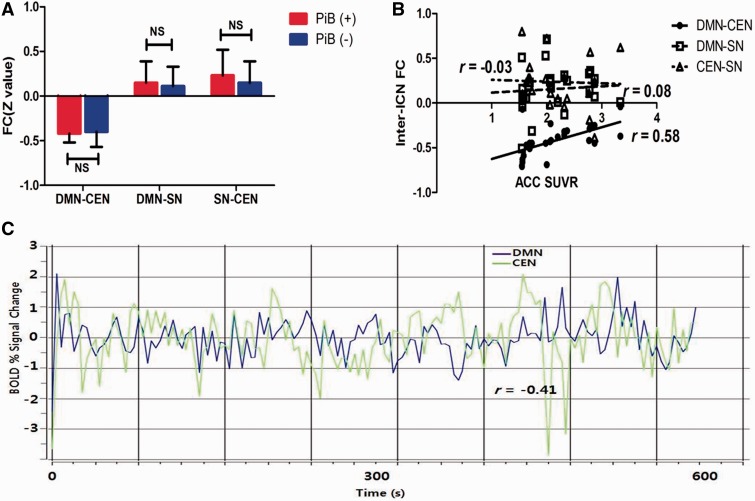
Both groups showed the negative correlation between the DMN functional connectivity and the CEN. As shown in Fig. 4C, the time course of blood oxygen level-dependent signal showed the anti-correlation between the DMN and the CEN. However, the SN showed no significant correlations between the DMN and the CEN. There were no significant inter-ICN functional connectivity differences between the PiB+ and the PiB− groups (Fig. 4A, Bonferroni corrected P < 0.05). In the PiB+ group, inter-ICN functional connectivity between the DMN and the CEN was positively correlated with the anterior cingulate cortex PiB level (Fig. 4B) indicating the DMN-CEN anti-correlation strength was negatively correlated with the anterior cingulate cortex PiB level. However, no significant correlations with PiB levels were observed in the other inter-ICN functional connectivities in the PiB+ group.
Figure 4.
(A) Group analysis results of the inter-ICN functional connectivity indicating no significant inter-ICN functional connectivity differences between the PiB+ and the PiB−groups. (B) Association between the inter-ICN functional connectivity and the anterior cingulate cortex standardized uptake value ratio in the PiB+ group indicating significant positive correlations between the anterior cingulate cortex standardized uptake value ratio and the DMN-CEN inter-ICN functional connectivity (r = 0.52, Holm-Bonferroni corrected P < 0.05) in the PiB+ group. (C) Single subject blood oxygen level-dependent time series for the DMN (blue) and the CEN (green). Note anti-correlations between the DMN and the CEN as evidenced by significant correlation coefficient (r = −0.41 Holm-Bonferroni corrected P < 0.05). FC = functional connectivity; ACC = anterior cingulate cortex; BOLD = blood oxygen level-dependent; NS = not significant; SUVR = standardized uptake value ratio.
Discussion
To the best of our knowledge, this is the first study to examine the effect of amyloid burden on the three large scale ICNs (the DMN, SN and CEN) in older adults with normal cognition. Furthermore, we describe a distinctive pattern of associations between functional connectivity, regional amyloid deposition and episodic memory in PiB+ individuals.
Functional connectivity differences between the PiB+ and PiB− groups
We found that the functional connectivities of (i) the DMN were greater; (ii) the SN were not different; and (iii) the CEN were lower in the PiB+ group, compared to the PiB− group. These results differed from previous studies that have found lower DMN functional connectivities in Alzheimer’s disease, amnestic MCI and cognitively normal subjects with amyloid burden (Hedden et al., 2009; Sheline et al., 2010; Mormino et al., 2011; Agosta et al., 2012). These discrepancies might be attributable to biphasic changes in functional connectivity across the longitudinal process of amyloid-β deposition, and therefore may not necessarily be contradictory (Ewers et al., 2011; Sperling et al., 2011). In these regards, we modified the two previous models proposed on the higher metabolic activity or functional connectivity according to amyloid deposition in Alzheimer’s disease trajectories (Cohen et al., 2009). One is the ‘acceleration’ hypothesis suggesting that once amyloid-β deposition is initiated by independent events, a milieu of higher functional connectivity hastens this deposition, which eventually leads to the functional disconnection or metabolic deterioration in the subjects with amyloid burden (Cohen et al., 2009; Johnson et al., 2014). During the period, there might be possibilities of the toxic excitation of affected neurons and compensatory higher functional connectivity induced by the amyloid retention (Mormino et al., 2011). Although not verified in humans in vivo, previous work in animal models has shown that intermediate levels of amyloid-β enhance synaptic activity presynaptically (Abramov et al., 2009), whereas abnormally high levels of amyloid-β impair synaptic activity by inducing post-synaptic depression (Palop and Mucke, 2010). In addition, a recent functional imaging study using FDG-PET showed greater metabolic activity in the superior temporal area and the medial thalamus in the PiB+ group compared to the PiB− group (Johnson et al., 2014). They proposed that this higher metabolism in the PiB+ group may represent an early event in the Alzheimer’s disease cascade in reaction to the presence of amyloid, in a process that eventually leads to metabolic decline. The other one is the ‘brain reserve’ hypothesis insisting that higher functional connectivity reflects a stable trait that imparts the capacity to withstand amyloid-β deposition and maintain normal cognition (Cohen et al., 2009). By selecting for normal cognition, we could have created a bias towards higher DMN functional connectivity in our PiB+ subjects that would not be required in the PiB− subjects. As the present study was cross-sectional and not suitable for explaining causality, prospective longitudinal studies would be needed to distinguish these possibilities.
