Abstract
The first six months of life reflects a time of high susceptibility to severe disease following respiratory virus infection. While this could be significantly improved by immunization, current vaccines are not approved for use in these very young individuals. This is the result of the combined effects of poor immune responsiveness and safety concerns regarding the use of live attenuated vaccines or potent adjuvants in this population. Vaccines to effectively combat respiratory viral infection would ideally result in robust CD4+ and CD8+ T cell responses as well high affinity antibody. Inclusion of TLR agonists or single cycle viruses are attractive approaches for provision of signals that can act as potent stimulators of DC maturation as well as direct activators of T and/or B cells. Here we discuss the challenges associated with generation of a robust immune response in neonates and the potential for adjuvants to overcome these obstacles.
Infant immune response to respiratory virus infections
Respiratory infections are one of the leading causes of morbidity and mortality throughout the world. Among the most prevalent are infections with respiratory syncytial virus (RSV), rhinovirus (RV) and influenza virus (1). These infections are particularly problematic for infants, resulting in increased morbidity and mortality compared to older children and adults. There are an estimated 11.9 million episodes of severe acute lower respiratory tract infection (ALRI) in young children each year (2). Children under one year of age account for 6.4 million instances of severe ALRI and nearly 3 million cases that are grave enough to be considered very severe (2). Further, children less than 12 months of age exhibit a three-fold increase in the rate of fatality following infection compared to children 12–59 months (2). Not surprisingly, the likelihood of severe disease decreases as age increases. For example, in the case of RSV infection, approximately half of children requiring hospitalization are ≤3 months of age (3) and infants under 27 days have the highest incidence of ALRI-associated disease (2). Together these findings demonstrate the extreme susceptibility of the newborn to disease caused by respiratory pathogens.
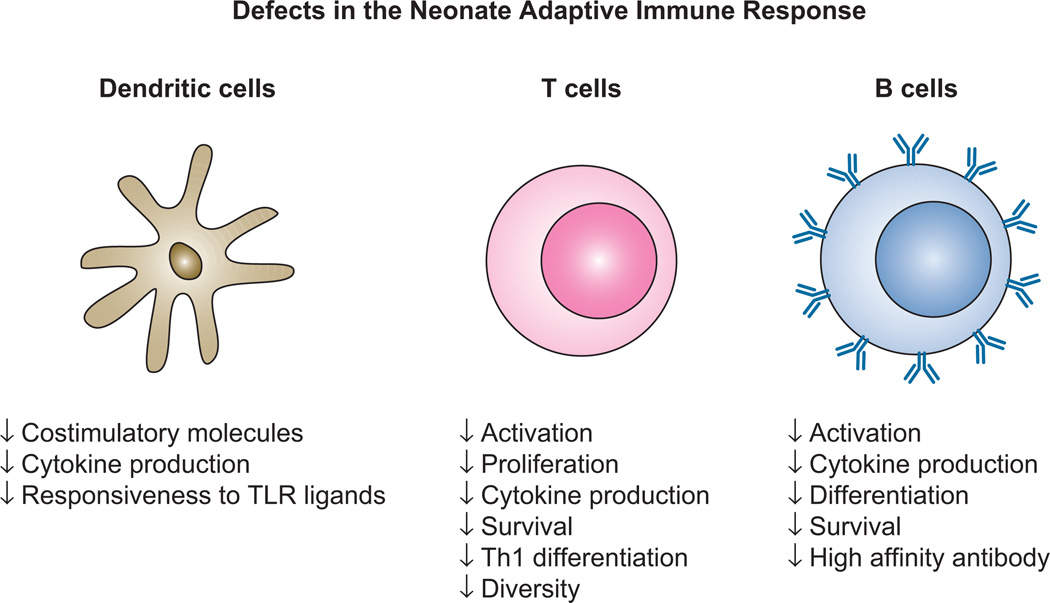
The increased disease severity associated with respiratory infection in infants is the result of both the naïve status of these individuals as well as the reduced ability of the immune system to respond to infection. Defects in infant immunity span both innate and adaptive components, both of which are critical contributors to immune mediated clearance of infection (4–6). Reported defects in the innate response include reduced migration, phagocytosis, and bactericidal activity (6, 7). Adaptive immune defects include decreased cytokine production and costimulatory molecule expression by antigen presenting cells, reduced T cell sensitivity following ligand engagement, decreased T cell repertoire diversity, decreased T cell effector function, a bias towards Th2 development, and impaired B cell differentiation and survival (4–7) (Fig. 1).
Figure 1. Neonates exhibit multiple adaptive immune defects that contribute to poor responses following infection or vaccination.
Potent adaptive immune responses are dependent on the capacity of DC to undergo maturation together with the robust activation, differentiation and survival of T and B cells. Defects encompassing a broad range of these attributes have been reported in cells from neonates. As a result responses following infection and vaccination are qualitatively and quantitatively reduced in these individuals compared to adults.
Effective control of respiratory virus infection begins with a robust innate antiviral response that is dominated by the production of type I IFN. The production of this critical innate antiviral mediator is diminished in neonates as a result of both decreased production on a per cell basis as well as a reduction in the number of plasmacytoid dendritic cells (DC) (3, 8, 9), the cell type specialized for high level type I IFN production. Beyond type I IFN, the innate response to virus infection that results the production of cytokines and chemokines that promote inflammation and immune cell recruitment is decreased in infants (10).
Innate immune responses to virus infection are dependent on activation through toll like receptors (TLR) as well as cytoplasmic innate sensors, e.g. RIG-I and MDA-5. Both TLR and RIG-I mediated responses are impaired in neonates (3, 9,11–13). The reduced activity of these innate sensors has implications for the generation of the adaptive immune response as they are important mediators of DC maturation that promotes competence for naïve T cell activation. Specifically, DC from neonates produce low amounts of IL-12 and are impaired in their ability to upregulate costimulatory molecules, e.g. CD80 and CD86, following exposure to virus-derived signals (e.g. (9)). These deficiencies comprise the first obstacle in generation of an efficacious adaptive immune response in the neonate.
