Abstract
Background.
Overweight and obesity have negative health effects. Primary care clinicians are best placed to intervene in weight management. Previous reviews of weight loss interventions have included studies from specialist settings. The aim of this review was to estimate the effect of behavioural interventions delivered in primary care on body weight in overweight and obese adults.
Methods.
The review included randomized controlled trials (RCTs) of behavioural interventions in obese or overweight adult participants in a primary care setting, with weight loss as the primary outcome, and a minimum of 12 months of follow-up. A systematic search strategy was implemented in Medline, Embase, Web of Science and the Cochrane Central Registry of Controlled Trials. Risk of bias was assessed using the Cochrane Risk of Bias tool and behavioural science components of interventions were evaluated. Data relating to weight loss in kilograms were extracted, and the results combined using meta-analysis.
Results.
Fifteen RCTs, with 4539 participants randomized, were selected for inclusion. The studies were heterogeneous with respect to inclusion criteria and type of intervention. Few studies reported interventions informed by behavioural science theory. Pooled results from meta-analysis indicated a mean weight loss of −1.36kg (−2.10 to −0.63, P < 0.0001) at 12 months, and −1.23kg (−2.28 to −0.18, P = 0.002) at 24 months.
Conclusion.
Behavioural weight loss interventions in primary care yield very small reductions in body weight, which are unlikely to be clinically significant. More effective management strategies are needed for the treatment of overweight and obesity.
Key words. General practice, obesity, overweight, primary health care.
Introduction
Obesity is associated with an increased risk of morbidity from diabetes, cancer and cardiovascular disease (1). Recent estimates suggest that 64% of women and 74% of men in the United States are overweight or obese (2). In the UK, 58% of women and 65% of men are overweight or obese (3). National recommendations identify primary care as central to the management and prevention of obesity in the general population. Primary care physicians (PCPs) are advised to screen all adults for obesity, and to spearhead individual and community-wide programmes to tackle overweight and obesity (4,5). However, some research suggests that PCPs may consider themselves ill-equipped to treat obese participants without additional training, such as nutrition counselling, and support from other health professionals (6).
Reviews of the effectiveness of behavioural interventions designed to reduce body weight and improve clinical outcomes have suggested positive results overall (7). Previous systematic reviews on primary care management of obesity have included studies conducted in specialist hospital or academic settings, with a view that they are directly transferrable to the primary care setting (8,9). Studies from specialist settings are likely to differ from those conducted within primary care in several important aspects. Firstly, the study population is unlikely to be representative of the general population due to higher rates of eating disorders, past experience of weight loss attempts and differences in motivation to lose weight (10). The interventions themselves are likely to be more intensive than those in primary care, with more time dedicated to ensuring protocol adherence and better follow-up rates. To illustrate this, one study included in a previous review (8) investigated a year-long programme for post-menopausal women. Participants were selected to maximize the effectiveness of the intervention by excluding smokers, those on hormone replacement therapy and those with diabetes or pre-diabetes. Recruitment took place via advertizing and participants had to undergo a telephone call and three baseline clinic visits to determine interest and eligibility before randomization, ensuring high motivation. The intervention itself consisted of exercise sessions with a physiologist three times per week for 3 months at a University facility, followed by 9 months of weekly sessions at the facility plus home-based activities (11). Inclusion of this, and similar studies, in previous reviews may have overstated the potential effect of weight loss interventions in routine practice in primary care. This review aimed to address this by applying a rigorous setting criterion.
In addition to differences in the definition of the primary care setting, we have identified a number of relevant studies that have been undertaken since the publication of previous reviews. Therefore, the aim of this study was to undertake a systematic review of randomized controlled trials (RCTs) of behavioural weight loss interventions for overweight and obese adults that were conducted within primary care. This was defined by participants being selected from the practice patient list, and the intervention being mainly delivered from within the practice. The outcome was weight change in kilograms (kg) and results were combined using meta-analysis.
Methods
Data sources and search
A literature search was implemented in the online databases Medline, Embase, Web of Science and the Cochrane Central Registry of Controlled Trials to search for English language papers from 1990 to the 17th September, 2012. The search was repeated on the 19th March 2014 and no new papers for inclusion were identified. The search terms are presented in the Supplementary material. Studies using drug treatment were included in the main search strategy as part of a larger project, but excluded at the full text stage from this review. Hand-searching of reference lists of relevant systematic reviews and included papers were used to search for further articles (5,7–9,12). Searching and selection of articles was conducted by one researcher. Resource constraints meant that grey literature, unpublished works and articles not in English were not considered for inclusion.
Study selection
Interventions promoting behaviour change for weight loss were eligible for inclusion in the review. Titles and abstracts of all retrieved articles were evaluated using pre-defined criteria and the full text was consulted where study eligibility was unclear. The following inclusion criteria were used to select studies from their titles and abstracts: participants were overweight and/or obese adults in primary care, weight loss was a primary outcome and an RCT design was used. Interventions did not have to be delivered by a PCP, but participants had to be selected from their practice patient list and the intervention conducted within the primary care setting. This is reflective of the multidisciplinary teams that now deliver primary care services. Follow-up was for a minimum of 12 months.
