Accumulation of amyloid-beta leads to loss of functional synapses in Alzheimer’s disease. Dorostkar et al. report that immunotherapy against oligomeric amyloid-beta in the Tg2576 mouse model attenuates synapse loss near plaques, and abolishes it elsewhere. Sequestering oligomeric amyloid-beta may counteract synaptic pathology, even while fibrillar amyloid load remains unchanged.
Keywords: Alzheimer’s disease pathology, immunotherapy, amyloid-β oligomers
Abstract
Cognitive decline in Alzheimer’s disease is attributed to loss of functional synapses, most likely caused by synaptotoxic, oligomeric forms of amyloid-β. Many treatment options aim at reducing amyloid-β levels in the brain, either by decreasing its production or by increasing its clearance. We quantified the effects of immunotherapy directed against oligomeric amyloid-β in Tg2576 mice, a mouse model of familial Alzheimer’s disease. Treatment of 12-month-old mice with oligomer-specific (A-887755) or conformation-unspecific (6G1) antibodies for 8 weeks did not affect fibrillar plaque density or growth. We also quantified densities of DLG4 (previously known as PSD95) expressing post-synapses and synapsin expressing presynapses immunohistochemically. We found that both pre- and post-synapses were strongly reduced in the vicinity of plaques, whereas distant from plaques, in the cortex and hippocampal CA1 field, only post-synapses were reduced. Immunotherapy alleviated this synapse loss. Synapse loss was completely abolished distant from plaques, whereas it was only attenuated in the vicinity of plaques. These results suggest that fibrillar plaques may act as reservoirs for synaptotoxic, oligomeric amyloid-β and that sequestering oligomers suffices to counteract synaptic pathology. Therefore, cognitive function may be improved by immunotherapy even when the load of fibrillar amyloid remains unchanged.
Introduction
Alzheimer’s disease is characterized by specific neuropathological changes, which are thought to be caused by a cascade of events (Hardy and Selkoe, 2002), initiated by an imbalance of amyloid-β production and clearance, leading to amyloid plaque deposition, ultimately leading to neuron loss and brain atrophy. However, cognitive deficits may be present several years before the onset of neuron loss and gross brain atrophy and these can be attributed to morphological alterations and loss of synapses (DeKosky and Scheff, 1990; Scheff et al., 1990). It is unclear how exactly amyloid-β harms neurons, though the main effectors seem to be soluble, oligomeric amyloid-β species, which were shown to have a variety of harmful effects (Gilbert, 2013).
As an imbalance between amyloid production and catabolism leads to the formation of plaques (Saido, 1998), one treatment strategy aims at sequestering amyloid-β from the CNS. For instance, in animal studies anti-amyloid immunotherapy reduced amyloid load and improved cognitive performance (Wilcock et al., 2004; Wang et al., 2011). On the other hand, trials in patients were largely unsuccessful, partly because of side effects and lack of cognitive improvement (Doody et al., 2014; Salloway et al., 2014). Nevertheless, a number of clinical trials are ongoing using adjusted experimental or treatment paradigms (Karran and Hardy, 2014).
We studied the effects of passive immunotherapy directed against oligomeric amyloid-β on synaptic pathology and plaque growth in an animal model of familial Alzheimer’s disease. Pan-amyloid-β antibodies as well as oligomer-specific antibodies alleviated the synaptic pathology, while amyloid deposition was unaffected by the treatment regimen.
Materials and methods
Animals
All animal procedures had been approved by the animal welfare official of the Ludwig-Maximilian University and the Government of Upper Bavaria.
For in vivo imaging experiments, female, 12–18 month old, Tg2576 mice (Hsiao et al., 1996), which had been obtained from Taconic, were used and non-transgenic littermates served as controls. Detailed protocols and settings are listed in the Supplementary material.
Antibody treatment
A weekly dose of 500 µg antibody per animal was given eight times, starting after the first imaging session, at 12 months of age. Animals received one of the following antibodies by intraperitoneal injection: 6G1, which is pan-amyloid-β specific and has similar binding characteristics as the diagnostic 6E10 antibody (Barghorn et al., 2005); A-887755, a globulomer-derived antibody which specifically detects oligomeric, but not monomeric or fibrillar amyloid-β (Hillen et al., 2010); IgG2a as a non-amyloid specific control. A fourth cohort was not treated. The experimenter was blind to the type of antibody administered.
