Abstract
This scientific commentary refers to ‘Regional amyloid burden and intrinsic connectivity networks in cognitively normal elderly subjects’ by Lim et al. (doi:10.1093/brain/awu271).
This scientific commentary refers to ‘Regional amyloid burden and intrinsic connectivity networks in cognitively normal elderly subjects’ by Lim et al. (doi:10.1093/awu271).
Connectivity in the brain is dynamic. From development through normal ageing or as a result of pathological processes, structural and functional connectivity are in a constantly changing state. Some changes may occur rapidly as a consequence of learning, for example, but others may take years or decades. Our ability to witness and measure changes in structural connectivity relies on the diffusion properties of water (e.g. diffusion tensor imaging, DTI). As for functional connectivity, detected as active regions with temporal contiguity, this relies on changes in blood oxygenation (e.g. resting state functional MRI). In this issue of Brain, Lim et al. (2014) examine the effects of amyloid deposition on the intrinsic functional connectivity networks in cognitively normal elderly populations. They report that the presence of amyloid, as measured with the PET tracer Pittsburgh Compound B (PiB), is associated with changes in network connectivity and also with regional interactions within those networks.
These measures of connectivity are important in the quest to better understand normal and compromised brain function. Connectivity, when combined with other morphological and physiological measures, provides a far more comprehensive picture of the brain in health and disease than its shape alone. Anatomically, many fascicles, bundles and tracts have been mapped in exquisite detail in mostly normal populations and also in a number of cohorts with neurodegenerative diseases, psychiatric illnesses, and even infectious diseases. Although subpopulation variability is largely unknown, there are efforts underway to assess that and relate it to genetics and other factors (Jahanshad et al., 2013; http://www.humanconnectomeproject.org/). Studies of structural brain connectivity in hundreds of subjects are beginning to reveal characteristic changes in brain networks with development (Dennis et al., 2013), and more subtle differences with regard to sex and genotype. Early in life, short-range structural connections tend to mature more quickly than long-range connections, and the brain develops a relatively ‘modular’ organization. van den Heuvel and Sporns (2011) suggest that the brain also develops a ‘rich club’ organization, in which very highly connected ‘hubs’ integrate local information and communicate with other hubs via long-range connections, such as the language pathway, or interhemispheric commissures. Similar patterns of hubs arise in airline and other transport networks, and hubs improve the communication efficiency of the network as a whole. The gradual loss of structural connections in Alzheimer’s disease may progress in the opposite sequence to normal development, in a first-in, first-out sequence, with gradual loss of characteristic features of efficient networks, such as ‘small-world organization’.
Functional connectivity, as determined using resting state functional MRI, has revealed a number of networks of coordinated activity (Biswal et al., 1995). Based upon either modelled or model-free approaches, at least three canonical networks have been identified: (i) the default mode network (Raichle et al., 2001), which includes the ventromedial prefrontal cortex and posterior cingulate cortex; (ii) a salience network, which includes the ventrolateral prefrontal cortex and anterior insula (sometimes called the fronto-insular cortex), as well as the anterior cingulate cortex; and (iii) a central executive network, whose key nodes include the dorsolateral prefrontal cortex and posterior parietal cortex. Figure 1 illustrates these three networks anatomically using tractography seeded with resting state functional MRI data. These networks have been examined in groups of patients with neurological diseases and psychiatric disorders such as Alzheimer’s disease, Parkinson’s disease, epilepsy, schizophrenia and autism. One early landmark study mapped the emergence of functional networks in children, and found that the age of the child could be predicted surprisingly well from networks extracted from resting state functional MRI (Dosenbach et al., 2010).
Figure 1.