Data extraction and quality assessment
Participant and intervention characteristics from the included studies were tabulated. Information defined a priori included sample sizes, baseline characteristics, inclusion criteria, intervention details, study duration and number of contacts. Outcome data for weight loss were extracted into a pre-prepared form by one reviewer and checked by an additional reviewer. Study quality was assessed using the Cochrane Risk of Bias tool (13), which assesses evidence of selection, performance, attrition, detection and reporting biases by testing six key domains plus an additional option for any other bias. The seven domains are judged to be at high or low risk of bias, or unclear due to lack of information. Publication bias or other asymmetry in the published results due to quality issues was assessed using funnel plots (14). Data relating to behavioural science theory underpinning the interventions and techniques used in the interventions for behaviour change were also extracted and assessed using published tools (15,16).
Data synthesis and analysis
The primary outcome was the difference in mean (MD) weight change between the control and intervention groups, measured in kilograms (kg). Variation in the results was considered using the standard error of the difference in means (SEMD). SEMD was calculated from the standard deviation or standard error of change in the two arms, or from P or t values where applicable. If there was inadequate information to calculate SEMD, the pooled standard deviation from the complete studies was used to impute SEMD as part of a sensitivity analysis. The results at 12 months and 24 months post-baseline were combined in separate random effects meta-analyses. Random effects meta-analysis is appropriate if study heterogeneity may be present as it allows an assumption that individual studies are not estimating a fixed treatment effect, but one that is variable (17).
Where data were reported separately for sub-groups, for example men and women, the groups were combined for the meta-analysis, and if trials were three-armed only the higher intensity arm was included, for example intervention delivered face-to-face rather than by telephone. This avoided combining the two intervention arms, thus potentially showing an attenuated treatment effect, or including the control arm in the meta-analysis twice (13). Cluster randomized trials were included in the review, but the methods used to account for the cluster design in their statistical analysis were assessed prior to their inclusion in the meta-analyses. Analysing cluster trials as though they are individually randomized can lead to unsuitably small P values and narrow confidence intervals (18).
Results
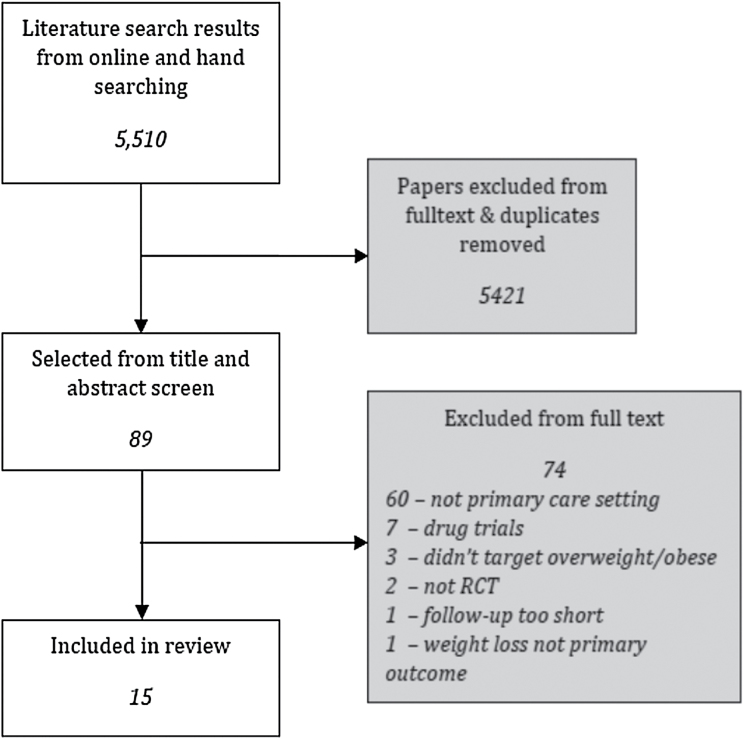
The initial database search identified 5449 unique studies, from which 28 were selected for inclusion in the review. A further 61 papers were identified from the reference lists of relevant systematic reviews, papers and clinical guidelines. On examination of the full text, 15 papers were eligible for inclusion in the review (see Figure 1). Some papers met more than one criterion for exclusion, but only one is reported. The majority of exclusions at this stage were made for studies not being conducted in a primary care setting.
Figure 1.
Flow diagram of study selection
Participant and study characteristics
Participant characteristics are outlined in Table 1 and intervention characteristics in Table 2. A total of 4539 participants took part in the studies, with 13 recruiting men and women, and 2 women only (27,31). The mean age of participants in the studies at baseline ranged from 41.8 to 60 years. Ten studies included overweight and obese participants (21–28,31,32) and five obese participants only (19,20,29,30,33). Ten studies recruited participants with specified co-morbidities or risk factors. Two of these were conducted in participants with type 2 diabetes (21,28) and three in participants with hypertension (20,22,23). One study recruited participants with at least one of hypertension, hypercholesterolaemia or diabetes (19), and one study recruited participants with two or more components of the metabolic syndrome (33).