In addition to the impaired function of DC, T lymphocytes from neonates exhibit inherent defects in their ability to undergo activation and differentiation (14–16). Reported defects include reduced levels of the signaling molecules lck and ZAP-70 (17) as well as a decrease in AP-1 mediated transcription (18). The combined deficiencies in DC maturation and T cell responsiveness are likely contributors to impaired T cell responses observed in vivo following infection or vaccination (19, 20).
Antibody responses are also significantly decreased in neonates (4, 21). Antibody responses in young infants are largely IgM, with IgG production generally weak for the first year of life (22). While increased relative to IgG, IgM responses are also impaired as exemplified by RSV infection of human infants where both IgM and IgG responses are poor (23). Similar findings have been reported in murine models (24). Isotype analysis showed a skewing towards IgG1, indicating a Th2 biased response (24). Important contributors to the poor antibody response in infants are impaired accessory cells, i.e. TFH (25) and follicular DC (26), as well as inherent defects in B cell survival and differentiation (27). A potential contributor to the latter is the reduced expression of BCMA and BAFF-R on neonate B cells (28). Both of these receptors bind to BAFF, with BCMA having an additional ligand APRIL (29). Engagement of BAFFR or BCMA on B cells promotes survival through upregulation of anti-apoptotic bcl-2 family members together with downregulation of the pro-apoptotic factors bim and bad (30). The survival of plasmablasts and differentiation into long lived antibody secreting cells in the neonate is likely hampered by decreased levels of APRIL and BCMA (31). In the context of influenza virus infection, the absence of BAFF and APRIL has been shown to result in an overall reduction in antiviral IgG (32). Whether there are defects in the initial activation of neonatal B cells is a matter of debate, although there are reports from studies of cord blood cells that suggest competence in this arena (33).
Infant immune response to vaccination against respiratory viruses
While data from the analysis of respiratory virus vaccine responses in very young infants is limited, what is available supports the inadequacy of current vaccine approaches for use in this population. These studies have predominantly analyzed the antibody response, undoubtedly for practical reasons associated with sampling in this population. Delivery of the trivalent inactivated influenza vaccine in infants between 3–5 months old results in poor generation of antibody (34, 35). An initial dose of vaccine was not capable of inducing seroconversion for most strains (as defined by a fold-fold increase in antibody) (34). This low responsiveness was not the result of maternal antibody, as all individuals had pre-vaccination titers of <1:8. A second dose resulted in seroconversion rate of 27–32% for H1N1 strains and 17–93% across H3N2 strains. Not surprisingly, a correlation was observed between age and the rate of conversion with older infants converting at a higher rate than younger infants (34). In a second study, conversion was assessed following completion of two doses of vaccine with a reported conversion rate of 42–43% for H1N1 and 39–67% for H3N2 strains (35). For comparison, published studies assessing responses in older children reported the percent individuals between 11 and 16 years of age with a 4-fold rise in titer was >90% after a single vaccination (36).
Vaccine responsiveness in young infants has also been evaluated for measles and mumps. This vaccine is routinely given at 12 months of age. Administration at an earlier time, i.e. 6 or 9 months of age, resulted in a significantly reduced antibody response (37). A parallel impairment in the T cell response was also observed, with virus-specific cells exhibiting a reduction in the amount of IFNγ produced in response to stimulation (37). Importantly, as with influenza virus, the reduced responsiveness in these individuals could not be accounted for by the presence of maternal antibody. The reduction in responsiveness in 6 and 9 month old infants suggests the immune system continues to be impaired to some extent throughout the first 9 months of life. It is difficult to state definitively when the immune system of the infant reaches full maturity. For example, four doses of the oral polio vaccine given to infants resulted in a reduced IFNγ producing T cell responses compared to adults receiving a single dose (38), whereas infants administered BCG vaccine had responses similar to adults (39). These findings suggest the time at which responses in children can approximate adults varies with the nature/strength of the challenge (27) Although there is often significant impairment in immunity in young infants, the ability to obtain some degree of responsiveness following vaccination and instances where responsiveness is relatively robust provides hope that the provision of additional stimulatory agents in the context of vaccination may be able to boost the response to levels that are protective in these individuals.
Desirable attributes of immune responses elicited by respiratory virus-specific vaccines and lung-specific challenges
The goal of vaccination is the generation of long-lived protective immunity. In the case of respiratory virus infection, this ideally includes the generation of high affinity neutralizing antibody, central memory T cells and tissue resident memory cells in the in the lung. This is a tall order in the context of adult vaccination and even more challenging in the neonate.
We have an increasing appreciation for the importance of lung resident memory T cells in the control of virus infection (40–42). The presence of these cells is the result of the combined effects of production in the local lymph node and in the bronchus associated lymphoid tissue (BALT). While the benefit of vaccination strategies that could induce local immune responses in BALT is clear, approaches that could achieve this goal are less so. Lung targeted delivery of a vaccine is certainly a difficult undertaking in a neonate. A further point of caution is that while induction of BALT to facilitate immune responses is potentially advantageous, it is unclear how/whether the presence at very early ages of the strong inflammatory signals necessary for the induction of BALT (43) would impact establishment of regulatory processes in the lung. For example, an overly robust inflammatory challenge has been shown to induce long-term changes in airway macrophages, i.e. these cells exhibit decreased responsiveness to TLR agonists, reduced phagocytosis and increased production of IL-10 (44). These changes can be conferred to newly recruited macrophages, thereby maintaining altered function for extended times (44). This is clearly an area where additional studies are needed in order to assess the potential for direct targeting of the lung for vaccination in this population.