The brain penetration of antibodies, as tested in different cohorts, was in the range of 0.1% of plasma levels.
Statistics
Synapse densities in relation to the distance from plaques were fit using one-phase association curves and the best-fit values for the plateaus were compared using F-tests. Synapse densities distant from plaques were compared using t-tests. Plaque densities, volumes and growth rates were compared using Kruskal-Wallis tests with Dunn’s post hoc test. Unless specified otherwise, error bars show standard deviation (SD).
Results
Fibrillar plaques are unaffected by immunotherapy
In vivo imaging was performed once a week and the antibody treatment was administered at the end of the treatment session. Thereafter, the animals were sacrificed and the brains removed and processed for immunohistochemistry (Supplementary material and Fig. 1A).
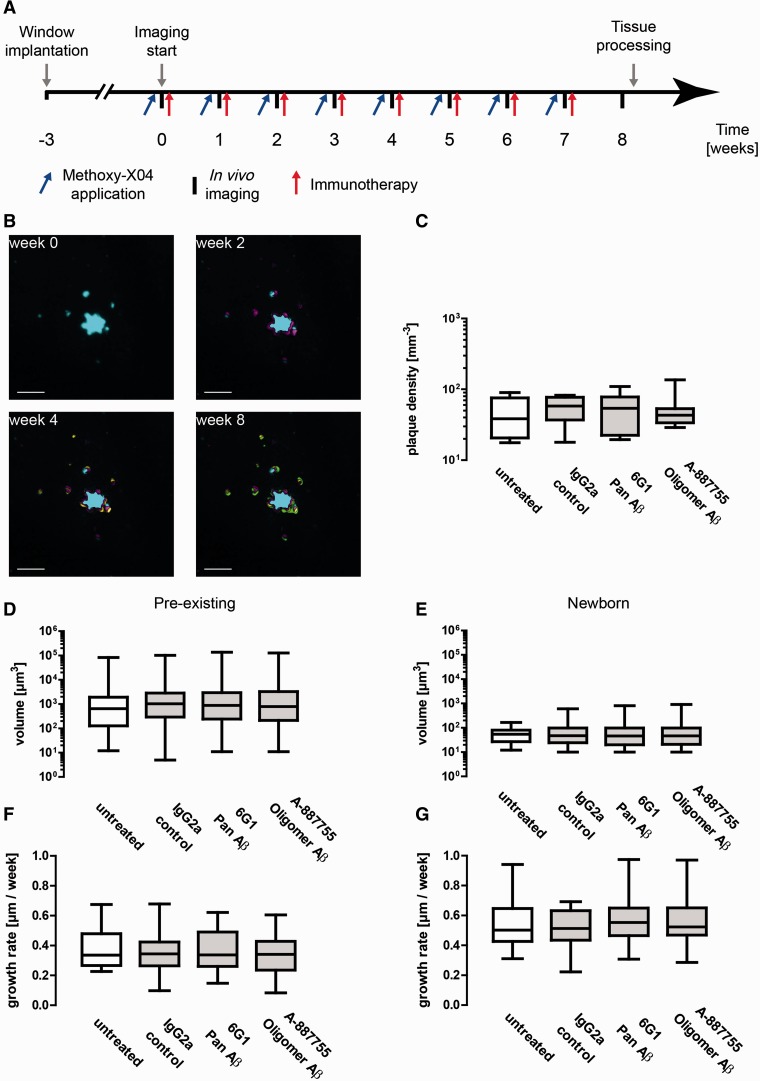
Figure 1.