Table 1.
Participant characteristics
| Study | Country | Participants randomized (% female) | Eligible age range (mean) | Eligible weight range | Mean BMI (kg/m 2 ) (and weight (kg) at baseline) | Participant inclusion criteria | Participants completed (%) |
|---|---|---|---|---|---|---|---|
| Appel et al. (19) | United States | 415 (63.6%) | Aged ≥21 (54.0) | Obese | 36.6 103.4 |
≥1 of: hypertension, hypercholesterolaemia, diabetes | 355 (86%) |
| Bennett et al. (20) | United States | 365 (68.5%) | Aged ≥21 (54.5) | BMI 30–50kg/m2 but weight <180 kg | 37.0 100.1 |
On anti-hypertensive medication and ≥1 medical visit in 12 months prior to study, fluent in Spanish and English | 283 (76%) |
| Christian et al. (21) | United States | 310 (66.5%) | Aged 18–75 (53.2) | BMI of ≥25kg/m2 | 35.1 92.4 |
Latino/Hispanic ethnicity, diabetes type 2 and uninsured or eligible for/ with Medicaid | 273 (88%) |
| Cohen et al. (22) | United States | 30 (73.3%) | Aged 20–75 (59.5) | BMI > 27.8kg/m2 in males or 27.3kg/m2 in females | 34.1 91.8 |
Hypertensive | 30 (100%) |
| Jalkanen (23) | Finland | 50 (not clear) | Aged 35–39(49) | BMI of 27–34 kg/m2 | Not given 82.9 |
Diastolic BP ≥ 95 mmHg | 49 (98%) |
| Karvetti and Hakala (24) | Finland | 243 (not clear) | Aged 17–65 (48.5) | BMI of ≥27 kg/m2 | Not clear 92.6 |
189 (78%) | |
| Kumanyika et al. (25) | United States | 261 (84%) | Aged 18–70(47) | BMI 27–55kg/ m2 but weight <182 kg | 37.2 101.2 |
Patient at practice for ≥ 1 year or seen at the practice ≥2 times | 187 (72%) |
| Logue et al. (26) | United States | 665 (not clear) | Aged 40–69 (not clear) | BMI of ≥27kg/m2 or an elevated waist-hip ratio | Not clear | 579 (87%) | |
| Martin et al. (27) | United States | 144 (100%) | Aged 18–65 (41.8) | BMI of ≥25 kg/m2 | 38.9 102.0 |
Low income (<$16,000 pa), attendee at clinic for >1 year | 109 (76%) |
| Mayer-Davis et al. (28) | United States | 187 (not clear) | Aged ≥45 (60) | BMI of ≥25 kg/m2 | 36.7 97.3 |
Diagnosis of diabetes | 152 (81%) |
| Moore et al. (29) | United Kingdom | 843 (74%) | Aged 16–64 (48.6) | BMI of ≥30 kg/m2 | 36.9 100.5 |
565 (67%) | |
| Munsch et al. (30) | Switzerland | 70 (74%) | Not stated, (48.4) | BMI of ≥30 kg/m2 | 36.8 94.8 |
49 (70%) | |
| Rapoport et al. (31) | United Kingdom | 76 (100%) | Aged 18–65 (46.9) | BMI of ≥28 kg/m2 | 35.3 94.4 |
Not involved in any other method of weight management | 58 (76%) |
| Ross et al. (32) | United States | 490 (70%) | Adults (52.8) | BMI 27–39 kg/m2 and abdominal obesity | 32.3 90.2 |
396 (81%) at 24 months | |
| Wadden et al. (33) | United States | 390 (79.7%) | Aged ≥21 (51.6) | BMI of 30–50 kg/m2 | 38.5 107.7 |
≥2 of metabolic syndrome components | 12 months: 332 (85%) 24 months: 336 (86%) |
Table 2.