In standard vaccine delivery approaches, resulting memory T cells must be recruited to the lung airway following reactivation in the draining lymph node, an event that is critical component of effective clearance and protection (45). In this regard, analysis of influenza infection of mouse neonates showed that T cells were impaired in their ability to migrate from the interstitium to the airways (46). Thus, efficient trafficking of effector T cells may pose an added obstacle to efficacious responses following infection.
An additional challenge in the lung is the apparent ability of the lung environment to negatively regulate effector cell function. The loss of function in effector cells has been reported to occur in the context of a number of infectious processes, including respiratory syncytial virus (47, 48), parainfluenza virus 5 (49), and murine pneumovirus (50). Our work suggests this is an intrinsic property of the lung (51, 52). Functional inactivation is observed even in the face of the inflammatory environment present following infection. That said, negative regulation is less apparent at early times postinfection, when viral load and inflammation are high, suggesting the presence of an inflammatory environment may dampen the inhibitory effect (47, 49). The extent to which this effect occurs in the lungs of neonates has not been explored. It seems likely, however, that it will be in play or even enhanced given the propensity for negative regulation of the immune response in this population.
Approaches to overcome the reduced responsiveness to vaccination in neonates
Virus infection often results in long-lasting, protective immunity. Arguably, the closest we have come to achieving this goal in the context of vaccination against viral pathogens is through the use of live attenuated constructs. This approach has resulted in the eradication of smallpox and large reductions in infections with viral pathogens previously associated with childhood disease, e.g. measles, mumps, rubella and chicken pox. The success of live attenuated vaccines is likely a consequence of elicitation of both robust antibody and CD8+ T cell responses (53–55), a goal not yet realized with inactivated/subunit vaccines. However, while the ability to achieve both humoral and cell mediated immune responses is highly desirable, the use of live attenuated constructs in neonates is undesirable due to safety concerns, including the potential for undiagnosed immune deficiencies. Consequently, alternative approaches that can elicit both arms of the immune response combined with a superior safety profile are sorely needed.
Generation of potent cell mediated and humoral immune responses requires the participation of multiple cell types- at a minimum dendritic cells, CD4+ T cells, CD8+ T cells and B cells. Optimal activation of these populations can be facilitated by mediators that act directly on individual cells (direct) as well as those that modulate function in accessory cells that subsequently provide activating signals to T cells in the form of cell surface molecules or cytokines (indirect). For example, vaccines that target DC maturation will promote T cell activation. However, T and B cells can also receive direct signals via stimulatory cell surface receptors. Targeting T and B cells by the combination of direct and indirect activation signals in the context of vaccination may aid in overcoming defects associated with neonatal immune responsiveness.
Vaccine responsiveness can be significantly improved by inclusion of adjuvants. Approved adjuvants in the US and/or Europe include aluminum salts, oil-in-water emulsions (MF59, AS03, an AF03), virosomes, and AS04 (monophoshporykllipis A preparation (MPL) with aluminum salt) (56). Excellent reviews on the actions of adjuvants have recently been published (56, 57). An area of intense focus in adjuvant development is the use of TLR agonists. TLR are sensors of pathogen associated molecular patterns (PAMPS) that survey the environment through residence at both the cell membrane and the endosome. Ten TLR have been characterized in humans (58). Cell surface TLR including TLR1/2 and TLR2/6 heterodimers together with TLR4, TLR5, and TLR10 homodimers recognize a variety of PAMPS associated with bacterial or viral infection. Endosomal TLR (TLR3, 7, 8, 9) sense pathogen derived nucleic acids and are key players in the context of virus infection (59). Respiratory viruses contain ligands for multiple TLR, a portion of which are shared across many viruses and thus are attractive for vaccine development. For example, influenza virus and RSV, pathogens of major clinical importance in neonates, activate TLR3 and TLR7 (60, 61). Ligands have been identified for each TLR, with the exception of TLR10, and not surprisingly work is underway to exploit these ligands in the context of vaccination.
Individual TLR can be widely distributed on immune cells. For example, TLR7 is expressed by human T cells (62), B cells (63), and pDC (64) and TLR8 is expressed on monocytes/macrophages and myeloid DC (65, 66). As such, a TLR7/8 agonist would provide both direct and indirect activation signals for the elicitation of T and B cells following vaccination. In contrast, in humans TLR5 is expressed on DC and T cells, but not B cells (67). As a result, agonists for this receptor would deliver activating signals to a more limited number of cells. Thus, the choice of TLR agonist will determine the cells targeted during vaccination.
The importance of TLR engagement in the generation of efficacious vaccine responses is suggested by studies from Polack and colleagues (68). The enhanced respiratory disease that occurred following vaccination of children with formalin-inactivated RSV was a major setback in vaccine development. The low avidity antibody generated was found to be the result of deficient TLR stimulation (68). These data strongly support the critical role for TLR in the generation of protective immune responses in the context of vaccination.
There are a number of ongoing trials to assess the efficacy of TLR agonists in the context of adult vaccination with promising results. The question then arises as to whether this is a valuable avenue of investigation in the context of neonates. At present, our understanding of the neonate response to TLR engagement is far from complete. Available data suggest the neonate expresses TLR at levels that are relatively similar to that of adults (69, 70). In spite of this, many studies using cells from cord blood or neonatal mice have reported hyporesponsiveness to TLR engagement. Analysis of DC from neonates revealed impaired maturation as measured by cytokine production and upregulation of the costimulatory molecules CD80 and CD86 in response to TLR agonists (9, 11, 12). The decreased responsiveness is associated with reduced expression of MyD88, suggesting impaired signaling may contribute to the failure to undergo appropriate maturation (69). While admittedly decreased, the ability to promote some degree of maturation following TLR engagement allows for the possibility that increasing the strength of signaling through these receptors may be able to overcome the observed deficits. In support of this, there are instances where increasing the level of TLR agonist results in cytokine production that is similar to levels observed in adults (12, 71).
