Abstract
Based on accumulating post-mortem evidence of abnormalities in Purkinje cell biology in essential tremor, we hypothesized that regressive changes in dendritic morphology would be apparent in the Purkinje cell population in essential tremor cases versus age-matched controls. Cerebellar cortical tissue from 27 cases with essential tremor and 27 age-matched control subjects was processed by the Golgi-Kopsch method. Purkinje cell dendritic anatomy was quantified using a Neurolucida microscopic system interfaced with a motorized stage. In all measures, essential tremor cases demonstrated significant reductions in dendritic complexity compared with controls. Median values in essential tremor cases versus controls were: 5712.1 versus 10 403.2 µm (total dendrite length, P = 0.01), 465.9 versus 592.5 µm (branch length, P = 0.01), 22.5 versus 29.0 (maximum branch order, P = 0.001), and 165.3 versus 311.7 (number of terminations, P = 0.008). Furthermore, the dendritic spine density was reduced in essential tremor cases (medians = 0.82 versus 1.02 µm−1, P = 0.03). Our demonstration of regressive changes in Purkinje cell dendritic architecture and spines in essential tremor relative to control brains provides additional evidence of a pervasive abnormality of Purkinje cell biology in this disease, which affects multiple neuronal cellular compartments including their axon, cell body, dendrites and spines.
Keywords: essential tremor, cerebellum, biology, Purkinje cell, neurodegenerative
Recent studies have documented structural changes in the cerebellum in patients with essential tremor. Louis et al. study the structure of Purkinje cells in control brains and in those with essential tremor. They report a consistent pattern of abnormalities including reduced dendritic arborization and loss of dendritic spines.
Introduction
Recent post-mortem studies have systematically documented numerous structural changes in the essential tremor cerebellum (Louis et al., 2006; Louis, 2014), mainly involving the Purkinje cells. In some, though not all studies, these changes include reductions in Purkinje cell counts (Louis et al., 2007, 2012a; Rajput et al., 2012) and reductions in Purkinje cell linear density (Axelrad et al., 2008; Louis et al., 2013, 2014; Symanski et al., 2014). Other findings include increased numbers of torpedoes (Louis et al., 2009, 2012b), a broad range of changes in Purkinje cell axonal morphology (Babij et al., 2013), increased numbers of Purkinje cell dendritic swellings (Yu et al., 2012), increased numbers of heterotopic Purkinje cells (Kuo et al., 2011), and changes in the basket cell axonal processes surrounding Purkinje cells (Erickson-Davis et al., 2010).
Patho-mechanistically, torpedoes and other Purkinje cell axonal changes are considered markers of neurons in extremis. Neurons with neurofilament misaccumulations (torpedoes) have impaired axonal transport, which may lead to neuronal strangulation and degeneration (Liem et al., 2003). Before degeneration, the metabolic economy of such neurons is severely challenged, and they are unable to maintain their extensive cytoskeleton. This may manifest as regressive changes in dendritic morphology (e.g. a truncation of the dendritic arbor) (Nakano and Hitano, 1987; Ma and Vacca-Galloway, 1991).
Based on accumulating evidence of abnormal Purkinje cell biology in essential tremor, we hypothesized that regressive changes in dendritic morphology might occur in Purkinje cells in essential tremor cases versus age-matched controls, and serve as an additional, more subtle, and more proximate biological marker than frank cell loss. Using the large collection of clinically and pathologically well characterized essential tremor brains obtained through the Essential Tremor Centralized Brain Repository, we applied the Golgi method to provide detailed morphometric study of both the Purkinje cell dendritic arbor and dendritic spine density, and application of rigorous quantitative methods to measure dendrite abnormalities.
Materials and methods
Clinical evaluation
Essential tremor cases were collected prospectively through the Essential Tremor Centralized Brain Repository, New York Brain Bank, Columbia University Medical Centre (Babij et al., 2013). Cases signed informed consent approved by the University Ethics Board.
Essential tremor diagnoses were assigned using three sequential methods (Babij et al., 2013). Cases underwent a standardized, videotaped neurological examination, which included assessments of postural, kinetic and intention tremors (Louis et al., 2007). Action tremor was rated using a clinical rating scale, resulting in a total tremor score (range = 0–36) (Louis et al., 2011). The videotaped examination included the motor portion of the Unified Parkinson’s Disease Rating Scale (Fahn et al., 1987). All data were reviewed (E.D.L.) and essential tremor diagnoses carefully reassessed using published diagnostic criteria (Babij et al., 2013).