Study characteristics
| Study and design | Delivery method and focus of the intervention | Setting | Intervention delivered by (number of contacts) | Length of intervention | Theory-base and number of behavioural techniques | Analysis type | Mean difference in weight |
|---|---|---|---|---|---|---|---|
| Appel et al. (19), RCT—3 arms | Face-to-face (group and individual), telephone and online. Diet and exercise | 6 primary care practices | Project staff and private health coaches (57 in-person plus phone/email) | 24 months | Social cognitive theory (11) | ITT from baseline (exc protocol- defined censoring events). Indicators for missing data | 12 months: −4.3kg 24 months: −4.3 kg |
| Bennett et al. (20), RCT | Telephone and optional face-to- face (group). Diet, exercise, lifestyle and hypertension management | 3 community health centres | Community health educators (18 phone, 12 optional group) | 24 months | None specified (10) | ITT from baseline. 1 ppt censored for bariatric surgery. Missing data treated as MAR | 12 months: −1.05kg 24 months: −1.03 kg |
| Christian et al. (21), RCT | Face-to-face (individual). Diet and exercise | 2 community health centres | Physician (4 visits) | 12 months | None specified (4) | ITT from baseline using last-record- carried-forward | 12 months: −0.68 kg |
| Cohen et al. (22), Cluster RCT | Face-to-face (individual). Diet and hypertension management | 1 family health centre | Physician (12 visits) | 12 months | None specified (4) | Not clear | 12 months: −2.18 kg |
| Jalkanen (23), RCT | Face-to-face (group). Diet and exercise | 2 primary hypertension clinics | Nurses (31 sessions) | 12 months | None specified (0) | Completers only analysis | 12 months: −5.0 kg |
| Karvetti and Hakala (24), RCT | Face-to-face (group). Diet | Health centres | Public health nurses (16 sessions) | 12 months | None specified (0) | Completers only analysis | 12 months: −7.05 kg |
| Kumanyika et al. (25), RCT | Face-to-face (group and individual). Diet and exercise | 5 primary care centres | PCPs (4 visits) and lifestyle coach (practice staff) (12 visits) | 12 months | None specified (5) | ITT as randomized. Assumed missing data was MAR | 12 months: −0.99 kg |
| Logue et al. (26), RCT | Face-to-face (individual) and telephone. Diet and exercise | 15 primary care centres | Dietician, weight loss advisors (4 visits) | 24 months | Trans-theoretical model (2) | ITT as randomized. Used MAR assumption | 12 months: −0.23kg 24 months: −0.23 kg |
| Martin et al. (27), Cluster RCT | Face-to-face (individual). Diet and exercise | 2 primary care clinics | Physician (6 visits) | 6 months | None specified (4) | ITT carrying forward baseline values | 12 months: −1.22 kg |
| Mayer-Davis et al. (28), RCT—3 arm | Face-to-face (group and individual). Diet and exercise | 2 primary care clinics | Nutritionist (26 group/individual) | 12 months | None specified (4) | Completers | 12 months: −2.0 kg |
| Moore et al. (29), Cluster RCT | Face-to-face (individual). Diet and exercise | 44 general practices | Physician (unclear, seen every 2 weeks then every 1-2 m once weight lost) | Variable up to 1 year | None specified (5) | ITT where possible | Unclear |
| Munsch et al. (30), RCT | Face-to-face (group). Diet and exercise | 14 general practices and 1 clinical centre (not included) | Physician, dietician and psychologist (16 sessions) | Not clear | None specified (5) | Unclear, probably completers only | 12 months: −4.3 kg |
| Rapoport et al. (31), RCT | Face-to-face (group). Diet and exercise | General practices and local health centres | Dietician and health psychologist (10 sessions) | 10 weeks | “Used basic behavioural and cognitive principles, but also included elements from psycho- educational, non-dieting and feminist approaches (9)” | Completers only analysis | 12 months: −1.6 kg |
| Ross et al. (32), RCT | Face-to-face (individual). Diet and exercise | 3 family medicine clinics | Physician (control group), health educator (intervention) (33 sessions) | 24 months | Trans-theoretical and social cognitive models (10) | ITT as randomized | 24 months: −0.58 kg |
| Wadden et al. (33), RCT—3 arm | Face-to-face (individual) and telephone. Diet and exercise | 6 primary care practices | Physician (8 visits) and lifestyle coach (26 visits—6 by phone if preferred) | 24 months | Social cognitive and behavioural self-management theories (8) | ITT from baseline | 12 months: −1.1kg 24 months: −1.2 kg |
Three of the studies were cluster-randomized, and one had a cluster element (a third arm conducted in a hospital clinic), but results from this arm were not included in the review (30). Two of the cluster trials reported accounting for clustering in their analysis (27,29), the paper that did not account for clustering was not included in the meta-analysis (22). Three 3-armed trials were included in the review. Only the higher intensity arm was considered in two (19,28), and in the third the arm that incorporated weight loss drugs was excluded to leave just the behavioural arm (33). The interventions focused on lifestyle change through diet and physical activity using behavioural methods.
Whilst all of the studies reported outcomes to at least 12 months the interventions had different durations. Seven were 12 months long (21–25,28,29), five lasted for 24 months (19,20,26,32,33), one for 6 months (27) and another 10 weeks (31). One study consisted of 16 sessions, but the time period over which these were delivered was not specified (30). In one paper, the intervention and control groups were switched for the purpose of the review; this paper was investigating modified cognitive behavioural therapy (CBT) for weight maintenance compared to standard CBT for weight loss (31).
Behavioural science components of the interventions
In four of the studies, the intervention was delivered solely by the PCP (21,22,27,29), in one it was delivered by the PCP and other practice staff (33), and in another two by the PCP and other health professionals, for example weight loss advisors, health psychologists (25,30). In the remaining studies the intervention was delivered by other health professionals including public health nurses, psychologists, health educators, nutritionists and the study staff.