Flagellin is a potent TLR5 agonist that has shown great promise as an adjuvant in the context of adult vaccination (67) and is currently in clinical trails as a component of vaccines against plague and influenza. In human adult-derived cells, TLR5 was found to both promote efficient maturation of DC (72) and increase activation of T lymphocytes (62). In the context of the neonate, when purified cord blood T cells were treated with flagellin together with TCR ligation, increased proliferation and higher levels of IFNγ and lytic components was observed (73). Flagellin exposure has also been reported to induce CD45RO and CCR4 expression in cord blood derived T cells demonstrating TLR stimulation can trigger maturation and may alter trafficking in T cells (74). Surprisingly, adult T cells did not show these same changes suggesting inherent differences in neonate versus adult responses. The efficacy of flagellin as an adjuvant in the setting of infant vaccination has been explored in young (4–6 month old) monkeys (75). Inclusion of flagellin in a vaccine against Pseudomonas aeruginosa resulted in increased antibody responses and significantly reduced pathogen loads following challenge (75). Thus, flagellin holds promise and further studies to evaluate its utility in neonates are merited.
There is evidence that TLR2 agonists may also be beneficial in neonates. Inclusion of the TLR2 agonist Pam3Cys increased activation of T cells in this population (73). Further, select TLR ligands can induce maturation of APC, approaching the level observed in adults (12, 76). For example, the TLR8 agonist 3M-002 induces potent upregulation of CD40, CD80, CD83 and CD86 as well as production of the Th1-polarizing cytokine IL-12p70 in cells from neonates (12). A contributor to the effectiveness of TLR8 agonists in the context of neonate cells appears to be the resistance of this pathway to inhibition by adenosine (12), a known inhibitory immune modulator in the blood of newborns (77).
An alternative strategy for increasing efficacy of these immune modulators is the delivery of multiple TLR agonists. Simultaneous engagement of several TLR has been shown to change dendritic cell maturation in both a qualitative and quantitative fashion (78, 79). T cells derived from human cord blood stimulated concurrently with TLR2 and TLR5 agonists underwent greater proliferation and cytokine production compared to cells stimulated with either agonist alone (73). While unknown, studies of TB vaccination may suggest this approach is useful in the context of newborn vaccination. The tuberculosis vaccine (BCG: Bacillus Calmette-Guerin), which is routinely delivered within 48h of birth, is one of a limited number of vaccines that has shown success in very young infants. BCG contains ligands for 5 distinct TLR (1, 2, 4, 6, and 9) (80). It is tempting to speculate that a contributing factor to the ability of this vaccine to induce immune responses in these very young infants may be the engagement of multiple TLR.
Although TLR agonists hold great promise as adjuvants, other approaches are under active investigation. Given the remarkable success of live attenuated viruses as vaccines, much effort has focused on the production of viral constructs that can achieve similar levels of immune stimulation while obviating the safety concerns associated with replicating virus. One promising area is the generation of single cycle virus constructs. These constructs enter cells and produce significant amounts of viral RNA as well as protein (81). This results in effective antigen presentation as well as DC maturation and inflammatory cytokine production (82). While not yet tested, it seems likely that a contributor to the efficacy of single cycle vaccines is the induction of innate immune responses similar to those resulting from virus infection, i.e. activation of endosomal TLR (TLR3, TLR7 and TLR8) and RLR (e.g. RIG-I or MDA-5). However, as these constructs are incapable or making infectious virus, and as such do not result in spread of virus beyond the initially infected cell, they have significantly reduced safety concerns.
Vaccines generated using single cycle virus constructs have shown utility in mice and nonhuman primates, eliciting both cell mediated and antibody responses (82–86). In addition they show promise in the context of the neonate. Infant mice vaccinated with a single cycle HSV-1 variant within 24 hours of birth had dramatically improved CD4+ and CD8+ T cell responses and were protected from virus challenge (87). In addition, infant rhesus macaques vaccinated with chimeric Venezuelan equine encephalitis/Sindbis virus replicon particles generated neutralizing antibody and were protected from disease following virus exposure even at 1 year post vaccination (88). Protection from virus challenge at this time is consistent with the generation of long lasting memory responses in the infants, a goal that has proven challenging in the setting of the neonate. Despite their success in experimental models, from a practical standpoint there may be hurdles to the use of these adjuvants given public wariness. This presents a challenge for the real-world application of these approaches.
Experimental challenges to forward movement in development of vaccines that are effective in neonates
One of the significant challenges associated with a more complete understanding of the defects in the neonatal immune response and the development of vaccines is the limitations associated with current experimental models. Neonatal mice, while extremely tractable, have a highly abbreviated period of infancy, making assessment of responses following prime-boost strategies difficult. A limitation to their use in the development of TLR agonists as adjuvants is the differential distribution and function of these molecules in mice versus humans. For example, in contrast to humans, murine T cells do not respond to TLR5 agonists (89). Further, mice do not have a functional TLR10 (90), while expressing TLR for which functional human equivalents appear to be lacking. The distribution of TLR among DC subsets and B cells also differs between mice and humans (91, 92).
With regard to T cell differentiation, there is evidence that mice may overestimate the Th2 bias of the neonate. Specifically, the strong Th2 bias apparent in neonatal mice following vaccination with acellular pertussis is not replicated in human infants where a more balanced Th1/Th2 response is observed (93).