Data on lifetime exposure to medications known to cause cerebellar damage were collected. In cases with essential tremor, the average number of daily drinks of beer, wine or liquor during adult lifetime was quantified. Heavy ethanol use was defined previously (Babij et al., 2013). Every 6 months, a follow-up evaluation was performed (Babij et al., 2013).
Normal elderly age-matched control brains (n = 11) were control subjects from the New York Brain Bank (Babij et al., 2013). Additional normal elderly age-matched controls were obtained from the University of Kentucky Alzheimer’s Disease Centre Biobank (n = 13) and Harvard Brain Tissue Resource Centre (McLean Hospital, Belmont, MA) (n = 3).
Neuropathological assessment
Each brain underwent a comprehensive neuropathological assessment (nybb.hs.columbia.edu), including assessment of neurofibrillary degeneration with Bielschowsky silver stain, and immunostains to alpha-synuclein, amyloid-β and hyperphosphorylated tau (Babij et al., 2013). Paraffin-embedded blocks from standardized brain regions were sectioned at 7 μm and stained with Luxol Fast blue, and haematoxylin and eosin. Brain weight (g) and post-mortem interval (hours between death and placement of brain in a cold room or upon ice) were recorded. Brains underwent Braak and Braak Alzheimer’s Disease staging for neurofibrillary tangles (Braak et al., 2006), Braak Parkinson’s disease staging of Lewy bodies (Braak et al., 2003), and Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) ratings for neuritic plaques (Mirra, 1997). A standard 3 × 20 × 25 mm parasagittal, formalin-fixed, tissue block was harvested from the neocerebellum; the block included the cerebellar cortex, white matter and dentate nucleus, and corresponded to the anterior and posterior quadrangulate lobules in the anterior lobe of the cerebellar cortex (lobules IV–VI), which are involved in motor control (Stoodley et al., 2012). A senior neuropathologist (P.L.F.), blinded to clinical information, counted torpedoes throughout one entire Luxol Fast blue and haematoxylin and eosin stained 7-µm thick section and, when available, a Bielschowsky stained 7-µm thick section. Purkinje cells were counted and averaged from 15 × 100 fields (Luxol Fast blue and haematoxylin and eosin stained section) and this count was divided by the length of the Purkinje cell layer centred in the microscopic field to derive Purkinje cell linear density (cells/mm).
Golgi studies
As brains were formalin-fixed for ≥15 months, the Golgi-Kopsch procedure is the optimal Golgi method (Jacobs et al., 2003). The standard cerebellar cortical tissue block was Golgi-Kopsch stained, and serially sectioned parallel to the long axis of the cerebellar gyri at 200-µm thickness with a vibrotome to increase the number of Purkinje cells with parasagittal orientation and full representation of their dendrite arbor. Purkinje cells selected for analysis followed a strict protocol. Four isolated Purkinje cells per block were chosen by a diagnostically-blinded technician based on previously published criteria (Jacobs et al., 1997): (i) cell body located centrally within the section depth to minimize cutting of distal dendritic branches; (ii) dark homogeneous impregnation throughout the extent of the neuronal dendrites, indicating adequacy of the Golgi staining; (iii) neurons were relatively unobscured by adjacent neuronal structures; and (iv) higher order branches had natural terminations. We further used a fifth selection criterion requiring optimal orientation (e.g. the dendritic arbor seen en face, with the visualization of at least two secondary dendrites). The first four cells in each block that met the above criteria were quantified using a Neurolucida system (Microbrightfield Inc) interfaced with a motorized microscope stage (Ludl Electronic Products) and ×20 objective lens for dendritic measurements and ×60 objective lens for spine density. Tracings proceeded in a specific sequence, beginning with the cell body followed by primary dendrites and their higher order branches, with tracking of branch nodes and dendrite lengths, as provided by the Neurolucida software. Five quantitative measures, as proposed by others (Jacobs et al., 1997; Rosoklija et al., 2000), were examined: (i) total dendrite length (i.e. the summed length of dendritic system) in µm; (ii) branch length (i.e. the average length of all dendritic segments between branch points) in µm; (iii) maximum branch order (i.e. the maximum number of branch bifurcations); (iv) number of terminations (i.e. the number of distal branch endings); and (v) dendritic spine density (i.e. the average number of spines/1 µm of dendritic length). For the determination of dendritic spine density, all terminal segments that at ×20 magnification were at least 5 µm long, had visible spines, and were unobscured by nearby branches were marked. Scholl’s method of concentric circles (Sholl, 1953) was used to divide the Purkinje cell dendritic arbor into two circles. The first (inner) circle was half the diameter of the second (outer) circle. Within each circle, the marked terminal segments were numbered in a clockwise sequence from a line drawn from the primary dendrite. Five of the marked proximal and distal terminal segments (10 segments per cell) were randomly selected. Using a ×60 oil immersion lens, spines were counted either to the nearest branch point or to the point at which the segment was obscured; the length of the segment was measured using the contour feature of StereoInvestigator (MicroBrightField). Dendritic spine density was the average spine density of the 10 segments.