Anti-amyloid immunotherapy with 6G1 and A-887755 antibodies has no effect on fibrillar plaque growth and density. 6G1 recognize monomeric, oligomeric and fibrillar amyloid-β (Aβ), A-887755 recognizes only oligomeric amyloid-β. (A) Timeline of experimental procedures. Methoxy-X04 was applied 24 h before in vivo imaging, immunotherapy was administered immediately afterwards. (B) Chronic in vivo imaging of fibrillar plaques. Maximum intensity projections of volumes are shown, while analyses were performed in 3D. Changes from the previous time points are colour-coded. Scale bars = 10 µm. (C) Overall plaque densities at the last imaging session (8 weeks). (D) Volumes of pre-existing plaques, i.e. plaques that were present at the first imaging session, and (E) volumes of newborn plaques, i.e. plaques that appeared during the treatment period. (F) Linear growth rates of pre-existing plaques. Linear growth is proportional to the cube root of the differences of plaque volumes. (G) Linear growth rates of newborn plaques. (C–G) Box-and-whiskers plots show median, 25th and 75th percentiles (box) as well as minimum and maximum values (whiskers). Groups were compared using Kruskal-Wallis tests. No significant differences were found between groups (P > 0.05).
We quantified the number, size and growth rates of fibrillar plaques in vivo by two-photon imaging of methoxy-X04 stained fibrillar deposits in the somatosensory cortex during the treatment period (Burgold et al., 2014). First, we quantified the plaque densities in the volumes imaged. Because of the heterogeneity of amyloid deposition in Tg2576 mice, the resulting data showed large variances. At the end of the treatment period, overall plaque densities did not differ significantly between groups (Kruskal-Wallis test, n = 4–8; Fig. 1C). Because amyloid deposition is a gradual process, the majority of the plaques may have been deposited before the treatment had an effect. Therefore, we analysed the volumes of pre-existing plaques, i.e. plaques that had been present before the treatment was started (Fig. 1D) and newborn plaques, i.e. plaques that appeared during the treatment period, separately (Fig. 1E). Neither group were altered by immunotherapy (Kruskal-Wallis test, n = 4–8; Fig. 3B and C). Finally, we analysed the linear growth rate of pre-existing and newborn plaques, which is proportional to the cube root of the difference in volumes between two imaging sessions (Hefendehl et al., 2011; Burgold et al., 2014). Again, immunotherapy had no effect on the growth rates of fibrillar plaques (Kruskal-Wallis test, n = 4–8; Fig. 1 F and G). To assess the efficacy of anti-amyloid-β antibodies, we first quantified the levels of total soluble and oligomeric amyloid-β in Tg2576 mice from 4.5 to 15 months of age, using quantitative immunoprecipitation with 6E10 and A-887755 antibodies, respectively (Supplementary Fig. 1A). Then we quantified amyloid-β levels in animals, which had been immunized at 3 months with either amyloid-β1-42 monomer or with amyloid-β20-42 globulomer. The latter generate a monospecific immune response resembling the antibody specificity of A-887755. At 12 months, the brain levels of total soluble and oligomeric amyloid-β were quantified (Supplementary Fig. 1B and C). Immunization with amyloid-β1-42 monomer caused a significant reduction in both total soluble amyloid-β as well as oligomeric amyloid (ANOVA with Bonferroni’s post hoc test; n = 11–14; Supplementary Fig. 1B and C), whereas immunization with amyloid-β20-42 globulomer caused a significant reduction only in oligomeric amyloid (ANOVA with Bonferroni’s post hoc test; n = 11–14; Supplementary Fig. 1B and C). Thus, immunotherapy caused a reduction in the respective amyloid isoforms.
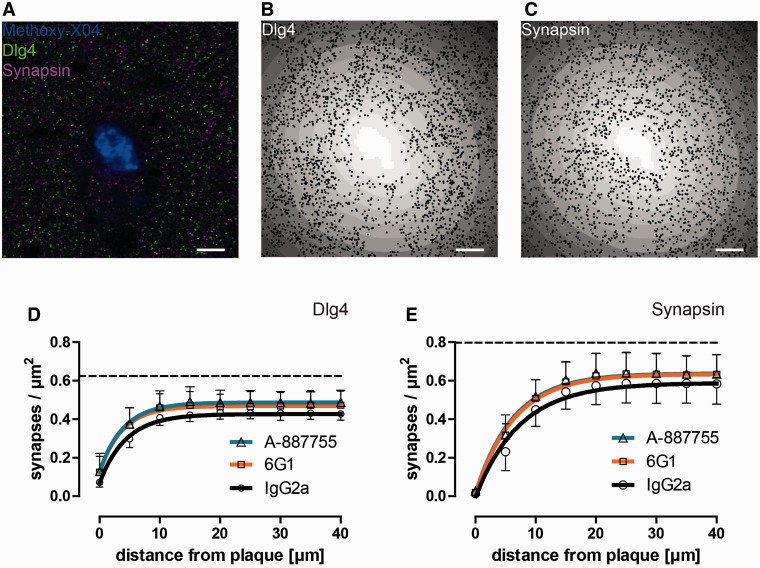
Figure 3.