Among the DMN areas showing aberrant functional connectivity, the PiB+ group showed higher bilateral hippocampal functional connectivity compared with the PiB− group. In contrast to our results, Sheline et al. (2010) found that PiB+ showed lower hippocampal functional connectivity within the DMN. They suggested that lower hippocampal functional connectivity dysfunction in the DMN might be due to its extensive connection with the posterior cingulate/precuneus cortex, and their approach of posterior cingulate seed-based analysis would be particularly sensitive to that. In the region of interest-to-region of interest connectivity within the DMN (Supplementary material), we found that the PiB+ individuals have greater functional connectivity between the hippocampus. In related work, Huijbers et al. (2014), used task-based functional MRI. They found that in cognitively unimpaired older adults, the presence of amyloid is associated with decreased deactivations; and the effect was localized to the entorhinal cortex. They suggest that the entorhinal cortex may be the most important medial temporal lobe structure, as it is more affected by amyloid-β retention due in part to their extensive connection with the DMN in the cognitively normal older adults (Huijbers et al., 2014). The entorhinal and hippocampal functional connectivity changes in older adults are of particular interest because they are directly linked to the episodic memory functions which are pathognomonic signs of the Alzheimer’s disease (Huijbers et al., 2014). Besides the amyloid-β retention, tau pathology has also been linked with these medial temporal memory systems (Braak and Braak, 1991; Huijbers et al., 2014). Hence, as the recent development of the tau PET in vivo imaging (Villemagne and Okamura, 2014), future multimodal analyses of functional connectivity, PiB and tau imaging could clarify the relationship between the Alzheimer’s disease pathology and medial temporal system functional changes.
In addition to the functional connectivity change in the DMN, we also observed decreased functional connectivities of the CEN and no significant change of the functional connectivity in the SN in the PiB+ group relative to the PiB− group. The DMN is known to be involved in the retrieval of autobiographical episodic memory as well as self-referential processing (Spreng et al., 2009; Whitfield Gabrieli et al., 2011). The CEN is critical for active maintenance and manipulation of information in working memory, and for judgement and decision-making in the context of goal-directed behaviour (Petrides, 2005; Muller and Knight, 2006; Koechlin and Summerfield, 2007). In addition, the SN is known to be involved in cognitive control functions such as attention, working memory, and response selection, and also serves the dynamic switching between the DMN and the CEN (Menon et al., 2001; Menon and Uddin, 2010). During tasks that demand attention and mental control, the DMN is usually deactivated, but the CEN and the SN are activated (Fox et al., 2005; Uddin et al., 2009; Menon and Uddin, 2010). This reciprocal relationship between these ICNs has been supported by several studies that have found negative correlations (anti-correlations) between the DMN and the CEN or SN (Uddin et al., 2009; Menon and Uddin, 2010). Agosta et al. (2012) found greater functional connectivities of the CEN and lower functional connectivities of the DMN in Alzheimer’s disease (Agosta et al., 2012). They also reported lower functional connectivities in the DMN, but not in the CEN and SN in subjects with amnestic MCI (Agosta et al., 2012). Another resting state functional MRI study by Machulda et al. (2011) revealed lower functional connectivities in the DMN and relatively greater functional connectivities in the SN in cognitively normal older adults who carry the ApoE4 allele. They suggested that the greater frontal lobe functional connectivities (the CEN and the SN) might be due to a compensatory mechanism associated with DMN impairments (Machulda et al., 2011). Our results, which show greater DMN functional connectivity and lower CEN, which were reciprocally anti-correlated, differ from other studies. This might be due to aberrant hyperactivity of the DMN and its anti-correlation to the CEN (i.e. a compensatory process).
