Idiopathic REM sleep behaviour disorder is characterized by dream-enactment behaviour and loss of REM atonia, and is considered a prodromal symptom of Parkinson’s disease. Wu et al. identify an abnormal brain metabolic network associated with the disorder, and show that evolution of this network occurs with progression to Parkinson’s disease.
Keywords: rapid eye movement sleep behaviour disorder, Parkinson’s disease, 18F-fluorodeoxyglucose, positron emission tomography, network biomarkers
Abstract
Rapid eye movement sleep behaviour disorder has been evaluated using Parkinson’s disease-related metabolic network. It is unknown whether this disorder is itself associated with a unique metabolic network. 18F-fluorodeoxyglucose positron emission tomography was performed in 21 patients (age 65.0 ± 5.6 years) with idiopathic rapid eye movement sleep behaviour disorder and 21 age/gender-matched healthy control subjects (age 62.5 ± 7.5 years) to identify a disease-related pattern and examine its evolution in 21 hemi-parkinsonian patients (age 62.6 ± 5.0 years) and 16 moderate parkinsonian patients (age 56.9 ± 12.2 years). We identified a rapid eye movement sleep behaviour disorder-related metabolic network characterized by increased activity in pons, thalamus, medial frontal and sensorimotor areas, hippocampus, supramarginal and inferior temporal gyri, and posterior cerebellum, with decreased activity in occipital and superior temporal regions. Compared to the healthy control subjects, network expressions were elevated (P < 0.0001) in the patients with this disorder and in the parkinsonian cohorts but decreased with disease progression. Parkinson’s disease-related network activity was also elevated (P < 0.0001) in the patients with rapid eye movement sleep behaviour disorder but lower than in the hemi-parkinsonian cohort. Abnormal metabolic networks may provide markers of idiopathic rapid eye movement sleep behaviour disorder to identify those at higher risk to develop neurodegenerative parkinsonism.
Introduction
Rapid eye movement sleep behaviour disorder (RBD) is a parasomnia characterized by dream enactment behaviours emerging during REM sleep and associated with a loss of normal skeletal muscle atonia (Boeve, 2010). Abnormal neural circuits resulting from damage to brainstem areas may have precipitated idiopathic RBD leading to the loss of motor inhibition during rapid eye movement (REM) sleep (Boeve et al., 2007; Lu and Saper, 2011). Idiopathic RBD is regarded as a prodromal stage of synucleinopathies such as Parkinson’s disease, dementia with Lewy bodies and multiple system atrophy because it often precedes the development of motor, cognitive, neuropsychiatric and autonomic features of these syndromes by years or decades (Iranzo et al., 2006; Postuma et al., 2009; Claassen et al., 2010; Schenck et al., 2013). Functional neuroimaging studies with Single Photon Emission Computed Tomography (SPECT) or PET provided further support with reduced nigrostriatal dopaminergic activity (Eisensehr et al., 2000; Iranzo et al., 2011), decreased cardiac adrenergic activity (Miyamoto et al., 2006; Fujishiro et al., 2010) and impaired brain perfusion (Hanyu et al., 2011; Vendette et al., 2011) found in patients with idiopathic RBD.
Metabolic imaging with FDG PET and spatial covariance analysis has provided valuable information concerning the abnormal regional brain function underlying neurodegenerative disorders (Eidelberg, 2009). Abnormal metabolic brain networks have been identified for Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy for accurate classification of these parkinsonian disorders at the single-patient level, even at early disease stages (Ma et al., 2007; Eckert et al., 2008; Tang et al., 2010). We have also identified a Parkinson’s disease-related covariance pattern (PDRP) in a Chinese cohort (Wu et al., 2013) that closely resembled the original topography identified in an analogous North American cohort (Niethammer and Eidelberg, 2012).
Abnormal changes in PDRP expression have recently been reported in two idiopathic RBD cohorts and, on an individual basis, higher baseline expression was associated with a greater likelihood of developing Parkinson’s disease or dementia with Lewy bodies (Holtbernd et al., 2014). It is not known, however, whether idiopathic RBD is itself associated with a unique metabolic topography that would evolve before symptom onset. We sought to identify a RBD-related covariance pattern (RBDRP) and determined its relationships with concurrent PDRP measurements using our network-based algorithm (Ma et al., 2007; Spetsieris et al., 2013). We also assessed the evolution of these network expressions in patients with idiopathic RBD and in patients with very early and moderate Parkinson’s disease.