Synapse density measurements in the proximity of fibrillar plaques. (A) Fibrillar plaques were stained with methoxy-X04, glutamatergic post-synapses were immunostained with anti-DLG4 (PSD-95) antibodies and presynapses with anti-synapsin antibodies. (B) Automatically detected DLG4-positive punctae overlaid on a distance transform highlighting the distances from the fibrillar plaque (white) in 5-µm wide bins (grey scale). (C) Automatically detected synapsin-positive punctae overlaid on a distance transform. (D) Quantification of the densities of DLG4-positive punctae as a monophasic association function of the distance from the fibrillar plaque. (E) Quantification of the densities of synapsin-positive punctae as a monophasic association function of the distance from the fibrillar plaque. (A–C) Scale bars = 10 µm. (D–E) Dashed lines indicate wild-type levels.
Synaptic pathology distant from plaques
The occurrence of fibrillar amyloid deposits is highly heterogeneous within the brains of Tg2576 mice, so that relatively large areas can be entirely free of plaques. We quantified the densities of DLG4 (previously known as PSD95) and synapsin-positive punctae in cortical and hippocampal areas which were at least 50 µm and typically ∼1 mm distant to the closest plaque to study the effects of non-fibrillar amyloid-β. DLG4 and synapsin-positive punctae were detected using morphological image analysis routines (Dorostkar et al., 2010), automatically counted and related to the tissue area after subtraction of blood vessels and neuronal cell bodies (Fig. 2A–E).
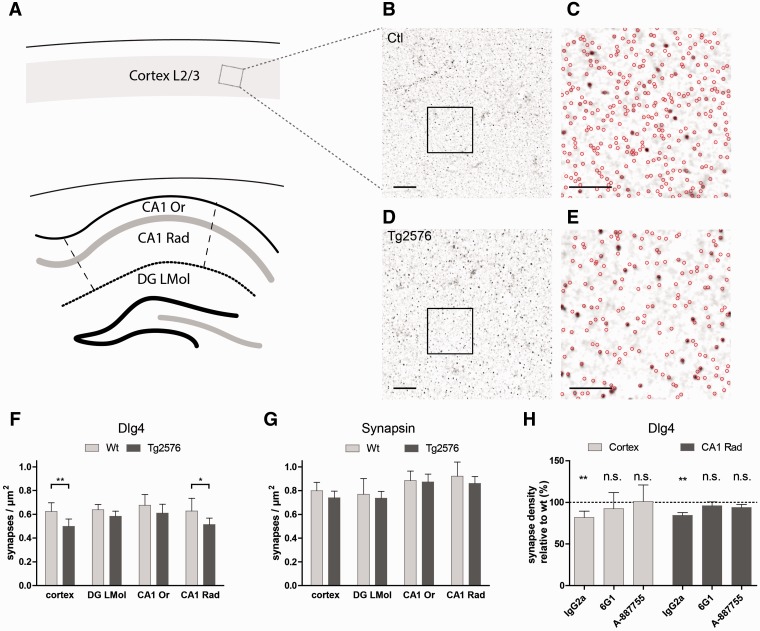
Figure 2.