Five studies stated a behavioural theory base for the intervention. Three studies used social cognitive theory; one as the sole theory (19), one alongside the transtheoretical model (32), and another with ‘behavioural self-management theories (33)’. Another paper based its intervention on the transtheoretical model (26), and one reported using cognitive approaches with elements from other fields including feminist theory (31). Detailed descriptions of the behavioural components of the interventions were limited in the published papers, possibly by specified word count, and protocols providing fuller descriptions were available online for two papers (19,33).
Thirteen of the papers used between two and eleven techniques from the published checklist to change behaviour (19–22, 25–33), and two did not report using any techniques (23,24). The most commonly reported technique was ‘prompt self-monitoring of behaviour’, which was used in nine studies. This was followed by behavioural goal setting and barrier identification/problem solving, both used in eight papers. Papers which reported using behavioural theory included, on average, double the number of behavioural techniques than papers that reported no theory (mean number of techniques 8 vs. 4.1). Four papers made links between the techniques used and the theoretical constructs underlying them that are understood to influence behaviour (19,30,32,33); three of these reported their theory-base. Further details are presented in Table 2.
Quality and risk of bias
Assessment of study quality using the Cochrane risk of bias tool found that many of the studies did not report their methodology clearly enough for the risk of bias under each heading to be established. It was generally only possible to assess the risk of bias for two or three out of a possible six pre-defined areas assessed by the tool. Only one study gave sufficient information for allocation concealment to be assessed (21), and three for whether outcomes had been selectively reported (19,29,33). Adequacy of blinding was found to cause a high risk of bias in the five papers that provided relevant information (20,21,26,29,32). The nature of the interventions meant it was not possible to blind interventionists or participants, therefore differential treatment or performance in the intervention and control groups may have occurred. The method of random sequence generation and blinding measures were reported most often. No studies that met the inclusion criteria were excluded from the review on the basis of poor quality or poor reporting. Further information on risk of bias in the studies is provided in Supplementary Table S1.
Effects of the intervention
Weight change at 12 months
All of the studies reported change in weight at 12 months in the control and intervention groups, but not all of these could be included in a meta-analysis. The cluster trial which had not accounted for clustering in its analysis was excluded (22), and a second cluster trial reported mean baseline and follow-up weights, but these values couldn’t be used to calculate weight loss due to a drop-out rate of almost 40% (29). One additional paper reported an MD of −1.56kg, but no sample size at 12 months (32).
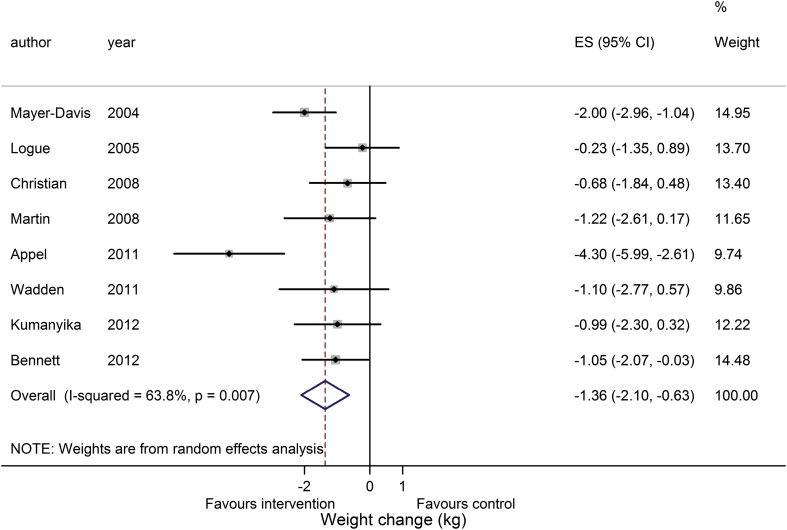
In four of the remaining studies it was not possible to calculate the SEMD (23,24,30,31). The meta-analysis was conducted without these studies, and a sensitivity analysis performed with the pooled standard deviation from complete studies used to impute missing SEMD. The studies with missing SEMD had large effect sizes, poor methodological reporting according to the Cochrane tool and used completer’s only analysis. These factors, considered alongside the incomplete reporting of outcomes, raised concerns over their quality. The meta-analysis of weight change at 12 months in eight studies is presented in Figure 2. The pooled estimate from these studies was −1.36kg (−2.10 to −0.63; P < 0.0001) favouring the intervention over the control. The I 2 value shows a good deal of heterogeneity between the studies. A funnel plot was not produced as the number of studies was insufficient based on Cochrane recommendations (13). If SEMD was imputed for the four studies with missing values the result would be −2.29kg (−3.44 to −1.14; P < 0.0001).
Figure 2.