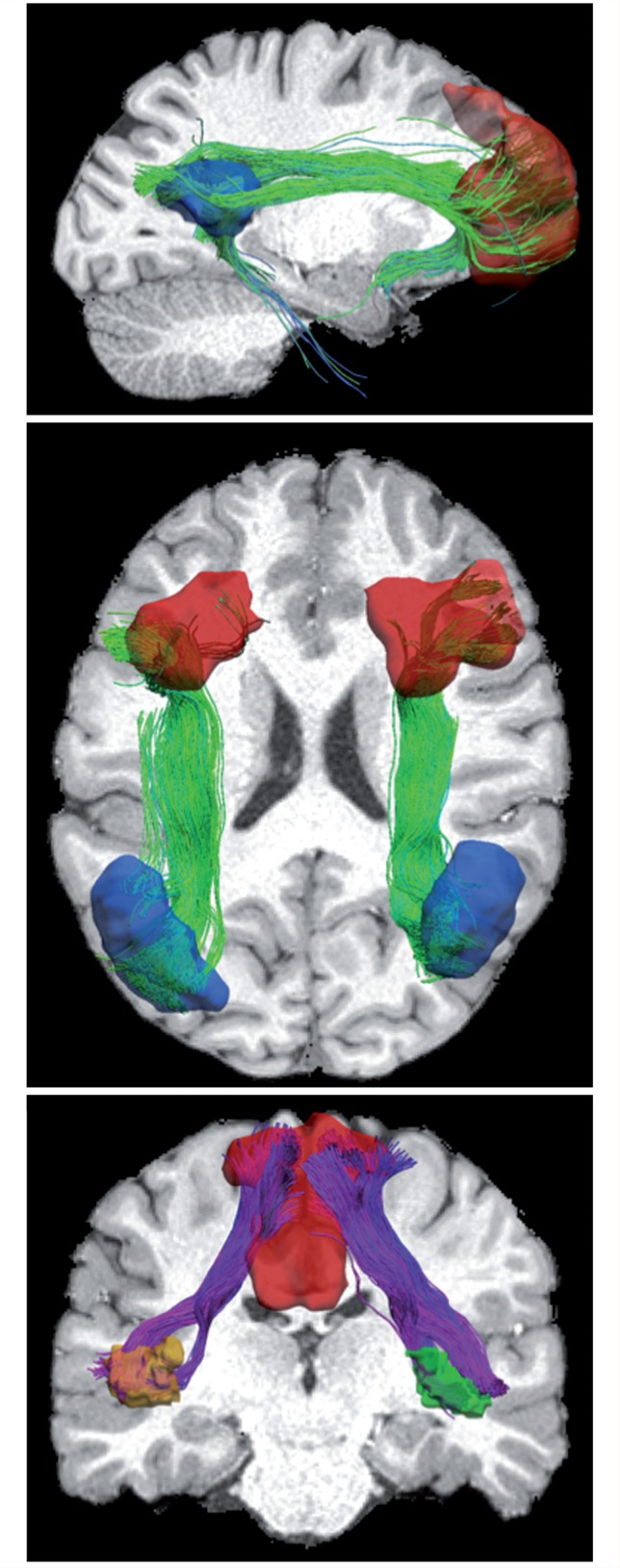
The anatomical substrate of intrinsic functional networks derived from tractography seeded with sites from resting state functional MRI. For each network, regions of interest were warped from the functional region of interest atlas to one random subject selected from the Human Connectome Project database. Note that because the regions of interest are generated from functional images (using ICA), there is no anatomical constraint. Using the fibre orientation distribution already computed, probabilistic tractography was performed between a pair of regions of interest to generate the fibre bundles. Outliers were removed with simple length-based thresholding. Finally, visualizations were generated to overlay the surface representation of regions of interest and fibre bundles. Top: Default mode network: ventromedial prefrontal cortex and posterior cingulate cortex. Middle: Central executive network: dorsolateral prefrontal cortex and posterior parietal cortex in both left and right hemispheres. Bottom: Salience network: ventrolateral prefrontal cortex and anterior insula in both hemispheres. Only the part of the salience network between insula and cingulate is shown here.
Numerous resting state studies suggest some overlap between the classical pattern of amyloid accumulation in the brain (Braak and Braak, 1991) and hubs of the default mode network (Hedden et al., 2009). Using independent component analysis (ICA), Greicius (2008) found decreased connectivity across the default mode network in Alzheimer’s disease—in frontal, parietal, and temporal cortices, and the hippocampus. In one multi-modal study of 93 adults aged 18–93 years, Andrews-Hanna et al. (2010) found a significant reduction in functional connectivity between anterior and posterior hubs of the default mode network (medial prefrontal cortex and posterior parietal cortex) with normal ageing, but this reduction was not solely explainable by increases in brain amyloid accumulation. Mormino et al. (2011) also used an ICA approach to investigate amyloid-β deposition, and found significant overlap with regions of the default mode network: elevated PiB was associated with decreased network connectivity in the ventral-medial prefrontal cortex, right angular gyrus, and left middle and superior frontal gyri. Lim and colleagues’ study suggests a possible interdependency in network connectivity among the default mode network, salience network and central-executive network (Lim et al., 2014). The attempt to determine how these networks respond to amyloid burden, and their relationship to episodic memory, is a more whole brain approach to age-related changes in cognition than that of earlier work. Their study suggests a complex cascade of changes with increasing amyloid, and that compensatory mechanisms or reserve capacity may have varying effects on different networks over time.