Materials and methods
Subjects
For identification of RBDRP, 21 patients with idiopathic RBD [male/female: 17/4, age: 65.0 ± 5.6 (mean ± standard deviation, SD) years] and 21 gender/age-matched healthy control subjects (male/female: 17/4, age: 62.5 ± 7.5 years) were recruited from Huashan Hospital for 18F-Fluorodeoxyglucose (FDG) PET imaging (Cohort A). Diagnosisof idiopathic RBD was made by sleep medicine experts (H.Y. and Y.D.) requiring a history of dream-enacting behaviours and video-polysomnographic demonstration of either increased EMG activity or abnormal behaviours during REM sleep (Boeve, 2010). Detailed polysomnographic procedures are included in the online Supplementary material.
For prospective validation of RBDRP network expression we included Cohort B with 15 patients with idiopathic RBD (male/female: 12/3, age: 65.5 ± 6.0 years) and 15 gender/age-matched healthy control subjects (male/female: 10/5, age: 60.5 ± 7.6 years). This idiopathic RBD group comprised nine patients from Cohort A who were scanned again at 1 year (male/female: 7/2, age: 65.2 ± 6.9 years) and six newly recruited subjects with idiopathic RBD (male/female: 5/1, age: 66.0 ± 5.0 years). Clinical and polysomnographic parameters did not differ significantly between the patients with idiopathic RBD in Cohorts A and B (Table 1).
Table 1.
Clinical characteristics and polysomnographic measures in patients with idiopathic RBD
| Cohort A | Cohort B | |
|---|---|---|
| Sex (male/female) | 17/4 | 12/3 |
| Age (years) | 65.0 ± 5.6 | 65.5 ± 6.0 |
| Age at onset of RBD (years) | 59.3 ± 6.9 | 60.1 ± 7.6 |
| RBD duration (years) | 5.7 ± 3.5 | 5.5 ± 4.0 |
| UPDRS III | 2.4 ± 2.1 | 2.8 ± 1.9 |
| Body mass index (kg/m2) | 23.8 ± 2.7 | 23.8 ± 2.5 |
| Polysomnographic data | ||
| Total sleep time (min) | 329.2 ± 47.0 | 325.3 ± 39.5 |
| Sleep efficiency (%) | 74.9 ± 9.4 | 73.5 ± 10.7 |
| Sleep onset latency(min) | 26.7 ± 32.2 | 21.1 ± 24.9 |
| REM sleep latency(min) | 81.4 ± 38.0 | 96.1 ± 37.2 |
| Non-REM sleep 1 (%) | 17.2 ± 8.7 | 17.1 ± 9.4 |
| Non-REM sleep 2 (%) | 46.2 ± 9.2 | 47.7 ± 7.5 |
| Non-REM sleep 3 (%) | 19.5 ± 8.2 | 18.0 ± 8.5 |
| REM sleep (%) | 17.2 ± 5.1 | 17.1 ± 5.8 |
| Microarousals index | 15.2 ± 7.4 | 16.1 ± 4.9 |
| Phasic EMG activity index/chin | 17.9 ± 9.8 | 17.5 ± 8.5 |
| Tonic EMG activity index /chin | 16.0 ± 11.9 | 17.8 ± 16.4 |
| Phasic EMG activity index /legs | 12.7 ± 7.1 | 13.5 ± 8.2 |
| Tonic EMG activity index /legs | 8.1 ± 6.3 | 8.0 ± 7.3 |
| Patients with either abnormal phasic or tonic chin EMG activity in REM sleep (%) | 10 (47.6%) | 7 (46.7%) |
| Apnoea-hypopnoea index | 3.0 ± 4.0 | 3.8 ± 2.7 |
| Mean SaO2 | 94.5 ± 0.9 | 94.5 ± 1.2 |
Values are given as means ± SD.