Final sample
Excluded were essential tremor brains with concomitant neurodegenerative disorders diagnosed on post-mortem examination [e.g. Parkinson’s disease (n = 2), progressive supranuclear palsy (n = 2), corticobasal degeneration (n = 1)]. After these exclusions, tissue was available on a final sample of 27 essential tremor brains, which were age-matched to 27 control brains. Three cases and three controls had been included in our earlier report (Louis et al., 2007).
Statistical analyses
Statistical analyses were performed in SPSS (version 21.0). Dendritic architecture and spine density variables were not normally distributed; hence, case-control data were presented as both means and medians, and non-parametric statistical testing (Mann-Whitney tests, Spearman’s correlation coefficients) was performed. Each dendritic architecture and spine density variable was carefully specified in an a priori manner during the design phase of the study; hence, correction for multiple and/or post hoc comparisons was not required. Similarly, each dendritic architecture and spine density variable was compared to only one clinical variable, the total tremor score, which had been prespecified during the design phase of the study.
In a subanalysis, we excluded 12 cases and two control subjects whose Braak Alzheimer’s disease score was >2. Because this resulted in a marked reduction in sample size and analytical power, for this subanalysis, we reported percentage case-control differences in the absence of statistical comparisons.
Results
The 27 essential tremor cases and 27 controls were similar in age at death, brain weight, Braak Alzheimer’s disease score, and CERAD score, but differed by gender and post-mortem interval (Table 1). Age of tremor onset was >65 years in four (14.8%) cases and ≤65 years in the remainder. No cases were heavy ethanol users and none had lifetime exposure to medications known to cause cerebellar damage. None of the cases had both a Braak (i.e. neuronal tangle) score = 6 and a CERAD score = 3, although three, with lower scores, had intermediate likelihood of Alzheimer’s disease. Lewy bodies (alpha-synuclein: dorsal vagal nucleus, locus ceruleus, substantia nigra) were detected in none.
Table 1.
Clinical and pathological features of 27 essential tremor cases and 27 controls
| Essential tremor cases | Controls | Significance | |
|---|---|---|---|
| Age at death (years) | 87.7 ± 7.4 | 85.0 ± 6.7 | P = 0.16a |
| Female gender | 14 (51.9) | 21 (77.8) | P = 0.046b |
| Age of tremor onset (years) | 40.3 ± 26.3 | Not applicable | Not applicable |
| Duration of tremor (years) | 47.5 ± 26.4 | Not applicable | Not applicable |
| Median post-mortem interval (h) | 2 | 5.25 | P = 0.001 |
| Brain weight (g) | 1212 ± 156 | 1176 ± 136 | P = 0.36a |
| Braak Alzheimer’s disease scorec | P = 0.16b | ||
| 0 | 2 (7.4) | 2 (18.2) | |
| 1 | 9 (33.3) | 3 (27.3) | |
| 2 | 4 (14.8) | 4 (36.4) | |
| 3 | 11 (40.7) | 1 (9.1) | |
| 4 | 1 (3.7) | 0 (0.0) | |
| 5 | 0 (0.0) | 0 (0.0) | |
| 6 | 0 (0.0) | 1 (9.1) | |
| CERAD scorec | P = 0.79b | ||
| 0 | 11 (40.7) | 4 (36.4) | |
| A | 10 (37.0) | 5 (45.5) | |
| B | 4 (14.8) | 2 (18.2) | |
| C | 2 (7.4) | 0 (0.0) | |
| Torpedo count (Luxol Fast blue and counterstained with haematoxylin and eosin) | 17.7 ± 16.6 | 6.1 ± 7.5 | P = 0.018a |
| Torpedo count (Bielschowsky) | 25.8 ± 22.8 | 10.3 ± 10.3 | P = 0.02a |
| Purkinje cell count | 6.8 ± 1.8 | 8.5 ± 2.1 | P = 0.025a |
| Purkinje cell linear density (cells/mm) | 3.1 ± 0.8 | 3.9 ± 1.0 | P = 0.025a |
Values are mean ± standard deviation or number (percentage) unless specified otherwise.