Human studies are also challenging as a result of the understandable difficulty in obtaining cells from neonates and restriction to in vitro analyses. Cells derived from cord blood have been used as a surrogate, but responses in these cells may not reflect the newborn past the first few days of birth as their function is likely modulated by transient changes associated with delivery, e.g. the increase in adenosine (10). New models, e.g. nonhuman primates, could markedly benefit this area of research as innate sensor structure and function more closely resemble that of humans. In addition the more extended period of infancy allows assessment of prime-boost regimens.
Conclusions
While there is still much to learn with regard to the infant immune system, there is evidence that under the right circumstances, the neonate can make a strong and effective response to challenge. At its core, the goal of a vaccine is to reproduce the quantity and quality of the immune response that is generated following pathogen clearance. As our understanding of the receptors and pathways involved in pathogen recognition and immune activation continues to expand, we will undoubtedly gain an appreciation for new targets and strategies that can be exploited to generate vaccines capable of providing protection in the highly vulnerable neonate population.
ACKNOWLEDGEMENTS
The author thanks Dr. Karen Haas for helpful comments regarding this manuscript.
Footnotes
This work was supported by NIH grant R01AI098339 (to M.A.A.-M.)
LITERATURE CITED
- 1.Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair H, Simoes EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JS, Feikin DR, Mackenzie GA, Moisi JC, Roca A, Baggett HC, Zaman SM, Singleton RJ, Lucero MG, Chandran A, Gentile A, Cohen C, Krishnan A, Bhutta ZA, Arguedas A, Clara AW, Andrade AL, Ope M, Ruvinsky RO, Hortal M, McCracken JP, Madhi SA, Bruce N, Qazi SA, Morris SS, El Arifeen S, Weber MW, Scott JA, Brooks WA, Breiman RF, Campbell H G. Severe Acute Lower Respiratory Infections Working. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381:1380–1390. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marr N, Wang TI, Kam SH, Hu YS, Sharma AA, Lam A, Markowski J, Solimano A, Lavoie PM, Turvey SE. Attenuation of respiratory syncytial virus-induced and RIG-I-dependent type I IFN responses in human neonates and very young children. J. Immunol. 2014;192:948–957. doi: 10.4049/jimmunol.1302007. [DOI] [PubMed] [Google Scholar]
- 4.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 5.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghazal P, Dickinson P, Smith CL. Early life response to infection. Curr. Opin. Infect. Dis. 2013;26:213–218. doi: 10.1097/QCO.0b013e32835fb8bf. [DOI] [PubMed] [Google Scholar]
- 7.Cuenca AG, Wynn JL, Moldawer LL, Levy O. Role of innate immunity in neonatal infection. Am. J. Perinatol. 2013;30:105–112. doi: 10.1055/s-0032-1333412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roux X, Remot A, Petit-Camurdan A, Nahori MA, Kiefer-Biasizzo H, Marchal G, Lagranderie M, Riffault S. Neonatal lung immune responses show a shift of cytokines and transcription factors toward Th2 and a deficit in conventional and plasmacytoid dendritic cells. Eur. J. Immunol. 2011;41:2852–2861. doi: 10.1002/eji.201041224. [DOI] [PubMed] [Google Scholar]
- 9.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur. J. Immunol. 2009;39:26–35. doi: 10.1002/eji.200838391. [DOI] [PubMed] [Google Scholar]
- 10.Philbin VJ, Levy O. Developmental biology of the innate immune response: implications for neonatal and infant vaccine development. Pediatr. Res. 2009;65:98R–105R. doi: 10.1203/PDR.0b013e31819f195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, III, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal innate TLR-mediated responses are distinct from those of adults. J. Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philbin VJ, Dowling DJ, Gallington LC, Cortes G, Tan Z, Suter EE, Chi KW, Shuckett A, Stoler-Barak L, Tomai M, Miller RL, Mansfield K, Levy O. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J. Allergy Clin. Immunol. 2012;130:195–204 e199. doi: 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Wit D, Tonon S, Olislagers V, Goriely S, Boutriaux M, Goldman M, Willems F. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J. Autoimmun. 2003;21:277–281. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Harris DT, Schumacher MJ, Locascio J, Besencon FJ, Olson GB, DeLuca D, Shenker L, Bard J, Boyse EA. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10006–10010. doi: 10.1073/pnas.89.21.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Cohen AC, Lewis DB. Impaired allogeneic activation and T-helper 1 differentiation of human cord blood naive CD4 T cells. Biol. Blood Marrow Transplant. 2006;12:160–171. doi: 10.1016/j.bbmt.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Nonoyama S, Penix LA, Edwards CP, Lewis DB, Ito S, Aruffo A, Wilson CB, Ochs HD. Diminished expression of CD40 ligand by activated neonatal T cells. J. Clin. Invest. 1995;95:66–75. doi: 10.1172/JCI117677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miscia S, Di Baldassarre A, Sabatino G, Bonvini E, Rana RA, Vitale M, Di VV, Manzoli FA. Inefficient phospholipase C activation and reduced Lck expression characterize the signaling defect of umbilical cord T lymphocytes. J. Immunol. 1999;163:2416–2424. [PubMed] [Google Scholar]