We also included Cohort C with 21 age-matched patients with hemi-Parkinson’s disease (male/female: 12/9, age: 62.6 ± 5.0 years, Hoehn and Yahr 1) and 16 patients with moderate Parkinson’s disease (male/female: 5/11, age: 56.9 ± 12.2 years, Hoehn and Yahr: 2.5 ± 0.5, range 2–3) to assess the correlations between the RBDRP and PDRP networks. All normal and parkinsonian subjects were recruited as described previously (Wu et al., 2013).
Ethical permission for the study was obtained from the Institutional Review Board of Huashan Hospital. Written consent was obtained from each subject after detailed explanation of the procedures.
PET imaging and processing
The imaging procedure and the data preprocessing were performed as described in detail previously (Wu et al., 2013). Briefly, FDG PET images of relative glucose metabolism were acquired with a Siemens Biograph 64 PET/CT (Siemens) and spatially normalized into a standard brain space by statistical parametric mapping software (SPM5; Institute of Neurology, London, UK).
Network analysis and prospective comparisons
RBDRP was identified from the combination of idiopathic RBD and control subjects in Cohort A by an automated voxel-based network analysis over the whole brain (Ma et al., 2007; Spetsieris et al., 2013). This was done within a grey matter mask defined with all FDG PET images by applying scaled subprofile model/principal component analysis (SSM/PCA; available on http://www.feinsteinneuroscience.org at the Centre for Neuroscience, the Feinstein Institute for Medical Research, Manhasset, NY, USA). The reliability of the resulting topography was evaluated by using a bootstrapping resample scheme described previously (Habeck et al., 2008; Peng et al., 2014).
The RBDRP was compared topographically to the PDRP (Wu et al., 2013) using volume- or voxel-wise correlations over the whole brain. The expressions of RBDRP or PDRP were quantified in all subjects with a voxel-based algorithm (Ma et al., 2007; Spetsieris et al., 2013). The network expression was Z-transformed using subject scores of the controls in the corresponding derivation cohort for each network.
Statistical analysis
Differences in network expression between patients and normal control subjects were assessed by Student’s t-tests with their diagnostic power for group discrimination evaluated by receiver operating characteristic analysis. One-way ANOVA was performed for each network measure across the subject groups with between-group differences assessed using post hoc Tukey’s HSD tests. Group × network activity interaction was evaluated by two-way ANOVA to determine whether any differences exist between changes for each of these network measures over the subject groups. Correlations between RBDRP and PDRP expressions as well as those with clinical measures in different subject groups were separately assessed by computing Pearson correlation coefficients. All analyses were performed using SPSS software (SPSS Inc.) and considered significant for P < 0.05.
Results
RBD-related pattern identification and disease discrimination
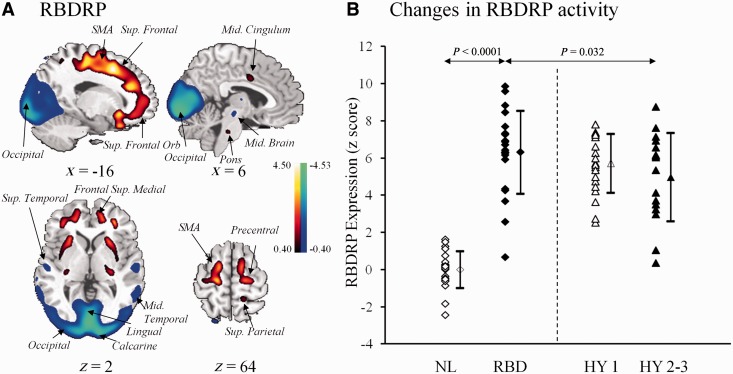
In Cohort A, the RBDRP was identified as the first principal component accounting for 14.0% of subject × voxel variance. The pattern was characterized by increased metabolic activity in pons, thalamus, precentral gyrus, supplementary motor area, medial frontal gyrus, hippocampus/parahippocampal gyrus, supramarginal and inferior temporal gyrus, and posterior cerebellar tonsil, associated with decreased metabolic activity in occipital regions, midbrain (red nucleus) and superior/middle temporal gyrus (Fig. 1A; Supplementary Table 1). RBDRP expression was abnormally elevated (P < 0.0001) in the patients with idiopathic RBD compared with the control subjects (Fig. 1B) and significantly discriminated (P < 0.0001) the subject groups, with an area under the curve of 0.989 ± 0.013 and a sensitivity of 95.2% and a specificity of 95.2%.