Relationships between the PiB retention and functional connectivity
We observed a significant negative correlation between the regional PiB retention in the anterior cingulate cortex and the functional connectivity of the posterior DMN (posterior cingulate/angular gyrus) in the PiB+ group. In addition, we also found a significant positive correlation between the anterior cingulate cortex PiB retention and the left fronto-parietal functional connectivity (within the CEN) in the PiB+ group. These are consistent with a previous study showing a negative correlation between the global PiB retention and the posterior DMN functional connectivity (Mormino et al., 2011). Moreover, this same study also reported positive correlations between the frontal DMN functional connectivities and global PiB retention (Mormino et al., 2011). Interestingly, our results show that the regional PiB accumulation in the anterior cingulate cortex was only significantly correlated with the functional connectivities (posterior DMN and fronto-parietal CEN) in the PiB+ group. As seen in the Fig. 1, the PiB+ group showed the weighted retention of the PiB in the anterior cingulate cortex, compared with the other brain areas. This weighted anterior cingulate cortex PiB retention was also observed in the validation study of our quantitative classification method of the PiB positivity (sparse k-means clustering method) in the cognitively normal older adults (Cohen et al., 2013), which was in accordance with pathological Braak stage I indicating earlier amyloid deposition in the brain (Braak and Braak, 1991). In these regards, as compared to retention in the other areas, the weighted retention of amyloid in the anterior cingulate cortex might cause a significant effect on the functional connectivities in the PiB+ group. In addition, the ‘distant’ correlation pattern was in-line with our previous study using FDG-PET in the Alzheimer’s disease group (Cohen et al., 2009). Although these results do not clarify the precise underlying neurobiological mechanisms, they do suggest that the ‘distant’ correlation pattern between the frontal PiB level and the posterior cingulate metabolism might be attributable to the closely inter-correlated PiB retention throughout the brain in the Alzheimer’s disease group. In this light, our result of ‘distant’ correlations should be interpreted cautiously because highly interrelated covariance of regional PiB levels (Supplementary Table 3) could generate apparent ‘distant’ correlations even when none exist. Overall, the negative correlation of lower functional connectivity with higher PiB retention, together with the observation of greater functional connectivity in the DMN of PiB+ subjects, does not distinguish between the compensation and high-reserve explanations presented above but prove the downstream effect of amyloid-β deposition on functional connectivity (Mormino et al., 2011). It could be that early compensatory increases fail with increased amyloid-β deposition or that baseline high functional connectivity (reserve) diminishes as amyloid-β load increases (downstream effect) (Mormino et al., 2011).
In this study, we also investigated the inter-ICN functional connectivity, which showed no significant group differences. In addition, we observed that anti-correlation strength between the DMN and the CEN was negatively correlated with anterior cingulate cortex PiB level in the PiB+ group. A previous study found that the DMN-CEN anti-correlation diminished according to the disease staging (normal, amnestic MCI and Alzheimer’s disease) (Brier et al., 2012). Other previous work has shown that the DMN and the CEN functional connectivities were negatively correlated with PiB level in the amnestic MCI group, but not in the control group (Myers et al., 2014). They proposed a graded network degeneration hypothesis that amyloid-β pathology deposition and connectivity-based spread will start in the DMN, followed in close succession by a number of other heteromodal networks, leading to a cross-network functional connectivity gradient according to Alzheimer’s disease progression (Myers et al., 2014). Our results expand previous studies to cognitively normal older adults by suggesting that the amyloid burden may be related to loss of the DMN-CEN anti-correlation, which may play an important role in maintaining normal cognition. However, further longitudinal analysis will be needed to confirm the effect of the amyloid on DMN-CEN anti-correlation according to Alzheimer’s disease cascade.