Synapse density measurements distant from plaques. (A) Synapse densities were quantified in floating sections stained with antibodies against DLG4 and synapsin. The regions analysed were cortical layers 2/3, hippocampal field CA1, stratum oriens (CA1 Or) and stratum radiatum (CA1 Rad) and the dentate gyrus molecular layer (DG LMol) in areas at least 50 µm distant from the closest fibrillar plaque. (B) DLG4 stained cortex of a wild-type mouse. Scale bar = 10 µm. (C) Automatically detected synaptic punctae in the square region highlighted in B. Scale bar = 5 µm. (D) DLG4 stained cortex of a Tg2576 mouse treated with control antibodies (IgG2a). Scale bar = 10 µm. (E) Automatically detected synaptic punctae in the square region highlighted in D. Scale bar = 5 µm. (F) Quantification of DLG4-positive punctae in the respective brain regions. (G) Quantification of synapsin-positive punctae in the respective brain regions. (H) Quantification of DLG4-positive punctae in the cortex and CA1 stratum radiatum of animals treated with control (IgG2a) antibodies, with conformation-unspecific (6G1) or oligomer-specific (A-887755) antibodies. Synapse densities were normalized to values in control animals treated with the same antibody. (F–H) n = 4 (wild-type) to 6 (Tg2576). (F) *P < 0.05; **P < 0.01 (t-test). (H) **P < 0.01, one-sample t-test.
Wt = wild-type; n.s. = not significant. PSD-95 is now known as DLG4.
In mice treated with control antibodies, the densities of DLG4-positive punctae were significantly reduced in certain brain regions of Tg2576 compared to non-transgenic controls. In the cortex these were reduced from 0.62 ± 0.05 µm−2 in controls to 0.50 ± 0.06 µm−2 in Tg2576 (P < 0.01, n = 4–6, t-test, Fig. 2F) and in the stratum radiatum of the hippocampal field CA1 from 0.63 ± 0.07 µm−2 in controls to 0.51 ± 0.05 µm−2 in Tg2576 (P < 0.05, n = 4–6, t-test, Fig. 2F). In the stratum oriens or dentate gyrus molecular layer, densities of DLG4-positive punctae were not significantly altered (Fig. 2F). We also analysed the densities of synapsin-positive punctae in the respective brain regions. However, significant alterations were not detectable in any region (Fig. 2G).
We focused on the cortex and stratum radiatum to study whether loss of post-synapses was alleviated by immunotherapy. Indeed, animals treated with either 6G1 or A-887755 antibodies showed no significant loss of DLG4-positive punctae (Fig. 2H), compared to wild-type littermates treated with the same antibody. Taken together, these results suggest that DLG4-positive post-synapses are lost as a consequence of the toxic effects of diffusible, oligomeric amyloid-β, whereas synapsin-positive presynapses remain unaffected.
Plaque-associated synaptic pathology
Next, we analysed if synapses were lost in the immediate vicinity of fibrillar plaques. Tissue sections were treated with methoxy-X04 to stain fibrillar amyloid (Fig. 3A), while immunostaining against DLG4 and synapsin marked glutamatergic post-synapses and presynapses, respectively (Fig. 3B and C). Plaque-associated synaptic pathology was analysed by quantifying the numbers of DLG4 and synapsin-positive punctae in 5 -µm wide bins around plaques (Fig. 3D and E).
The densities of DLG4-positive punctae around plaques followed monophasic association functions (Fig. 3D), with a plateau of 0.43 µm−2 [95% confidence interval (CI) 0.42–0.44]. The half-distance, a measure for the radius of influence of plaques, was 3.02 µm (95% CI 2.54–3.74 µm). Immunotherapy caused small, but significant, increases in synapse densities, while half-distances were not significantly altered. In animals treated with 6G1 and A-887755 antibodies, the maximal densities of DLG4-positive punctae were 0.47 µm−2 (95% CI 0.45–0.49, P < 0.001, F-test) and 0.49 µm−2 (95% CI 0.47–0.50, P < 0.001, F-test), respectively. Similar effects were seen on densities of synapsin-positive punctae (Fig. 3E): in animals treated with control antibodies, the maximal densities were 0.59 µm−2 (95% CI 0.56–0.61 µm−2) and half-distances were 5.09 µm (95% CI 4.03–6.90 µm). In animals treated with 6G1 and A-887755 antibodies, the maximal densities of synapsin-positive punctae were 0.63 µm−2 (95% CI 0.61–0.66 µm−2, P < 0.005, F-test) and 0.64 µm−2 (95% CI 0.62–0.66 µm−2, P < 0.005, F-test), respectively, while the half-distances were not significantly altered (P > 0.05, F-test).