Figure 1.
(A) RBDRP identified by network analysis of FDG PET scans from 21 patients with idiopathic RBD and 21 age-matched normal controls in Cohort A. This spatial covariance pattern was characterized by relative increases in sensorimotor, superior frontal, cingulate, thalamic, pontine, and cerebellar metabolism, associated with decreases in occipital, midbrain and superior temporal metabolism. The pattern was overlaid on a standard MRI brain template to display voxels that were reliable at P < 0.01 based on the bootstrapping algorithm. Voxels with positive region weights (metabolic increases) are colour-coded red; those with negative region weights (metabolic decreases) are colour-coded blue. (B) RBDRP expressions (subject scores) were increased in the patients with idiopathic RBD relative to the normal subjects in Cohort A as well as the patients with moderate Parkinson’s disease in Cohort C. These scores were also elevated when compared to those in the hemi-parkinsonian patients in Cohort C but did not reach significance. Subject scores of the parkinsonian patients in Cohort C were both elevated (P < 0.0001; post hoc tests) versus the normal controls. The error bars represent standard errors of the mean. NL = normal control; HY = Hoehn and Yahr; SMA = supplementary motor area.
Comparisons between spatial topographies of RBD- and Parkinson’s disease-related patterns
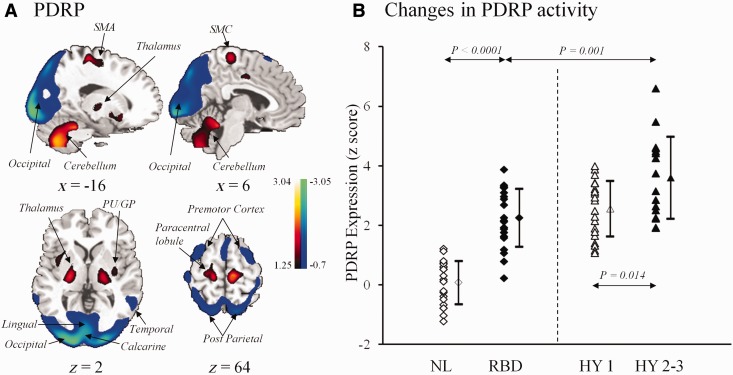
Both RBDRP and PDRP showed varying degrees of overlap in several brain areas including the thalamus, sensorimotor cortices and occipital regions (Figs 1A and 2A). Their region-weights correlated significantly with each other using both volumetric (r = 0.634, P < 0.002) and voxelized (r = 0.680; P < 0.0001) analyses.
Figure 2.
(A) PDRP identified by network analysis of FDG PET scans from 33 patients with Parkinson’s disease and 33 age-matched normal control subjects (Wu et al., 2013). This spatial covariance pattern was characterized by relative increases in sensorimotor, pallidothalamic, pontine, and cerebellar metabolism, associated with decreases in premotor and posterior parietal-occipital metabolism. The pattern was overlaid onto a standard MRI brain template to display voxels that were reliable at P < 0.001 based on the bootstrapping algorithm. Voxels with positive region weights (metabolic increases) are colour coded red; those with negative region weights (metabolic decreases) are colour coded blue. (B) PDRP expressions (subject scores) were increased in the patients with idiopathic RBD relative to the normal subjects in Cohort A but decreased relative to the moderate parkinsonian patients in Cohort C. These scores were also decreased slightly but not significantly when compared to the hemi-parkinsonian patients in Cohort C. Subject scores of the parkinonian patients in Cohort C were both elevated (P < 0.0001; post hoc tests) versus the normal controls. The error bars represent standard errors of the mean. GP = globus pallidus; PU = putamen; NL = normal control; HY = Hoehn and Yahr; SMA = supplementary motor area; SMC = sensorimotor cortex.