- 18.Palin AC, Ramachandran V, Acharya S, Lewis DB. Human neonatal naive CD4+ T cells have enhanced activation-dependent signaling regulated by the microRNA miR-181a. J. Immunol. 2013;190:2682–2691. doi: 10.4049/jimmunol.1202534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans IA, Jones CA. HSV induces an early primary Th1 CD4 T cell response in neonatal mice, but reduced CTL activity at the time of the peak adult response. Eur. J. Immunol. 2005;35:1454–1462. doi: 10.1002/eji.200425333. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez MA, Evans IA, Hassan EH, Carbone FR, Jones CA. Neonatal CD8+ T cells are slow to develop into lytic effectors after HSV infection in vivo. Eur. J. Immunol. 2008;38:102–113. doi: 10.1002/eji.200636945. [DOI] [PubMed] [Google Scholar]
- 21.Crowe JE, Jr, Williams JV. Immunology of viral respiratory tract infection in infancy. Paediatr. Respir. Rev. 2003;4:112–119. doi: 10.1016/s1526-0542(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 22.Randolph DA. The neonatal adaptive immune system. Neo Reviews. 2005;6:e454–e462. [Google Scholar]
- 23.Brandenburg AH, Groen J, van Steensel-Moll HA, Claas EC, Rothbarth PH, Neijens HJ, Osterhaus AD. Respiratory syncytial virus specific serum antibodies in infants under six months of age: limited serological response upon infection. J. Med. Virol. 1997;52:97–104. doi: 10.1002/(sici)1096-9071(199705)52:1<97::aid-jmv16>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 24.Tregoning JS, Wang BL, McDonald JU, Yamaguchi Y, Harker JA, Goritzka M, Johansson C, Bukreyev A, Collins PL, Openshaw PJ. Neonatal antibody responses are attenuated by interferon-gamma produced by NK and T cells during RSV infection. Proc. Natl. Acad. Sci. USA. 2013;110:5576–5581. doi: 10.1073/pnas.1214247110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debock I, Jaworski K, Chadlaoui H, Delbauve S, Passon N, Twyffels L, Leo O, Flamand V. Neonatal follicular Th cell responses are impaired and modulated by IL-4. J. Immunol. 2013;191:1231–1239. doi: 10.4049/jimmunol.1203288. [DOI] [PubMed] [Google Scholar]
- 26.Pihlgren M, Tougne C, Bozzotti P, Fulurija A, Duchosal MA, Lambert PH, Siegrist CA. Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. J. Immunol. 2003;170:2824–2832. doi: 10.4049/jimmunol.170.6.2824. [DOI] [PubMed] [Google Scholar]
- 27.Siegrist CA. The challenges of vaccine responses in early life: selected examples. J. Comp. Pathol. 2007;137(Suppl 1):S4–S9. doi: 10.1016/j.jcpa.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Kaur K, Chowdhury S, Greenspan NS, Schreiber JR. Decreased expression of tumor necrosis factor family receptors involved in humoral immune responses in preterm neonates. Blood. 2007;110:2948–2954. doi: 10.1182/blood-2007-01-069245. [DOI] [PubMed] [Google Scholar]
- 29.Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, Mackay F. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 2013;24:203–215. doi: 10.1016/j.cytogfr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol. Rev. 2011;244:115–133. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat AF, Bossen C, Schneider P, Huard B, Lambert PH, Siegrist CA. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111:2755–2764. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- 32.Wolf AI, Mozdzanowska K, Quinn WJ, 3rd, Metzgar M, Williams KL, Caton AJ, Meffre E, Bram RJ, Erickson LD, Allman D, Cancro MP, Erikson J. Protective antiviral antibody responses in a mouse model of influenza virus infection require TACI. J. Clin. Invest. 2011;121:3954–3964. doi: 10.1172/JCI57362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halista SM, Johnson-Robbins LA, El-Mohandes AE, Lees A, Mond JJ, Katona IM. Characterization of early activation events in cord blood B cells after stimulation with T cell-independent activators. Pediatr. Res. 1998;43:496–503. doi: 10.1203/00006450-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Groothuis JR, Levin MJ, Rabalais GP, Meiklejohn G, Lauer BA. Immunization of high-risk infants younger than 18 months of age with split-product influenza vaccine. Pediatrics. 1991;87:823–828. [PubMed] [Google Scholar]
- 35.Halasa NB, Gerber MA, Chen Q, Wright PF, Edwards KM. Safety and immunogenicity of trivalent inactivated influenza vaccine in infants. J Infect. Dis. 2008;197:1448–1454. doi: 10.1086/587643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neuzil KM, Dupont WD, Wright PF, Edwards KM. Efficacy of inactivated and cold-adapted vaccines against influenza A infection 1985 to 1990: the pediatric experience. Pediatr. Infect. Dis. J. 2001;20:733–740. doi: 10.1097/00006454-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Gans H, DeHovitz R, Forghani B, Beeler J, Maldonado Y, Arvin AM. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine. 2003;21:3398–3405. doi: 10.1016/s0264-410x(03)00341-4. [DOI] [PubMed] [Google Scholar]
- 38.Vekemans J, Ota MO, Wang EC, Kidd M, Borysiewicz LK, Whittle H, McAdam KP, Morgan G, Marchant A. T cell responses to vaccines in infants: defective IFNgamma production after oral polio vaccination. Clin. Exp. Immunol. 2002;127:495–498. doi: 10.1046/j.1365-2249.2002.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vekemans J, Amedei A, Ota MO, D'Elios MM, Goetghebuer T, Ismaili J, Newport MJ, Del Prete G, Goldman M, McAdam KP, Marchant A. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur. J. Immunol. 2001;31:1531–1535. doi: 10.1002/1521-4141(200105)31:5<1531::AID-IMMU1531>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 40.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 41.Richert LE, Harmsen AL, Rynda-Apple A, Wiley JA, Servid AE, Douglas T, Harmsen AG. Inducible bronchus-associated lymphoid tissue (iBALT) synergizes with local lymph nodes during antiviral CD4+ T cell responses. Lymphat. Res. Biol. 2013;11:196–202. doi: 10.1089/lrb.2013.