These results demonstrate that in the immediate vicinity of amyloid plaques, both pre- and post-synaptic structures are lost. Removal of amyloid-β oligomers alleviates this loss, though only by a small degree.
Discussion
Tg2576 mice express human amyloid precursor protein with the Swedish mutations and develop numerous amyloid plaques in the cortex and hippocampus as they age (Hsiao et al., 1996). Typically, growth of amyloid plaques is observed at the age of 12 months, while at ∼18 months plaque growth tapers off (Christie et al., 2001). We treated female, 12-month-old mice with one of three antibodies for 8 weeks: 6G1, which is pan-amyloid-β specific and recognizes monomeric, fibrillar and oligomeric forms (Barghorn et al., 2005); A-887755, a globulomer-derived antibody which specifically detects oligomeric, but not monomeric or fibrillar amyloid-β (Hillen et al., 2010); IgG2a as a non-amyloid specific control. Active immunization of animals in a pilot study with amyloid-β1-42 globulomer, which results in a monospecific immune response resembling the antibody specificity of A-887755, had led to a significant reduction of amyloid-β globulomer peptides after 9 months (Supplementary Fig. 1).
As loss of cognitive function in Alzheimer’s disease ultimately depends on loss of functional synapses (DeKosky and Scheff, 1990; Scheff et al., 1990) rather than on plaques themselves, we quantified the numbers of pre- and post-synapses as a readout to assess the efficacy of immunotherapy in a model of Alzheimer’s disease. In this context, we defined presynapses as spots immunoreactive for the presynaptic marker synapsin and post-synapses as spots immunoreactive for DLG4, essentially marking dendritic spines (Sorra and Harris, 2000). These were quantified using automated algorithms, based on morphologic spot detection (Dorostkar et al., 2010), thus eliminating any possible bias after image acquisition. In the immediate vicinity (up to ∼10–15 µm) of fibrillar, methoxy-X04-positive, amyloid plaques, the densities of both pre- and post-synapses were strongly reduced (Fig. 3G and H). However, even several tens of micrometres away from plaques, the densities of pre- and post-synapses were reduced compared to non-transgenic controls. This suggests that the concentration of synaptotoxic factors is highest at the fibrillar core of plaques and diminishes with the distance from plaques. Similar results were obtained previously in different amyloid mouse models, which showed loss of DLG4 positive post-synapses or dendritic spines around fibrillar (Kirkwood et al., 2013).
Plaques may be reservoirs of oligomeric amyloid-β, causing loss of DLG4-positive post-synapses near plaques (Koffie et al., 2009, 2011). Similarly, presynapses have been shown to be lost in various models of Alzherimer’s disease (Pozueta et al., 2013). Curiously, we found that in areas free of plaques, synapses were affected differently. On one hand, only DLG4-positive post-synapses, but not synapsin-positive presynapses, were reduced. Furthermore, not all brain areas were affected similarly: while we detected reductions in the densities of post-synapses in the cortex and stratum radiatum of the CA1 field, we were unable to detect any effects in the stratum oriens of the CA1 field or in the molecular layer of the dentate gyrus (Fig. 2F and G). This could mean that different types of neurons are affected to different degrees (Capetillo-Zarate et al., 2006), or that different amounts of amyloid-β oligomers (Sakono and Zako, 2010; Benilova et al., 2012) are present in different brain regions.