Effect of amyloid-β on the relationships between episodic memory and functional connectivities
In the present study we also compared the effect of amyloid-β on the relationships between episodic memory and functional connectivities of three ICNs. We found significant group × episodic memory interactions in the posterior and frontal DMN functional connectivity. Furthermore, the PiB+ group showed a positive correlation between functional connectivity and episodic memory in the posterior DMN, and negative correlations in the frontal DMN, which may be interpreted in two ways: (i) poorer episodic memory associated with higher functional connectivity in frontal DMN and lower functional connectivity in posterior DMN; and (ii) better episodic memory associated with higher functional connectivity in posterior DMN and lower functional connectivity in the frontal DMN in the PiB+ group. The former interpretation was in-line with the previous functional studies in cognitively normal older adults (Mormino et al., 2011) and subjects with amnestic MCI (Dunn et al., 2014), reflecting a downstream effect of the amyloid retention in the posterior DMN and compensation of the frontal DMN brain (Mormino et al., 2011). In addition, this interpretation could be supported by our findings that the DMN functional connectivity-episodic memory correlation patterns were similar to the DMN functional connectivity-PiB level correlation patterns. However, as there was only a negative trend between the PiB level and episodic memory (Supplementary Table 9), we should interpret these relationships cautiously. In terms of the latter interpretation, as discussed above, this relationship could represent a stable trait, reflecting brain reserve with efficient cognitive processing (Cohen et al., 2009). This was supported by several functional MRI studies showing that the posterior DMN was activated during episodic memory retrieval and the frontal DMN was strongly deactivated in younger cognitively normal adults (Buckner et al., 2008; Sestieri et al., 2011). Overall, our results show dissociation between the frontal and posterior DMN in relation to episodic memory, which is in-line with previous studies. However, the precise neurobiological mechanisms underlying the dissociation between the frontal and posterior DMN are still not clear. Future longitudinal analyses are needed to confirm the effect of amyloid on functional connectivity and cognitive decline.
Limitations and future perspectives
The limitations of our study include the following. First, as this study was cross-sectional, we can only report correlations, and have limited ability to infer causal pathways. Further longitudinal analysis on the trajectories of Alzheimer’s disease and normal ageing will be needed to clarify the causal effect of amyloid-β on the ICNs. Second, although the number of the subjects with the ApoE4 allele was low, there might be an effect of the ApoE4 allele on the functional connectivity of ICNs in the cognitively normal subjects as several previous studies indicated (Filippini et al., 2009; Machulda et al., 2011). Hence, exploring the interaction between the APOE gene, functional connectivities and PiB retention would be needed for an integrative model of neurobiological mechanisms of the normal ageing process. Third, as we recruited ICNs of interest that were most similar to the previously identified networks template, we could not observe other networks of interest, such as the right-side CEN. Last, we could not investigate the spatial correlation between amyloid burden and functional connectivity within the network boundaries. As indicated in the previous study in the subjects with amnestic MCI (Myers et al., 2014), these distinctive correlations within the boundary of network would be helpful to identify the more sophisticated neurobiological process of amyloid burden on functional connectivity in cognitively normal older adults.
In conclusion, we have shown altered functional connectivities of the three large functional ICNs and a distinctive pattern of correlations with episodic memory and regional PiB retention in cognitively normal older adults with amyloid burden. As recent clinical trials of disease-modifying agents in Alzheimer’s disease have failed, the focus in Alzheimer’s disease drug development is shifting from treatment to prevention (Vellas et al., 2011). Hence, the newer strategies will examine the potential neuroprotective activity of these drugs in the preclinical stage of Alzheimer’s disease, with the help of biomarkers that predict disease progression before development of overt dementia (Salomone et al., 2012). If our results of aberrant DMN hyperactivity/hyper-connectivity in the PiB+ group reflect a very early, reversible functional change in the longitudinal course of Alzheimer’s disease progression, these changes in functional connectivity in large ICNs could be a critical measure of therapeutic intervention. However, large and longitudinal prospective studies will be necessary to test this hypothesis
Funding
The following funding sources contributed to this study as a whole, including design and conduct, collection, management, analysis, and interpretation of the data, as well as preparation and review of this manuscript. Grant support included: P50 AG005133, R37 AG025516, P01 AG025204, 5K23AG038479, R21 NS060184, and R01 MH076079 from the National Institutes of Health.