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norton EB, Clements JD, Voss TG, Cardenas-Freytag L. Prophylactic administration of bacterially derived immunomodulators improves the outcome of influenza virus infection in a murine model. J. Virol. 2010;84:2983–2995. doi: 10.1128/JVI.01805-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, Kolls JK, Khader SA, Randall TD. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat. Immunol. 2011;12:639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 45.Slutter B, Pewe LL, Kaech SM, Harty JT. Lung airway-surveilling CXCR3hi memory CD8+ T cells are critical for protection against influenza A virus. Immunity. 2013;39:939–948. doi: 10.1016/j.immuni.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lines JL, Hoskins S, Hollifield M, Cauley LS, Garvy BA. The migration of T cells in response to influenza virus is altered in neonatal mice. J. Immunol. 2010;185:2980–2988. doi: 10.4049/jimmunol.0903075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang J, Braciale TJ. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat. Med. 2002;8:54–60. doi: 10.1038/nm0102-54. [DOI] [PubMed] [Google Scholar]
- 48.Vallbracht S, Unsold H, Ehl S. Functional impairment of cytotoxic T cells in the lung airways following respiratory virus infections. Eur. J. Immunol. 2006;36:1434–1442. doi: 10.1002/eji.200535642. [DOI] [PubMed] [Google Scholar]
- 49.Gray PM, Arimilli S, Palmer EM, Parks GD, Alexander-Miller MA. Altered function in CD8+ T cells following paramyxovirus infection of the respiratory tract. J. Virol. 2005;79:3339–3349. doi: 10.1128/JVI.79.6.3339-3349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Claassen EA, van der Kant PA, Rychnavska ZS, van Bleek GM, Easton AJ, van der Most RG. Activation and inactivation of antiviral CD8 T cell responses during murine pneumovirus infection. J. Immunol. 2005;175:6597–6604. doi: 10.4049/jimmunol.175.10.6597. [DOI] [PubMed] [Google Scholar]
- 51.Arimilli S, Palmer EM, Alexander-Miller MA. Loss of function in virus-specific lung effector T cells is independent of infection. J. Leukoc.Biol. 2008;83:564–574. doi: 10.1189/jlb.0407215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arimilli S, Sharma SK, Yammani R, Reid SD, Parks GD, Alexander-Miller MA. Pivotal Advance: Nonfunctional lung effectors exhibit decreased calcium mobilization associated with reduced expression of ORAI1. J. Leukoc. Biol. 2010 doi: 10.1189/jlb.0809575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kremer M, Suezer Y, Volz A, Frenz T, Majzoub M, Hanschmann KM, Lehmann MH, Kalinke U, Sutter G. Critical role of perforin-dependent CD8+ T cell immunity for rapid protective vaccination in a murine model for human smallpox. PLoS Pathog. 2012;8:e1002557. doi: 10.1371/journal.ppat.1002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He XS, Holmes TH, Mahmood K, Kemble GW, Dekker CL, Arvin AM, Greenberg HB. Phenotypic changes in influenza-specific CD8+ T cells after immunization of children and adults with influenza vaccines. J. Infect. Dis. 2008;197:803–811. doi: 10.1086/528804. [DOI] [PubMed] [Google Scholar]
- 55.Fukazawa Y, Park H, Cameron MJ, Lefebvre F, Lum R, Coombes N, Mahyari E, Hagen SI, Bae JY, Reyes MD, 3rd, Swanson T, Legasse AW, Sylwester A, Hansen SG, Smith AT, Stafova P, Shoemaker R, Li Y, Oswald K, Axthelm MK, McDermott A, Ferrari G, Montefiori DC, Edlefsen PT, Piatak M, Jr, Lifson JD, Sekaly RP, Picker LJ. Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat. Med. 2012;18:1673–1681. doi: 10.1038/nm.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat. Med. 2013;19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 57.Perez O, Romeu B, Cabrera O, Gonzalez E, Batista-Duharte A, Labrada A, Perez R, Reyes LM, Ramirez W, Sifontes S, Fernandez N, Lastre M. Adjuvants are key factors for the development of future vaccines: lessons from the finlay adjuvant platform. Front. Immunol. 2013;4:407. doi: 10.3389/fimmu.2013.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 59.Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16:141–147. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- 60.Mukherjee S, Lukacs NW. Innate immune responses to respiratory syncytial virus infection. Curr. Top. Microbiol. Immunol. 2013;372:139–154. doi: 10.1007/978-3-642-38919-1_7. [DOI] [PubMed] [Google Scholar]
- 61.Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014;14:315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caron G, Duluc D, Fremaux I, Jeannin P, David C, Gascan H, Delneste Y. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J. Immunol. 2005;175:1551–1557. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- 63.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102:956–963. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 64.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 65.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 66.Marques JT, Williams BR. Activation of the mammalian immune system by siRNAs. Nat. Biotechnol. 2005;23:1399–1405. doi: 10.1038/nbt1161. [DOI] [PubMed] [Google Scholar]
- 67.Mizel SB, Bates JT. Flagellin as an adjuvant: cellular mechanisms and potential. J. Immunol. 2010;185:5677–5682. doi: 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yan SR, Qing G, Byers DM, Stadnyk AW, Al Hertani W, Bortolussi R. Role of MyD88 in diminished tumor necrosis factor alpha production by newborn mononuclear cells in response to lipopolysaccharide. Infect. Immun. 2004;72:1223–1229. doi: 10.1128/IAI.72.3.1223-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J. Immunol. 2004;173:4627–4634. doi: 10.4049/jimmunol.173.7.4627. [DOI] [PubMed] [Google Scholar]