Currently, no treatment for Alzheimer’s disease is established to be safe and efficacious. Initial approaches with immunotherapy have failed for various reasons including harmful side effects and lack of efficacy (Karran and Hardy, 2014). Nevertheless, immunotherapy seems to be one of the more promising approaches to combat Alzheimer’s disease, if nothing else because several preclinical trials showed promising results: some studies showed cognitive improvement as well as increased clearing of amyloid deposits in animal models as a result of immunotherapy (Wilcock et al., 2004; Wang et al., 2011; DeMattos et al., 2012), which was most effective when used preventatively (DeMattos et al., 2012). Thus, the amyloid clearing effect may depend on a number of factors, including the epitope recognized by therapeutic antibodies, the time relative to amyloid deposition when the antibody is administered, but also the animal model itself. Neither the oligomer-specific antibody had an effect on amyloid plaques, nor the conformation-unspecific antibody (Fig. 1). This was expected for the former, as A-887755 does not recognize fibrils. However, we observed no effect of the conformation-unspecific antibody, 6G1, which has an intermediate affinity for fibrils (Hillen et al., 2010), though in contrast to most other studies we quantified only fibrillar amyloid, as only this can be visualized in vivo. Nevertheless, the lack of effect even on newly appearing plaques suggests that amyloid deposition was unaffected by both antibodies. However, even in the absence of an amyloid clearing effect we found that synapse loss was alleviated in animals treated with either non-conformation specific or oligomer-specific antibodies (Fig. 2). Close to fibrillar plaques, the beneficial effect was small, whereas distant from plaques synapse densities were restored to similar levels as in control animals (Fig. 2H). Beneficial effects of A-887755 antibodies on spine loss have been reported previously (Hillen et al., 2010). These results suggest that synapse loss close to plaques is not reversible to the same degree as synapse loss distant from plaques. This may be the case because synapse loss at plaques occurs earlier, is more pronounced and is therefore irreversible by immunotherapy. Alternatively, fibrillar plaques may act as reservoirs for toxic amyloid-β species. For instance, oligomeric amyloid-β, which is widely accepted as a major neurotoxic effector of Alzheimer’s disease (Benilova et al., 2012; Gilbert, 2013), accumulates around plaques (Koffie et al., 2009). A-887755 only recognizes oligomeric, but not monomeric or fibrillar amyloid-β, whereas 6G1 recognizes all forms (Hillen et al., 2010). The fact that A-887755 had protective effects further corroborates the hypothesis that oligomers are synaptotoxic and that removal of oligomers suffices to confer a synaptoprotective effect. Additionally, our results signify that sequestering oligomers by immunotherapy suffices to counteract synaptic pathology, thus suggesting that cognitive function may be improved even when the load of fibrillar amyloid remains unchanged.
Acknowledgements
The authors would like to thank Sonja Steinbach and Kevin Keppler for their excellent technical assistance and Patrizia Bonert, Julia Vlcek and Mehdi Shakarami for their invaluable assistance in animal care.
Glossary
Abbreviation
- CA1
cornu ammonis 1
Funding
This work was in part funded by grants from the Deutsche Forschungsgemeinschaft (SFB 596, A13), the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, 01GZ0713, 13N9268, 13N12778), the German Federal Ministry of Economics and Technology (Bundesministerium für Wirtschaft und Technologie, 16IN0675) and the European Union (Neuro.GSK3, FP-7-223276).
Conflict of interest
This study was partially sponsored and funded by AbbVie. S Barghorn, C.K., H.H. are employees of AbbVie and may own AbbVie stocks. AbbVie contributed to the study design, research, and interpretation of data, writing, reviewing and approving the publication.
Supplementary material
Supplementary material is available at Brain online.