aStudent t-test.
bChi-square test.
cData available on 11 rather than 27 control subjects.
Essential tremor cases had higher torpedo counts [P = 0.018 (Luxol Fast blue and counterstained with haematoxylin and eosin) and P = 0.02 (Bielschowsky)] and lower Purkinje cell counts and Purkinje cell linear density (P = 0.025) than controls (Table 1). The two torpedo counts (Luxol Fast blue and counterstained with haematoxylin and eosin, and Bielschowsky) were highly consistent with one another (Pearson’s r = 0.85, P < 0.001).
In all measures, essential tremor cases demonstrated significant reductions in dendritic complexity compared with controls (Table 2 and Fig. 1). Reductions in essential tremor cases ranged from 21.4–47.0% (median = 33.8%, Table 2). Furthermore, the dendritic spine density was reduced in essential tremor cases compared to controls (Table 2).
Table 2.
Purkinje cell dendritic arborization and spine density in 27 essential tremor cases and 27 controls
| Essential tremor cases | Controls | Percentage differencea | Significance | |
|---|---|---|---|---|
| Total dendrite length (µm) | 8605.9 ± 6909.9 (5712.1) | 11 658.5 ± 5734.2 (10 403.2) | 45.1% | P = 0.01b |
| Branch length (µm) | 515.9 ± 174.3 (465.9) | 600.3 ± 134.2 (592.5) | 21.4% | P = 0.01b |
| Maximum branch order | 23.4 ± 8.4 (22.5) | 29.6 ± 6.5 (29.0) | 22.4% | P = 0.001b |
| Number of terminations | 211.0 ± 164.1 (165.3) | 311.1 ± 153.8 (311.7) | 47.0% | P = 0.008b |
| Dendritic spine density (µm − 1) | 0.79 ± 0.21 (0.82) | 0.97 ± 0.20 (1.02) | 18.6% | P = 0.03b |
Values are mean ± standard deviation (median).
aPercentage difference between the median value of cases versus the median value of controls.
bMann Whitney test.
In a subanalysis in which we excluded 12 cases and two controls whose Braak Alzheimer’s disease score was >2, the percentage difference between the median value of the cases versus controls persisted: total dendrite length (51.7%), branch length (22.8%), maximum branch order (25.8%), number of terminations (53.8%), and dendritic spine density (18.5%).
Figure 1.
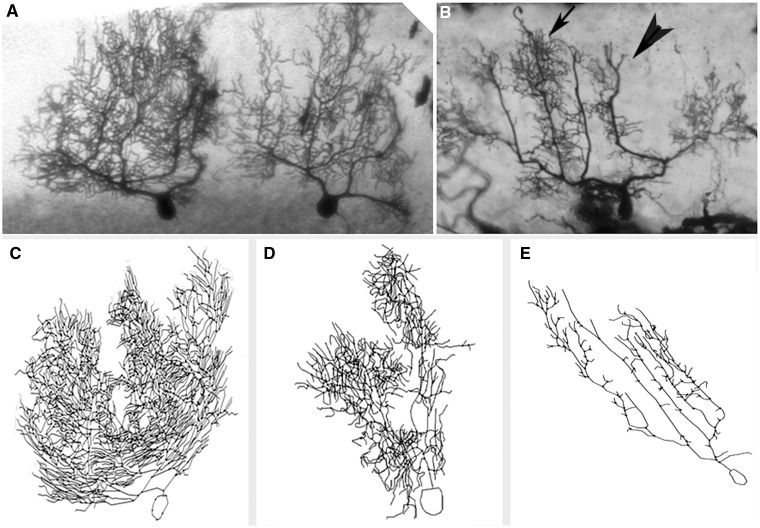
Reduction in Purkinje cell dendritic complexity in ET. (A and B) Cerebellar cortical sections stained with Golgi Kopsch method, ×2.5. Two adjacent Purkinje cells in a control (A) and one Purkinje cell in an essential tremor case (B). Arrow in B shows an area of relatively preserved dendritic complexity versus arrowhead in B which shows an area of reduced dendritic complexity in the essential tremor case. (C–E) Neurolucida tracings of Purkinje neurons in two controls (C and D) and one representative essential tremor case (E). The neurolucida tracings correspond to different neurons than those shown in A and B.