- 71.Dowling DJ, Tan Z, Prokopowicz ZM, Palmer CD, Matthews MA, Dietsch GN, Hershberg RM, Levy O. The ultra-potent and selective TLR8 agonist VTX-294 activates human newborn and adult leukocytes. PLoS One. 2013;8:e58164. doi: 10.1371/journal.pone.0058164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J. Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 73.McCarron M, Reen DJ. Activated human neonatal CD8+ T cells are subject to immunomodulation by direct TLR2 or TLR5 stimulation. J. Immunol. 2009;182:55–62. doi: 10.4049/jimmunol.182.1.55. [DOI] [PubMed] [Google Scholar]
- 74.Crespo M, Martinez DG, Cerissi A, Rivera-Reyes B, Bernstein HB, Lederman MM, Sieg SF, Luciano AA. Neonatal T-cell maturation and homing receptor responses to Toll-like receptor ligands differ from those of adult naive T cells: relationship to prematurity. Pediatr. Res. 2012;71:136–143. doi: 10.1038/pr.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weimer ET, Ervin SE, Wozniak DJ, Mizel SB. Immunization of young African green monkeys with OprF epitope 8-OprI-type A- and B-flagellin fusion proteins promotes the production of protective antibodies against nonmucoid Pseudomonas aeruginosa. Vaccine. 2009;27:6762–6769. doi: 10.1016/j.vaccine.2009.08.080. [DOI] [PubMed] [Google Scholar]
- 76.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–1290. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J. Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Warger T, Osterloh P, Rechtsteiner G, Fassbender M, Heib V, Schmid B, Schmitt E, Schild H, Radsak MP. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood. 2006;108:544–550. doi: 10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- 79.Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum. Immunol. 2007;68:813–822. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 80.Randhawa AK, Hawn TR. Toll-like receptors: their roles in bacterial recognition and respiratory infections. Expert Rev. Anti Infect. Ther. 2008;6:479–495. doi: 10.1586/14787210.6.4.479. [DOI] [PubMed] [Google Scholar]
- 81.Vander Veen RL, Harris DL, Kamrud KI. Alphavirus replicon vaccines. Anim. Health. Res. Rev. 2012;13:1–9. doi: 10.1017/S1466252312000011. [DOI] [PubMed] [Google Scholar]
- 82.Tonkin DR, Jorquera P, Todd T, Beard CW, Johnston RE, Barro M. Alphavirus replicon-based enhancement of mucosal and systemic immunity is linked to the innate response generated by primary immunization. Vaccine. 2010;28:3238–3246. doi: 10.1016/j.vaccine.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carroll TD, Matzinger SR, Barro M, Fritts L, McChesney MB, Miller CJ, Johnston RE. Alphavirus replicon-based adjuvants enhance the immunogenicity and effectiveness of Fluzone (R) in rhesus macaques. Vaccine. 2011;29:931–940. doi: 10.1016/j.vaccine.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herbert AS, Kuehne AI, Barth JF, Ortiz RA, Nichols DK, Zak SE, Stonier SW, Muhammad MA, Bakken RR, Prugar LI, Olinger GG, Groebner JL, Lee JS, Pratt WD, Custer M, Kamrud KI, Smith JF, Hart MK, Dye JM. Venezuelan equine encephalitis virus replicon particle vaccine protects nonhuman primates from intramuscular and aerosol challenge with ebolavirus. J. Virol. 2013;87:4952–4964. doi: 10.1128/JVI.03361-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caley IJ, Betts MR, Irlbeck DM, Davis NL, Swanstrom R, Frelinger JA, Johnston RE. Humoral, mucosal, and cellular immunity in response to a human immunodeficiency virus type 1 immunogen expressed by a Venezuelan equine encephalitis virus vaccine vector. J. Virol. 1997;71:3031–3038. doi: 10.1128/jvi.71.4.3031-3038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Widman DG, Ishikawa T, Giavedoni LD, Hodara VL, Garza Mde L, Montalbo JA, Travassos Da Rosa AP, Tesh RB, Patterson JL, Carrion R, Jr, Bourne N, Mason PW. Evaluation of RepliVAX WN, a single-cycle flavivirus vaccine, in a non-human primate model of West Nile virus infection. Am. J. Trop. Med. Hyg. 2010;82:1160–1167. doi: 10.4269/ajtmh.2010.09-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Franchini M, Abril C, Schwerdel C, Ruedl C, Ackermann M, Suter M. Protective T-cell-based immunity induced in neonatal mice by a single replicative cycle of herpes simplex virus. J. Virol. 2001;75:83–89. doi: 10.1128/JVI.75.1.83-89.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan CH, Greer CE, Hauer D, Legg HS, Lee EY, Bergen MJ, Lau B, Adams RJ, Polo JM, Griffin DE. A chimeric alphavirus replicon particle vaccine expressing the hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques from measles. J. Virol. 2010;84:3798–3807. doi: 10.1128/JVI.01566-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cottalorda A, Verschelde C, Marcais A, Tomkowiak M, Musette P, Uematsu S, Akira S, Marvel J, Bonnefoy-Berard N. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur. J. Immunol. 2006;36:1684–1693. doi: 10.1002/eji.200636181. [DOI] [PubMed] [Google Scholar]
- 90.Hasan U, Chaffois C, Gaillard C, Saulnier V, Merck E, Tancredi S, Guiet C, Briere F, Vlach J, Lebecque S, Trinchieri G, Bates EE. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J. Immunol. 2005;174:2942–2950. doi: 10.4049/jimmunol.174.5.2942. [DOI] [PubMed] [Google Scholar]
- 91.Kaisho T. Pathogen sensors and chemokine receptors in dendritic cell subsets. Vaccine. 2012;30:7652–7657. doi: 10.1016/j.vaccine.2012.10.043. [DOI] [PubMed] [Google Scholar]
- 92.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ryan M, Gothefors L, Storsaeter J, Mills KH. Bordetella pertussis-specific Th1/Th2 cells generated following respiratory infection or immunization with an acellular vaccine: comparison of the T cell cytokine profiles in infants and mice. Dev. Biol. Stand. 1997;89:297–305. [PubMed] [Google Scholar]