RBD-related pattern expressions in patients with Parkinson’s disease
RBDRP expressions in different patient cohorts were presented in Table 2 and Fig. 1B. RBDRP activity differed significantly among the multiple subject groups [F(3,75) = 51; F(3,63) = 32, P < 0.0001; one-way ANOVA across Cohorts A and C or B and C, respectively]. RBDRP expression in the patients with idiopathic RBD was similarly elevated (P < 0.0001) relative to the controls in Cohort A/B. RBDRP scores were also elevated (P < 0.0001) in the hemi-parkinsonian patients but slightly lower (P ≥ 0.18) than these patients with idiopathic RBD. However, these scores decreased significantly in the moderate parkinsonian patients compared to the idiopathic RBD patients in Cohort A/B (P = 0.032/0.014). RBDRP scores did not differ between the two parkinsonian groups (P ≥ 0.22).
Table 2.
Network expressions in normal controls, patients with idiopathic RBD and Parkinson’s disease
| n | RBDRP scores | PDRP scores | |
|---|---|---|---|
| Cohort A | |||
| Normal control | 21 | 0.00 ± 1.00 | 0.08 ± 0.72 |
| Idiopathic RBD | 21 | 6.31 ± 2.23 | 2.25 ± 0.97 |
| Cohort B | |||
| Normal control | 15 | 1.03 ± 1.48 | 0.47 ± 0.95 |
| Idiopathic RBD | 15 | 6.96 ± 1.54 | 2.52 ± 1.22 |
| Cohort C | |||
| Parkinson’s disease (Hoehn and Yahr 1) | 21 | 5.72 ± 1.59 | 2.57 ± 0.93 |
| Parkinson’s disease | 16 | 4.97 ± 2.38 | 3.60 ± 1.38 |
| (Hoehn and Yahr 2–3) | |||
Values are given as means ± SD.
Parkinson’s disease-related pattern expressions in patients with idiopathic RBD
PDRP expressions in different patient cohorts were presented in Table 2 and Fig. 2B. PDRP activity differed significantly among the multiple subject groups [F(3,75) = 42; F(3,63) = 21, P < 0.0001; one-way ANOVA across Cohorts A and C or B and C, respectively]. PDRP scores in the idiopathic RBD patients in Cohort A/B were similarly elevated (P < 0.0001) relative to the controls, decreased not significantly (P ≥ 0.74) versus the hemi-parkinsonian patients but significantly (P = 0.001/0.047) versus the moderate parkinsonian patients. PDRP scores increased significantly (P = 0.014/0.035) comparing the moderate to the hemi-parkinsonian groups.
Comparisons of network activities and clinical correlations in patients with idiopathic RBD and Parkinson’s disease
RBDPR and PDRP scores disclosed an effect of interaction across the multiple subject groups (F = 9.4/9.3, P = 0.003; two-way ANOVA across Cohorts A and C or B and C, respectively). RBDRP and PDRP scores correlated in the idiopathic RBD patients in Cohort A (r = 0.710, P < 0.0001) and in the parkinsonian cohort (r = 0.387, P = 0.018). UPDRS motor ratings correlated with PDRP (r = 0.481, P = 0.003) but not RBDRP (r = 0.14, P = 0.41) scores in the parkinsonian cohort. Moreover, neither network scores correlated with clinical and polysomnographic parameters in patients with idiopathic RBD.
Discussion
This report describes the first study to identify a characteristic metabolic brain network (i.e. RBDRP) with FDG PET in an idiopathic RBD population. RBDRP featured a highly symmetrical topography suggesting the existence of a widely-distributed brain network of abnormal metabolic activity underlying this disorder. RBDRP expression accurately discriminated patients with RBD from normal control subjects in both derivation and validation samples. Moreover, RBDRP activity decreased but PDRP activity increased in early to moderate parkinsonian patients compared to patients with idiopathic RBD.
Comparisons of brain network characteristics associated with idiopathic RBD and Parkinson’s disease
We have noted that RBDRP and PRDP share some similarities. Firstly, the two topographic patterns showed moderate overlap. Secondly, both RBDRP and PDRP expressions were elevated and correlated with each other in patients with RBD. Thirdly, elevated PDRP expression in the patients with idiopathic RBD was lower or comparable to the hemi-parkinsonian patients, in agreement with the recent report (Holtbernd et al., 2014).
We have also observed unique differences between RBDRP and PRDP. Firstly, the RBDRP included several specific regions such as hippocampus and midbrain, but excluded the putamen and pallidum (Figs 1A and 2A). Secondly, RBDRP activity declined while PRDP activity increased steadily in patients with idiopathic RBD, hemi- and moderate Parkinson’s disease, given the elevation of both network activities in each of these patient cohorts relative to the controls. Thirdly, not surprisingly, subject scores of PDRP (not RBDRP) correlated moderately with clinical motor ratings in the combined patient cohort of very early to moderate Parkinson’s disease.