The dendritic arborization and spine density variables were all highly-intercorrelated (i.e. changes in one variable were reflected in changes in other variables), indicating a system-wide set of changes rather than single, isolated changes (Table 3).
Table 3.
Correlation between dendritic arborization and spine density variables in 27 essential tremor cases and 27 controls
| Total dendrite length (µm) | Branch length (µm) | Maximum branch order | Number of terminations | Dendritic spine density (µm−1) | |
|---|---|---|---|---|---|
| Total dendrite length (µm) | r = 0.78, P < 0.001 | r = 0.83, P < 0.001 | r = 0.93, P < 0.001 | r = 0.52, P = 0.002 | |
| Branch length (µm) | r = 0.61 P < 0.001 | r = 0.58, P < 0.001 | r = 0.39, P = 0.026 | ||
| Maximum branch order | r = 0.90, P < 0.001 | r = 0.58, P < 0.001 | |||
| Number of terminations | r = 0.59, P < 0.001 | ||||
| Dendritic spine density (µm−1) |
r = Spearman’s correlation coefficients.
The dendritic architecture and spine density variables were not correlated to any consistent degree with age, gender, post-mortem interval, CERAD score, Braak Alzheimer’s disease score, or brain weight (Supplementary Table 1). Therefore, these variables could not have been confounders. In essential tremor cases, the average number of daily drinks (beer, wine and liquor combined) was not associated with total dendrite length (Spearman’s r = 0.11, P = 0.63), branch length (Spearman’s r = −0.04, P = 0.87), maximum branch order (Spearman’s r = −0.13, P = 0.55), number of terminations (Spearman’s r = 0.04, P = 0.84), or dendritic spine density (Spearman’s r = 0.09, P = 0.73).
Dendritic architecture and spine density variables were not correlated to a significant degree with Braak Alzheimer’s disease score (Supplementary Table 1); hence, the observed case-control differences in these variables were not likely due to confounding effects of Alzheimer’s type changes. Nonetheless, we performed an additional subanalysis in which we excluded 12 cases and two controls whose Braak Alzheimer’s disease score was >2. Case-control differences persisted, and for four of five variables, the magnitude of the case-control differences was slightly higher than seen when analyses included the full sample (Table 2).
The Purkinje cell count (Luxol Fast blue and counterstained with haematoxylin and eosin) was marginally associated with maximum branch order (i.e. brains with fewer Purkinje cells had a lower maximum branch order, Spearman’s r = 0.33, P = 0.06). Total tremor score was inversely associated with branch length (Spearman’s r = −0.44, P = 0.047), maximum branch order (Spearman’s r = −0.41, P = 0.058), and number of terminations (Spearman’s r = −0.43, P = 0.054) (i.e. more severe tremor was associated with more marked dendritic pruning). None of these variables (including total tremor score) was associated with disease duration.
Discussion
Using quantitative analyses of Purkinje cells stained by the Golgi method, we found significant reductions in Purkinje cell dendritic complexity and dendritic spine density in essential tremor cases versus controls. These dendritic arbor and spine density variables were all highly intercorrelated, indicating a system-wide set of regressive changes rather than isolated and unconnected changes.
A reduction in dendritic arborization is a structural change associated with dysfunction, and it is thought to precede neuronal death (Ferrer et al., 1984). This type of change is not specific to essential tremor, and it has been noted in Purkinje cells in patients with hereditary ataxias (Shintaku and Kaneda, 2009) as well as Purkinje cells in chronic alcoholics (Ferrer et al., 1984). Hence, these changes are a marker of neurons in extremis. Previous studies have shown a reduction of Purkinje cell dendritic arbor complexity in Alzheimer’s disease (Mavroudis et al., 2013). We conducted several additional analyses that indicated that concomitant Alzheimer’s type-changes in essential tremor cases do not seem to account for the observed essential tremor case-control differences.