We do not yet understand the mechanisms whereby Alzheimer pathology affects the hubs of the brain’s functional networks. Early cellular dysfunction, including vascular insufficiency, metabolic compromise, inflammation, and myelin degradation may disrupt the synchrony of brain networks, followed later by overt loss of neurons and their axonal connections to other parts of the brain, along with Wallerian degeneration. Some speculate that the highly adaptive systems involved in learning and memory—with their high metabolic activity—may be more vulnerable to neuronal loss due to amyloid and tau pathology than the heavily myelinated primary sensorimotor and visual systems that mature the earliest in childhood. Typically, the primary cortices show least atrophy in ageing and Alzheimer’s disease, and appear functionally intact until the advanced stages of disease.
Balanced and symbiotic activity among the larger networks of the brain may also be important for normal functioning. Disturbance of equilibrium owing to pathological processes such as amyloid-β accumulation may produce increases and decreases in network connectivity as compensatory mechanisms. Agosta et al. (2012) examined the default mode, central executive, and salience networks in Alzheimer’s disease and mild cognitive impairment. Surprisingly, they found increased mean connectivity in Alzheimer’s disease in the central-executive component, and this was associated with cognitive scores. They suggested that the increased connectivity may be the brain’s attempt to limit the functional consequences of continuing tissue damage.
Several large-scale initiatives are underway that seek to understand how brain connectivity varies with age and genetic factors over the lifespan. The Human Connectome Project (http://www.humanconnectomeproject.org/), for example, is scanning large cohorts of normal twins and advancing the power of imaging methods to assess brain connectivity, such as diffusion spectrum imaging, and ultra-high field imaging. The quest to identify genetic variants that affect brain connectivity has also begun in earnest; international collaborations such as ENIGMA (http://enigma.ini.usc.edu/) have begun to identify heritable measures of brain connectivity across cohorts scanned around the world, aggregating these data into large-scale genome/connectome-wide screens. At the same time, large-scale neuroimaging studies in ageing and dementia, such as the Alzheimer’s Disease Neuroimaging Initiative (ADNI; http://www.adni-info.org/), GAAIN (http://www.gaain.org/) and DIAN (http://www.dian-info.org) are beginning to identify connectivity metrics that characterize and predict network breakdown as we age, along with prodromal changes in functional brain connectivity in autosomal dominant forms of Alzheimer’s disease.
The sensitivity of resting state functional MRI to acute changes in brain networks has also attracted the attention of clinical trial designers, who are keen to identify treatment effects over intervals that are as short as possible. Ultimately, defining which features of image acquisition and analysis promote reproducible studies and metrics of brain connectivity will make it easier to integrate, coordinate, and meta-analyse connectivity data across disparate studies that were initially designed independently.
The broad interest in methods to assess brain connectivity relative to other measures associated with ageing and dementia suggests that major advances are imminent, revealing new factors that influence brain network changes in the millisecond range, across the entire human lifespan and as a result of neurodegenerative disease.
Acknowledgements
The authors would like to thank Yonggang Shi of the Laboratory of Neuro Imaging for preparation of Fig. 1.
Funding
Work related to this commentary was supported by the National Institutes of Health (Rosen), R01MH094343, and P41EB015922 to A.W.T.
References
- Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol Aging. 2012;33:1564–78. doi: 10.1016/j.neurobiolaging.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–62. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Res Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;1:213–6. doi: 10.1111/j.1750-3639.1991.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Dennis EL, Jahanshad N, Toga AW, McMahon KL, de Zubicaray GI, Martin NG, et al. Development of brain structural connectivity between ages 12 and 30: a 4-tesla diffusion imaging study in 439 adolescents and adults. Neuroimage. 2013;64:671–84. doi: 10.1016/j.neuroimage.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–61. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KRA, Becker JA, Mehta A, Sperling RA, Johnson KA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–94. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshad N, Toga AW, McMahon KL, de Zubicaray GI, Martin NG, Wright MJ, et al. Connectome-wide genome-wide search discovers SPON1 gene variant influencing dementia severity. PNAS. 2013;110:4768–73. doi: 10.1073/pnas.1216206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HK, Nebes R, Snitz B, Cohen A, Mathis C, Price J, et al. Regional amyloid burden and intrinsic connectivity networks in cognitively normal elderly. Brain. 2014;137:3320–31. doi: 10.1093/brain/awu271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Smiljic A, Hayenga AO, Onami Sh, Greicius MD, Rabinovici GD, et al. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex. 2011;21:2399–407. doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. PNAS. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31:15775–86. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