Relationships with dementia with Lewy bodies or multiple system atrophy
Occipital and parietotemporal hypometabolism evident in both RBDRP and PDRP is also a finding frequently seen on FDG PET and perfusion SPECT in patients with probable dementia with Lewy bodies (Mosconi et al., 2008; Inui et al., 2014). Thus, a PDRP-like network may underlie dementia with Lewy bodies to a large degree and an elevated PDRP activity in idiopathic RBD may also point to the subsequent development of this disorder with disease progression (Holtbernd et al., 2014). Of the 17 subjects with idiopathic RBD followed clinically in that study, eight (47%) who had similarly elevated PDRP scores at baseline were diagnosed with Parkinson’s disease or dementia with Lewy bodies after 4.6 ± 2.5 years, in line with the estimated risk in the same period (Iranzo et al., 2006; Postuma et al., 2009). That said, it may still be necessary to assess other likely clinical outcomes using different spatial covariance patterns associated with Parkinson's disease-related cognitive impairment (Huang et al., 2007) and multiple system atrophy (Eckert et al., 2008; Teune et al., 2013).
Evolving metabolic brain networks and activities in patients with idiopathic RBD with disease progression
RBDRP depicts an insightful spatial covariance map for describing intrinsic connectivity between regional abnormalities and related pathways. The topography of RBDRP includes increased metabolic activity in the pons, thalamus and hippocampus, consistent with regional abnormalities measured with FDG PET and perfusion SPECT in several idiopathic RBD cohorts (Vendette et al., 2011; Dang-Vu et al., 2012; Holtbernd et al., 2014). Notably, in addition to increases in the putamen, the latter studies also found that increased metabolism and perfusion in the pons and/or hippocampus at baseline were predictive of the eventual onset of Parkinson’s disease or dementia with Lewy bodies in 3–5 years. Overall, the present study has revealed several dysfunctional neural circuits linking brainstem, midbrain, cerebellum, thalamus and fronto-occipital cortices, in line with the pathophysiology of idiopathic RBD and related disorders (Boeve et al., 2007; Lu and Saper, 2011; Del Tredici and Braak, 2013).
The findings that RBDRP activity decreased but PDRP activity increased in patients with advancing Parkinson’s disease compared to patients with idiopathic RBD (Figs 1B and 2B) suggest that RBDRP is perhaps relevant only for prodromal RBD cases and likely to break down with disease progression. This is supported by the lack of any correlations between RBDRP activity and clinical motor ratings in either RBD or parkinsonian cohort. Collectively these results pointed out the hypothesis of an evolving abnormal metabolic network with disease progression: from a RBDRP at very early stages (i.e. idiopathic RBD) to a PDRP at later stages when a neurodegenerative process is really advanced to that of Parkinson’s disease. This process is expected to coincide with the appearance in the resulting network of covarying subcortical regions in basal ganglia (i.e. putamen) with increased metabolic activity. This is a hypothesis to be validated by long-term clinical and imaging studies.
This study has some limitations. First, brain metabolism has been assessed not in REM sleep when the abnormal behaviour occurred. However, as currently reported in wake resting state, an increased perfusion in supplementary motor area during a RBD episode in a severe patient affected with multiple system atrophy was also reported in a SPECT study (Dauvilliers et al., 2011). Second, no absolute brain glucose metabolism was quantified to adequately propose a pathophysiological explanation on the network architecture underlying idiopathic RBD. Nevertheless, this study did disclose an abnormal metabolic network implying a specific brain circuitry. Third, elevated RBDRP/PDRP expressions in the patients with idiopathic RBD relative to the control subjects were replicated but not in a truly independent cohort. Regardless this result at least demonstrates that these metabolic network activities are highly reproducible across different idiopathic RBD subjects or metabolic images. Finally, idiopathic RBD was diagnosed by a visual scoring method with excellent sensitivity and specificity for both phasic and tonic chin EMG measures. However, despite a typical history of dream-enacting behaviours in all patients with idiopathic RBD, only ∼48% of them had abnormal REM sleep without atonia with the remainder presenting complex movements during REM sleep.