Importantly, we demonstrated that tremor of greater severity was correlated with more pruning of the Purkinje cell dendritic arbor, as observed in several quantitative measures including branch length, maximum branch order and number of terminations. In addition, the Purkinje cell count (Luxol Fast blue and counterstained with haematoxylin and eosin) was associated with maximum branch order (i.e. brains with fewer Purkinje cells had a lower maximum branch order).
This study provides the only report to date on the dendritic anatomy of the Purkinje neuron population in essential tremor. These findings may be added to the growing catalogue of changes documented in the Purkinje cell population in essential tremor (Grimaldi et al., 2013). The current study focused in detail on the Purkinje cell dendritic compartment, whereas our previous work has focused on the axonal compartment and cell body. In combination, these results indicate that the Purkinje cell is broadly affected across several cell compartments in essential tremor. Our current findings and previous findings (Babij et al., 2013) also indicate that the identifiable presence of a range of structural markers of neurons in extremis in essential tremor and, as discussed in detail elsewhere, frank Purkinje cell loss is likely to be only one part of a cascade of patho-mechanistic events involving the cerebellar cortex in essential tremor (Louis, 2014).
Regressive changes in Purkinje cell dendritic architecture seem to be a feature of essential tremor relative to control brains, and these changes provide new evidence of a pervasive abnormality of Purkinje cell biology in this disease.
Acknowledgements
We thank the Harvard Brain Tissue Resource Centre (supported in part by PHS grant number R24 MH068855) for providing control tissue. We similarly thank the University of Kentucky Alzheimer’s Disease Centre Biobank (P30 AG028383) for providing control tissue, and the patients, staff, and clinicians who have contributed to their efforts. We thank Bob Jacobs PhD, Colorado College, for providing guidance on Golgi methods. We thank Andrew Dwork, MD, Columbia University, for providing equipment and expertise with Golgi methods and Neurolucida microscopy.
Glossary
Abbreviation
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease
Funding
Dr Louis has received research support from the National Institutes of Health: NINDS #R01 NS042859 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #R01 NS086736 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R01 NS085136 (principal investigator), NINDS #T32 NS07153-24 (principal investigator), NINDS #R21 NS077094 (co-Investigator), NINDS #R01 NS36630 (co-Investigator) and NIEHS P30 ES09089 (co-investigator). He has also received support from Parkinson’s Disease Foundation, the Arlene Bronstein Essential Tremor Research Fund (Columbia University), and the Claire O'Neil Essential Tremor Research Fund (Columbia University). Dr Faust has received funding from NINDS #R21 NS077094 (principle Investigator) and NINDS #R01 NS39422 (co-investigator). Dr Vonsattel has received funding from NINDS #R01 NS39422 (co-investigator).
Supplementary material
Supplementary material is available at Brain online.
References
- Axelrad JE, Louis ED, Honig LS, Flores I, Ross GW, Pahwa R, et al. Reduced purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65:101–7. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babij R, Lee M, Cortés E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136(Pt 10):3051–61. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Erickson-Davis CR, Faust PL, Vonsattel JP, Gupta S, Honig LS, Louis ED. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69:262–71. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Elton RL. Members of the UPDRS development committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calen DB, Goldstein M, editors. Recent developments in Parkinson's disease. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–63. [Google Scholar]
- Ferrer I, Fabregues I, Pineda M, Gracia I, Ribalta T. A Golgi study of cerebellar atrophy in human chronic alcoholism. Neuropathol Appl Neurobiol. 1984;10:245–53. doi: 10.1111/j.1365-2990.1984.tb00357.x. [DOI] [PubMed] [Google Scholar]
- Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord. 2013;28:1759–61. doi: 10.1002/mds.25645. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Creswell J, Britt JP, Ford KL, Bogen JE, Zaidel E. Quantitative analysis of cortical pyramidal neurons after corpus callosotomy. Ann Neurol. 2003;54:126–30. doi: 10.1002/ana.10620. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol. 1997;386:661–80. [PubMed] [Google Scholar]