Meta-analysis of weight loss at 12 months
Weight change at 24 months
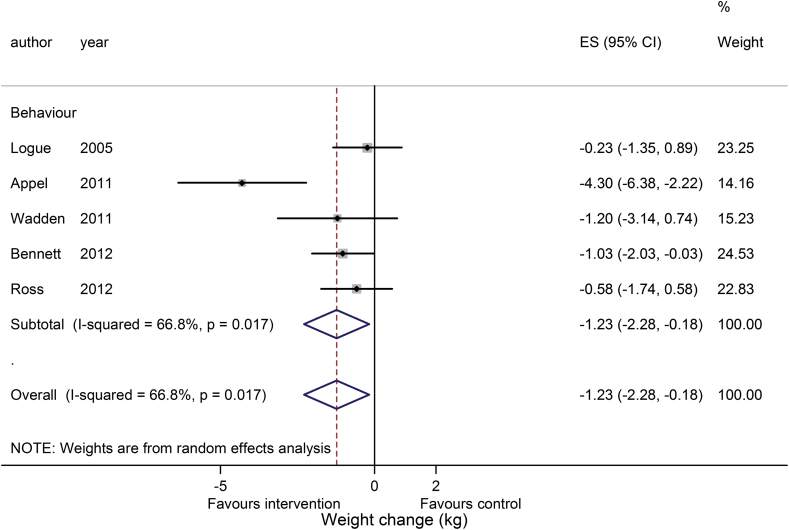
Five lifestyle studies reported weight change at 24 months, all of which provided complete data and were included in a meta-analysis. The pooled result, shown in Figure 3, was −1.23kg (−2.28 to −0.18; P = 0.002). The paper by Appel et al. (19) showed the greatest body weight loss, having also shown the highest weight reduction among papers included in the meta-analysis at 12 months. Study heterogeneity was again found to be high and no funnel plot was produced due to the small number of studies (14).
Figure 3.
Meta-analysis of weight loss at 24 months
Of the four studies that presented results at both 12 and 24 months, two found the same mean change from baseline at both time points (19,26), one showed a small decrease in weight lost (20), and the other a small increase (33). One of these studies had the aim of encouraging weight loss in the first six months of the trial, then maintaining body weight (19).
Discussion
The results of this review suggest that behavioural interventions conducted in primary care have a negligible effect on participants’ weight at 12 and 24 months. Weight loss interventions are generally considered to be clinically significant if participants lose ≥5% of their baseline total body weight (34). Based on the range of mean baseline body weights observed in this review (82.9–107.7kg) the pooled estimates did not reach this threshold. Whilst the key clinical finding from this review relates to the limited effectiveness of primary care behavioural interventions at achieving weight loss in obese participants, the results also highlight a number of questions of importance for research. These are outlined below in the ‘implications for future research and practice’ section.
The study that reported the highest number of behaviour change techniques and was based on social cognitive theory showed the greatest weight losses at both 12 and 24 months (19). However, other studies in the review reported similar use of evidence from theory with a lesser effect (20,31–33). This study also had the highest number of participant–interventionist contacts which may have enabled stronger reinforcement of the behavioural components, and increased participant motivation to lose weight.
Strengths and limitations
A key inclusion criterion for this review was the primary care setting. The majority of weight loss trials are conducted in university-based hospital clinics and demonstrate efficacy over effectiveness of interventions (35). These studies are likely to have more restrictive inclusion criteria and consequently poorer external validity than studies conducted in routine clinical settings (36). The studies in the present review only excluded participants with serious mental and physical conditions that would prevent participants from complying with the study routines safely, pregnancy and related conditions and use of other weight-related treatments. Just one study excluded diabetic participants (24). In addition to participant differences, studies based in the primary care setting may have routines more akin to standard practice, including less rigorous follow-up and management by clinicians rather than investigators with specialist training. As a result of these differences, the findings of this review are likely to be more generalizable than those of other systematic reviews. Ethnicity of the participants was not reported in some of the studies, however, the majority were white or black. As a result, the findings may be less applicable to other ethnic minority groups. A large number of the included studies were identified by hand-searching rather than the electronic search. This indicates that the search terms could have benefited from further refinement, and study selection could have been undertaken by an additional reviewer.
The trial results were pooled using meta-analysis, but a high level of heterogeneity was observed. Seven of the 15 studies could not be included in the meta-analysis at 12 months due to poor reporting of results, including missing sample sizes or a lack of information to calculate SEMD. Study reporting was found to be of a poor standard overall, with no evidence of improvement over time, making assessment of the study quality, risk of bias and isolation of the techniques or components that may have been effective or not in the intervention difficult. This contributed to the poor performance of the studies in the Cochrane Risk of Bias tool. The potential for bias in the studies reduces the reliability of the results and this should be considered in their interpretation.
Comparison with other studies
We identified two systematic reviews that investigated behavioural weight loss interventions for primary care (8,9). Both reviews included trials of weight loss drugs and trials that were conducted in specialist settings, but that the review authors considered were transferrable to primary care, making the present review the only one to include studies solely conducted in primary care. Only a third of studies included in the present review were incorporated into the two earlier reviews due to differences in inclusion criteria and date of publication. Five studies included in this review were published after the search dates for the Tsai and LeBlanc reviews (25,19,20,32,33). Our results were comparable with those of both published reviews. The meta-analysis by LeBlanc found a more favorable result than the present review, of 3kg body weight loss at 12–18 months after behavioural intervention. This difference is likely to be a result of the more stringent way in which we applied the primary care setting criteria. The Tsai review used narrative analysis, and found heterogeneous results from studies using ‘collaborative’ care, where the provider was someone other than the PCP. Reported weight losses in such studies ranged from 0.4 to 7.7 kg. Results from commercial weight loss programmes have similarly been associated with weight losses that reach statistical significance, but are unlikely to be clinically relevant, with one systematic review identifying a maximum reduction of 3.2% in body weight (37–39). Weight loss drugs have been found to elicit 5% body weight losses, however, the most effective drug, sibutramine, has been removed from the market due to safety concerns (40).