In conclusion, we have reported the first identification of RBDRP with FDG PET, which may be useful in differentiating patients with idiopathic RBD from normal controls and in revealing similarities and unique differences with PDRP. The close association of neuropathological processing between RBD and Parkinson’s disease suggests that idiopathic RBD is indeed a prodromal phase of Parkinson’s disease along a wide spectrum of disease progression. Further prospective studies are necessary to determine the relationships between baseline measurements in network expression and eventual clinical outcomes of idiopathic RBD patients.
Acknowledgements
Professor Dauvilliers helped and collaborated with the Sleep and Wake Disorders Centre, Department of Neurology, Huashan Hospital, Fudan University in Shanghai since its establishment in 2007.
Glossary
Abbreviations
- FDG
18F-fluorodeoxyglucose
- PDRP
Parkinson’s disease-related pattern
- RBD
rapid eye movement sleep behaviour disorder
- RBDRP
rapid eye movement sleep behaviour disorder-related pattern
- REM
rapid eye movement
Funding
Dr Wu was supported by the Scientific Research Project from Huashan Hospital at Fudan University. Drs Yu, Wang and Zuo were supported by the China-US Biomedical Collaborative Research Program (No. 81361120393) and grants (No. 81071018, No. 81171189, No. 81301136 and No. 81371413) from the National Natural Science Foundation of China, the Key Project (13JC1401103) from the Science and Technology Commission of Shanghai Municipality and the Project (XBR2013088 and 20134042) of Shanghai Municipal Health Bureau. Drs Ma, Peng and Eidelberg at the Feinstein Institute for Medical Research were supported by the Morris K Udall Centre of Excellence for Parkinson’s Disease Research (P50 NS071675) and the US-China Biomedical Collaborative Research Program (R01 NS083490) from the National Institute of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary material
Supplementary material is available at Brain online.
References
- Boeve BF. REM sleep behavior disorder: updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. 2010;1184:15–54. doi: 10.1111/j.1749-6632.2009.05115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–88. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology. 2010;75:494–9. doi: 10.1212/WNL.0b013e3181ec7fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang-Vu TT, Gagnon JF, Vendette M, Soucy JP, Postuma RB, Montplaisir J. Hippocampal perfusion predicts impending neurodegeneration in REM sleep behavior disorder. Neurology. 2012;79:2302–6. doi: 10.1212/WNL.0b013e318278b658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers Y, Boudousq V, Lopez R, Gabelle A, De Cock VC, Bayard S, et al. Increased perfusion in supplementary motor area during a REM sleep behaviour episode. Sleep Med. 2011;12:531–2. doi: 10.1016/j.sleep.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Braak H. Dysfunction of the locus coeruleus-norepinephrine system and related circuitry in Parkinson's disease-related dementia. J Neurol Neurosurg Psychiatry. 2013;84:774–83. doi: 10.1136/jnnp-2011-301817. [DOI] [PubMed] [Google Scholar]
- Eckert T, Tang C, Ma Y, Brown N, Lin T, Frucht S, et al. Abnormal metabolic networks in atypical parkinsonism. Mov Disord. 2008;23:727–33. doi: 10.1002/mds.21933. [DOI] [PubMed] [Google Scholar]
- Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci. 2009;32:548–57. doi: 10.1016/j.tins.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisensehr I, Linke R, Noachtar S, Schwarz J, Gildehaus FJ, Tatsch K. Reduced striatal dopamine transporters in idiopathic rapid eye movement sleep behaviour disorder. Comparison with Parkinson's disease and controls. Brain. 2000;123:1155–60. doi: 10.1093/brain/123.6.1155. [DOI] [PubMed] [Google Scholar]
- Fujishiro H, Iseki E, Murayama N, Yamamoto R, Higashi S, Kasanuki K, et al. Diffuse occipital hypometabolism on [18F]-FDG PET scans in patients with idiopathic REM sleep behavior disorder: prodromal dementia with Lewy bodies? Psychogeriatrics. 2010;10:144–52. doi: 10.1111/j.1479-8301.2010.00325.x. [DOI] [PubMed] [Google Scholar]