Conflict of interest
GE Healthcare holds a license agreement with the University of Pittsburgh based on the technology described in this manuscript. Drs Klunk and Mathis are co-inventors of PiB and, as such, have a financial interest in this license agreement. GE Healthcare provided no grant support for this study and had no role in the design or interpretation of results or preparation of this manuscript. All other authors have no conflicts of interest with this work and had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- CEN
central executive network
- DMN
default mode network
- ICN
intrinsic connectivity network
- MCI
mild cognitive impairment
- PiB
Pittsburgh compound B
- SN
salience network
References
- Abramov E, Dolev I, Fogel H, Ciccotosto G, Ruff E, Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12:1567–76. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni G, Filippi M. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol Aging. 2012;33:1564–78. doi: 10.1016/j.neurobiolaging.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Bressler S, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–90. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J Neurosci. 2012;32:8890–9. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cohen A, Price J, Weissfeld L, James J, Rosario B, Bi W, et al. Basal cerebral metabolism may modulate the cognitive effects of Abeta in mild cognitive impairment: an example of brain reserve. J Neurosci. 2009;29:14770–8. doi: 10.1523/JNEUROSCI.3669-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AD, Mowrey W, Weissfeld LA, Aizenstein HJ, McDade E, Mountz JM, et al. Classification of amyloid-positivity in controls: comparison of visual read and quantitative approaches. Neuroimage. 2013;71:207–15. doi: 10.1016/j.neuroimage.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS. Resting-state fMRI as a biomarker for Alzheimer's disease? Alzheimer's Res Ther. 2012:4–8. doi: 10.1186/alzrt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CJ, Duffy SL, Hickie IB, Lagopoulos J, Lewis SJ, Naismith SL, et al. Deficits in episodic memory retrieval reveal impaired default mode network connectivity in amnestic mild cognitive impairment. Neuroimage Clin. 2014;4:473–80. doi: 10.1016/j.nicl.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer's disease dementia. Trends Neurosci. 2011;34:430–42. doi: 10.1016/j.tins.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA. 2009;106:7209–14. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Snyder A, Vincent J, Corbetta M, Van Essen D, Raichle M. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C, Lazar N, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–94. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Huijbers W, Mormino EC, Wigman SE, Ward AM, Vannini P, McLaren DG, et al. Amyloid deposition is linked to aberrant entorhinal activity among cognitively normal older adults. J Neurosci. 2014;34:5200–10. doi: 10.1523/JNEUROSCI.3579-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132 (Pt 5):1355–65. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Christian BT, Okonkwo OC, Oh JM, Harding S, Xu G, et al. Amyloid burden and neural function in people at risk for Alzheimer's Disease. Neurobiol Aging. 2014;35:576–84. doi: 10.1016/j.neurobiolaging.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11:229–35. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Jones DT, Vemuri P, McDade E, Avula R, Przybelski S, et al. Effect of APOE epsilon4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch Neurol. 2011;68:1131–6. doi: 10.1001/archneurol.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer CC, Kinahan PE, Greer PJ, Nichols TE, Comtat C, Cantwell MN, et al. Comparative evaluation of MR-based partial-volume correction schemes for PET. J Nucl Med. 1999;40:2053–65. [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–43. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin L. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Smiljic A, Hayenga AO, Onami SH, Greicius MD, Rabinovici GD, et al. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cerebral Cortex. 2011;21:2399–407. doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J, Roe C, Grant E, Head D, Storandt M, Goate A, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–75. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Muller NG, Knight RT. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. 2006;139:51–8. doi: 10.1016/j.neuroscience.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Myers N, Pasquini L, Gottler J, Grimmer T, Koch K, Ortner M, et al. Within-patient correspondence of amyloid-beta and intrinsic network connectivity in Alzheimer's disease. Brain. 2014;137(Pt 7):2052–64. doi: 10.1093/brain/awu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop J, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–8. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005;360:781–95. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Klunk W, Lopresti B, Lu X, Hoge J, Ziolko S, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metabo. 2005;25:1528–47. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- Salomone S, Caraci F, Leggio GM, Fedotova J, Drago F. New pharmacological strategies for treatment of Alzheimer's disease: focus on disease modifying drugs. Br J Clin Pharmacol. 2012;73:504–17. doi: 10.1111/j.1365-2125.2011.04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 2011;31:4407–20. doi: 10.1523/JNEUROSCI.3335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67:584–7. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22:158–65. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–88. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar R, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Storandt M, Mintun M, Head D, Morris J. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–81. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L, Kelly AM, Biswal B, Castellanos FX, Milham M. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–37. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, Hedden T, Becker J, Sullivan C, Putcha D, Rentz D, et al. Age and amyloid-related alterations in default network habituation to stimulus repetition. Neurobiol Aging. 2012;33:1237–52. doi: 10.1016/j.neurobiolaging.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellas B, Aisen P, Sampaio C, Carrillo M, Scheltens P, Scherrer B, et al. Prevention trials in Alzheimer's disease: an EU-US task force report. Prog Neurobiol. 2011;95:594–600. doi: 10.1016/j.pneurobio.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Jones DT, Jack CR., Jr Resting state functional MRI in Alzheimer's disease. Alzheimer's Res Ther. 2012;4:2. doi: 10.1186/alzrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Okamura N. In vivo tau imaging: obstacles and progress. Alzheimer's Demen. 2014;10(3 Suppl):S254–64. doi: 10.1016/j.jalz.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Whitfield Gabrieli S, Moran JM, Nieto-Castañón A, Triantafyllou C, Saxe R, Gabrieli JD. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage. 2011;55:225–32. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]