A systematic review investigating the ‘active ingredients’ in obesity interventions found that an increasing number of behaviour change techniques was positively associated with weight loss (41). Techniques identified as being particularly useful for weight change were the provision of dietary instruction, self-monitoring of dietary behaviour and relapse prevention. The study in the present review that used the largest number of techniques, including those highlighted above, showed high weight loss (19). However, other studies also reporting these techniques showed less positive results, and some studies reporting high weight loss did not report using any techniques from the checklist.
Implications for future research and practice
Behavioural weight loss interventions conducted in primary care elicit very small reductions in body weight in overweight and obese participants’ which are unlikely to be clinically significant. Assessment of the behavioural science components of the interventions was inconclusive and hindered by poor study reporting. Stubbs et al. (10) have highlighted heterogeneity of participants, interventions, measurements and intervention constructs as a barrier to identifying predictors of weight loss. Standardization of these elements and detailed reporting should be a key area for improvement, as should long-term follow-up beyond the end of an intervention. Recent work by Kirk et al. (42) reviewing the evidence to identify key themes and practice points is valuable in terms of using the current literature to best effect.
The clinical impact of a review such as this could be extended by selecting a primary outcome other than weight loss. For instance, improvement in cardiovascular risk factors or changes in the incidence rate of weight-related morbidities such as type 2 diabetes are possible alternative endpoints. Recently, the publication of several studies investigating the use of commercial providers for weight loss have shown relatively successful results in the short-term and merit further evaluation (38,39,43). Additionally, disease prevention interventions should be considered, many of which have a weight loss component as an alternative. Reviews of diabetes or cardiovascular disease prevention studies have produced similarly mixed results to weight loss reviews (44–46). Comparing these bodies of work, along with those investigating weight loss drugs and surgery, will increase our understanding of the best strategies to reduce the burden of morbidity and mortality in overweight and obese populations.
Supplementary material
Supplementary material is available at Family Practice online.
Declaration
Funding: National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s, St Thomas’ NHS Foundation Trust and King’s College London, UK National Prevention Research Initiative (UKNPRI).
Ethical approval: none.
Conflicts of interest: none declared.
Supplementary Material
Acknowledgements
The authors would like to thank Mr Nawaraj Bhattarai, Dr Alex Dregan and Dr Alice Forster for their assistance in checking extracted data. This study is based in part on data from the Clinical Practice Research Datalink (CPRD) obtained under license from the UK Medicines and Healthcare products Regulatory Agency (UKMHRA).
References
- 1. Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009; 9: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012; 307: 491–7. [DOI] [PubMed] [Google Scholar]
- 3. Health and Social Care Information Centre. Statistics on Obesity, Physical Activity and Diet: England, 2013. London: National Statistics, 2013. [Google Scholar]
- 4. Moyer VA; U.S. Preventive Services Task Force. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 157: 373–8. [DOI] [PubMed] [Google Scholar]
- 5. National Institute for Health and Care Excellence. Obesity: Guidance on the Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: National Institute for Health and Care Excellence, 2006. [PubMed] [Google Scholar]
- 6. Bleich SN, Bennett WL, Gudzune KA, Cooper LA. National survey of US primary care physicians’ perspectives about causes of obesity and solutions to improve care. BMJ Open 2012; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Avenell A, Broom J, Brown TJ, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 2004; 8: iii–iv, 1–182. [DOI] [PubMed] [Google Scholar]
- 8. Leblanc ES, O’Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2011; 155: 434–47. [DOI] [PubMed] [Google Scholar]
- 9. Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med 2009; 24: 1073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stubbs J, Whybrow S, Teixeira P, et al. Problems in identifying predictors and correlates of weight loss and maintenance: implications for weight control therapies based on behaviour change. Obes Rev 2011; 12: 688–708. [DOI] [PubMed] [Google Scholar]
- 11. Irwin ML, Yasui Y, Ulrich CM, et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA 2003; 289: 323–30. [DOI] [PubMed] [Google Scholar]
- 12. Scottish Intercollegiate Guidelines Network. Management of Obesity: A National Clinical Guideline. Edinburgh: Scottish Intercollegiate Guidelines Network, 2010. [Google Scholar]
- 13. . Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2011. www.cochrane-handbook.org (accessed on 5 November 2012).