- Habeck C, Foster NL, Perneczky R, Kurz A, Alexopoulos P, Koeppe RA, et al. Multivariate and univariate neuroimaging biomarkers of Alzheimer's disease. Neuroimage. 2008;40:1503–15. doi: 10.1016/j.neuroimage.2008.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyu H, Inoue Y, Sakurai H, Kanetaka H, Nakamura M, Miyamoto T, et al. Regional cerebral blood flow changes in patients with idiopathic REM sleep behavior disorder. Eur J Neurol. 2011;18:784–8. doi: 10.1111/j.1468-1331.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- Holtbernd F, Gagnon JF, Postuma RB, Ma Y, Tang CC, Feigin A, et al. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology. 2014;82:620–7. doi: 10.1212/WNL.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson's disease. Neuroimage. 2007;34:714–23. doi: 10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui Y, Toyama H, Manabe Y, Sarai M, Iwata N. Comparison of 123I-MIBG myocardial scintigraphy, brain perfusion SPECT, and voxel-based MRI morphometry for distinguishing between dementia with Lewy bodies and Alzheimer's disease. Ann Nucl Med. 2014;28:796–804. doi: 10.1007/s12149-014-0873-2. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Molinuevo JL, Santamaria J, Serradell M, Marti MJ, Valldeoriola F, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–7. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Valldeoriola F, Lomena F, Molinuevo JL, Serradell M, Salamero M, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10:797–805. doi: 10.1016/S1474-4422(11)70152-1. [DOI] [PubMed] [Google Scholar]
- Lu J, Saper CB. Neuronal mechanisms of REM sleep and their role in REM sleep behavior disorder. In: Olanow CW, Stocchi F, Lang AE, editors. Parkinson's disease: non-motor and non-dopaminergic features. Oxford: Blackwell Publishing Ltd; 2011. pp. 240–5. [Google Scholar]
- Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson's disease: test-retest reproducibility. J Cereb Blood Flow Metab. 2007;27:597–605. doi: 10.1038/sj.jcbfm.9600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Miyamoto M, Inoue Y, Usui Y, Suzuki K, Hirata K. Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology. 2006;67:2236–8. doi: 10.1212/01.wnl.0000249313.25627.2e. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer's disease, and other dementias. J Nucl Med. 2008;49:390–8. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M, Eidelberg D. Metabolic brain networks in translational neurology: concepts and applications. Ann Neurol. 2012;72:635–47. doi: 10.1002/ana.23631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Ma Y, Spetsieris PG, Mattis P, Feigin A, Dhawan V, et al. Characterization of disease-related covariance topographies with SSMPCA toolbox: Effects of spatial normalization and PET scanners. Hum Brain Mapp. 2014;35:1801–14. doi: 10.1002/hbm.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72:1296–300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14:744–8. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Spetsieris P, Ma Y, Peng S, Ko JH, Dhawan V, Tang CC, et al. Identification of disease-related spatial covariance patterns using neuroimaging data. J Vis Exp. 2013;76:e50319. doi: 10.3791/50319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CC, Poston KL, Eckert T, Feigin A, Frucht S, Gudesblatt M, et al. Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet Neurol. 2010;9:149–58. doi: 10.1016/S1474-4422(10)70002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teune LK, Renken RJ, Mudali D, De Jong BM, Dierckx RA, Roerdink JB, et al. Validation of parkinsonian disease-related metabolic brain patterns. Mov Disord. 2013;28:547–51. doi: 10.1002/mds.25361. [DOI] [PubMed] [Google Scholar]
- Vendette M, Gagnon JF, Soucy JP, Gosselin N, Postuma RB, Tuineag M, et al. Brain perfusion and markers of neurodegeneration in rapid eye movement sleep behavior disorder. Mov Disord. 2011;26:1717–24. doi: 10.1002/mds.23721. [DOI] [PubMed] [Google Scholar]
- Wu P, Wang J, Peng S, Ma Y, Zhang H, Guan Y, et al. Metabolic brain network in the Chinese patients with Parkinson's disease based on 18F-FDG PET imaging. Parkinsonism Relat Disord. 2013;19:622–7. doi: 10.1016/j.parkreldis.2013.02.013. [DOI] [PubMed] [Google Scholar]