References
- Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, et al. Globular amyloid β-peptide1−42 oligomer − a homogenous and stable neuropathological protein in Alzheimer's disease. J Neurochem. 2005;95:834–47. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- Benilova I, Karran E, De Strooper B. The toxic A[beta] oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–57. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- Burgold S, Filser S, Dorostkar MM, Schmidt B, Herms J. In vivo imaging reveals sigmoidal growth kinetic of beta-amyloid plaques. Acta Neuropathol Commun. 2014;2:30. doi: 10.1186/2051-5960-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capetillo-Zarate E, Staufenbiel M, Abramowski D, Haass C, Escher A, Stadelmann C, et al. Selective vulnerability of different types of commissural neurons for amyloid β-protein-induced neurodegeneration in APP23 mice correlates with dendritic tree morphology. Brain. 2006;129:2992–3005. doi: 10.1093/brain/awl176. [DOI] [PubMed] [Google Scholar]
- Christie RH, Bacskai BJ, Zipfel WR, Williams RM, Kajdasz ST, Webb WW, et al. Growth arrest of individual senile plaques in a model of Alzheimer's disease observed by in vivo multiphoton microscopy. J Neurosci. 2001;21:858–64. doi: 10.1523/JNEUROSCI.21-03-00858.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- DeMattos Ronald B, Lu J, Tang Y, Racke Margaret M, DeLong Cindy A, Tzaferis John A, et al. A Plaque-specific antibody clears existing beta-amyloid plaques in Alzheimer's disease mice. Neuron. 2012;76:908–20. doi: 10.1016/j.neuron.2012.10.029. [DOI] [PubMed] [Google Scholar]
- Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:311–21. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- Dorostkar MM, Dreosti E, Odermatt B, Lagnado L. Computational processing of optical measurements of neuronal and synaptic activity in networks. J Neurosci Methods. 2010;188:141–50. doi: 10.1016/j.jneumeth.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert BJ. The role of amyloid β in the pathogenesis of Alzheimer's disease. J Clin Pathol. 2013;66:362–6. doi: 10.1136/jclinpath-2013-201515. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The Amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hefendehl JK, Wegenast-Braun BM, Liebig C, Eicke D, Milford D, Calhoun ME, et al. Long-term in vivo imaging of beta-amyloid plaque appearance and growth in a mous model of cerebral beta-amyloidosis. J Neurosci. 2011;31:624–9. doi: 10.1523/JNEUROSCI.5147-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen H, Barghorn S, Striebinger A, Labkovsky B, Müller R, Nimmrich V, et al. Generation and therapeutic efficacy of highly oligomer-specific β-amyloid antibodies. J Neurosci. 2010;30:10369–79. doi: 10.1523/JNEUROSCI.5721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–103. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Karran E, Hardy J. Antiamyloid therapy for Alzheimer's disease—are we on the right road? N Engl J Med. 2014;370:377–8. doi: 10.1056/NEJMe1313943. [DOI] [PubMed] [Google Scholar]
- Kirkwood CM, Ciuchta J, Ikonomovic MD, Fish KN, Abrahamson EE, Murray PS, et al. Dendritic spine density, morphology, and fibrillar actin content surrounding amyloid-[beta] plaques in a mouse model of amyloid-[beta] deposition. J Neuropathol Exp Neurol. 2013;72:791–800. doi: 10.1097/NEN.0b013e31829ecc89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie R, Hyman B, Spires-Jones T. Alzheimer's disease: synapses gone cold. Mol Neurodegener. 2011;6:63. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci USA. 2009;106:4012–17. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozueta J, Lefort R, Shelanski ML. Synaptic changes in Alzheimer’s disease and its models. Neuroscience. 2013;251:51–65. doi: 10.1016/j.neuroscience.2012.05.050. [DOI] [PubMed] [Google Scholar]
- Saido TC. Alzheimer's disease as proteolytic disorders: anabolism and catabolism of beta-amyloid. Neurobiol Aging. 1998;19(1 Suppl):S69–75. doi: 10.1016/s0197-4580(98)00033-5. [DOI] [PubMed] [Google Scholar]
- Sakono M, Zako T. Amyloid oligomers: formation and toxicity of Aβ oligomers. FEBS J. 2010;277:1348–58. doi: 10.1111/j.1742-4658.2010.07568.x. [DOI] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two Phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370:322–33. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol Aging. 1990;11:29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Harris KM. Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus. 2000;10:501–11. doi: 10.1002/1098-1063(2000)10:5<501::AID-HIPO1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Wang A, Das P, Switzer RC, Golde TE, Jankowsky JL. Robust amyloid clearance in a mouse model of Alzheimer's disease provides novel insights into the mechanism of amyloid-beta immunotherapy. J Neurosci. 2011;31:4124–36. doi: 10.1523/JNEUROSCI.5077-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Levkowitz G, Subbarao S, Alamed J, et al. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J Neurosci. 2004;24:6144–51. doi: 10.1523/JNEUROSCI.1090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]