- Kuo SH, Erickson-Davis C, Gillman A, Faust PL, Vonsattel JP, Louis ED. Increased number of heterotopic Purkinje cells in essential tremor. J Neurol Neurosurg Psychiatry. 2011;82:1038–40. doi: 10.1136/jnnp.2010.213330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem RK, Leung CL. Neuronal intermediate filament overexpression and neurodegeneration in transgenic mice. Exp Neurol. 2003;184:3–8. doi: 10.1016/s0014-4886(03)00291-7. [DOI] [PubMed] [Google Scholar]
- Louis ED. Re-thinking the biology of essential tremor: from models to morphology. Parkinsonism Related Disord. 2014;20(Suppl 1):S88–93. doi: 10.1016/S1353-8020(13)70023-3. [DOI] [PubMed] [Google Scholar]
- Louis ED, Agnew A, Gillman A, Gerbin M, Viner AS. Estimating annual rate of decline: prospective, longitudinal data on arm tremor severity in two groups of essential tremor cases. J Neurol Neurosurg Psychiatry. 2011;82:761–5. doi: 10.1136/jnnp.2010.229740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Babij R, Lee M, Cortés E, Vonsattel JP. Quantification of cerebellar hemispheric purkinje cell linear density: 32 ET cases versus 16 controls. Mov Disord. 2013;28:1854–9. doi: 10.1002/mds.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Faust PL. Purkinje cell loss in essential tremor. Mov Disord. 2014;29:1329–30. doi: 10.1002/mds.25965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor: towards a more mature understanding of pathogenesis. Parkinsonism Relat Disord. 2012a;18:1003–4. doi: 10.1016/j.parkreldis.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130(Pt 12):3297–307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ma K, Babij R, Cortés E, Liem RK, Vonsattel JP, et al. Neurofilament protein levels: quantitative analysis in essential tremor cerebellar cortex. Neurosci Lett. 2012b;518:49–54. doi: 10.1016/j.neulet.2012.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Vonsattel JP, Honig LS, Ross GW, Lyons KE, Pahwa R. Neuropathologic findings in essential tremor. Neurology. 2006;66:1756–9. doi: 10.1212/01.wnl.0000218162.80315.b9. [DOI] [PubMed] [Google Scholar]
- Louis ED, Yi H, Erickson-Davis C, Vonsattel JP, Faust PL. Structural study of Purkinje cell axonal torpedoes in essential tremor. Neurosci Lett. 2009;450:287–91. doi: 10.1016/j.neulet.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WY, Vacca-Galloway LL. Reduced branching and length of dendrites detected in cervical spinal cord motoneurons of Wobbler mouse, a model for inherited motoneuron disease. J Comp Neurol. 1991;311:210–22. doi: 10.1002/cne.903110204. [DOI] [PubMed] [Google Scholar]
- Mavroudis IA, Manani MG, Petrides F, Petsoglou K, Njau SD, Costa VG, et al. Dendritic and spinal pathology of the Purkinje cells from the human cerebellar vermis in Alzheimer's disease. Psychiatr Danub. 2013;25:221–6. [PubMed] [Google Scholar]
- Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer's disease: a commentary. Neurobiol Aging. 1997;18(Suppl 4):S91–4. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- Nakano I, Hirano A. Atrophic cell processes of large motor neurons in the anterior horn in amyotrophic lateral sclerosis: observation with silver impregnation method. J Neuropathol Exp Neurol. 1987;46:40–9. doi: 10.1097/00005072-198701000-00004. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Robinson CA, Rajput ML, Robinson SL, Rajput A. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord. 2012;18:626–8. doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann JJ, Latov N, et al. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–56. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- Shintaku M, Kaneda D. Chromosome 16q22.1-linked autosomal dominant cerebellar ataxia: an autopsy case report with some new observations on cerebellar pathology. Neuropathology. 2009;29:285–92. doi: 10.1111/j.1440-1789.2008.00947.x. [DOI] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59:1560–70. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symanski C, Shill HA, Dugger B, Hentz JG, Adler CH, Jacobson SA, et al. Essential tremor is not associated with cerebellar Purkinje cell loss. Mov Disord. 2014;29:496–500. doi: 10.1002/mds.25845. [DOI] [PubMed] [Google Scholar]
- Yu M, Ma K, Faust PL, Honig LS, Cortés E, Vonsattel JP, et al. Increased number of Purkinje cell dendritic swellings in essential tremor. Eur J Neurol. 2012;19:625–30. doi: 10.1111/j.1468-1331.2011.03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]