- 14. Sterne JAC, Harbord RM. Funnel plots in meta-analysis. STATA J 2004; 4: 127–41. [Google Scholar]
- 15. Michie S, Ashford S, Sniehotta FF, et al. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011; 26: 1479–98. [DOI] [PubMed] [Google Scholar]
- 16. Michie S, Prestwich A. Are interventions theory-based? Development of a theory coding scheme. Health Psychol 2010; 29: 1–8. [DOI] [PubMed] [Google Scholar]
- 17. Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998; 3: 486–504. [Google Scholar]
- 18. Campbell MK, Mollison J, Steen N, et al. Analysis of cluster randomized trials in primary care: a practical approach. Fam Pract 2000; 17: 192–6. [DOI] [PubMed] [Google Scholar]
- 19. Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med 2011; 365: 1959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bennett GG, Warner ET, Glasgow RE, et al. ; Be Fit, Be Well Study Investigators. Obesity treatment for socioeconomically disadvantaged patients in primary care practice. Arch Intern Med 2012; 172: 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christian JG, Bessesen DH, Byers TE, et al. Clinic-based support to help overweight patients with type 2 diabetes increase physical activity and lose weight. Arch Intern Med 2008; 168: 141–6. [DOI] [PubMed] [Google Scholar]
- 22. Cohen MD, D’Amico FJ, Merenstein JH. Weight reduction in obese hypertensive patients. Fam Med 1991; 23: 25–8. [PubMed] [Google Scholar]
- 23. Jalkanen L. The effect of a weight reduction program on cardiovascular risk factors among overweight hypertensives in primary health care. Scand J Soc Med 1991; 19: 66–71. [DOI] [PubMed] [Google Scholar]
- 24. Karvetti RL, Hakala P. A seven-year follow-up of a weight reduction programme in Finnish primary health care. Eur J Clin Nutr 1992; 46: 743–52. [PubMed] [Google Scholar]
- 25. Kumanyika SK, Fassbender JE, Sarwer DB, et al. One-year results of the Think Health! study of weight management in primary care practices. Obesity (Silver Spring) 2012; 20: 1249–57. [DOI] [PubMed] [Google Scholar]
- 26. Logue E, Sutton K, Jarjoura D, et al. Transtheoretical model-chronic disease care for obesity in primary care: a randomized trial. Obes Res 2005; 13: 917–27. [DOI] [PubMed] [Google Scholar]
- 27. Martin PD, Dutton GR, Rhode PC, et al. Weight loss maintenance following a primary care intervention for low-income minority women. Obesity (Silver Spring) 2008; 16: 2462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mayer-Davis EJ, D’Antonio AM, Smith SM, et al. Pounds off with empowerment (POWER): a clinical trial of weight management strategies for black and white adults with diabetes who live in medically underserved rural communities. Am J Public Health 2004; 94: 1736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moore H, Summerbell CD, Greenwood DC, et al. Improving management of obesity in primary care: cluster randomised trial. BMJ 2003; 327: 1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Munsch S, Biedert E, Keller U. Evaluation of a lifestyle change programme for the treatment of obesity in general practice. Swiss Med Wkly 2003; 133: 148–54. [DOI] [PubMed] [Google Scholar]
- 31. Rapoport L, Clark M, Wardle J. Evaluation of a modified cognitive-behavioural programme for weight management. Int J Obes Relat Metab Disord 2000; 24: 1726–37. [DOI] [PubMed] [Google Scholar]
- 32. Ross R, Lam M, Blair SN, et al. Trial of prevention and reduction of obesity through active living in clinical settings: a randomized controlled trial. Arch Intern Med 2012; 172: 414–24. [DOI] [PubMed] [Google Scholar]
- 33. Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med 2011; 365: 1969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guidance for Industry Developing Products for Weight Management. Rockville, MD: U.S. Department of Health and Human Services, 2007. [Google Scholar]
- 35. Foster GD, Makris AP, Bailer BA. Behavioral treatment of obesity. Am J Clin Nutr 2005; 82(1 Suppl): 230–5S. [DOI] [PubMed] [Google Scholar]
- 36. Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003; 290: 1624–32. [DOI] [PubMed] [Google Scholar]
- 37. Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med 2005; 142: 56–66. [DOI] [PubMed] [Google Scholar]
- 38. Jolly K, Lewis A, Beach J, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: lighten up randomised controlled trial. BMJ 2011; 343: d6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jebb SA, Ahern AL, Olson AD, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet 2011; 378: 1485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ara R, Blake L, Gray L, et al. What is the clinical effectiveness and cost-effectiveness of using drugs in treating obese patients in primary care? A systematic review. Health Technol Assess 2012; 16: iii–xiv, 1–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dombrowski SU, Knittle K, Avenell A, Araújo-Soares V, Sniehotta FF. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ 2014; 348: g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kirk SF, Penney TL, McHugh TL, Sharma AM. Effective weight management practice: a review of the lifestyle intervention evidence. Int J Obes (Lond) 2012; 36: 178–85. [DOI] [PubMed] [Google Scholar]
- 43. Dixon KJ, Shcherba S, Kipping RR. Weight loss from three commercial providers of NHS primary care slimming on referral in North Somerset: service evaluation. J Public Health (Oxf) 2012; 34: 555–61. [DOI] [PubMed] [Google Scholar]
- 44. Gillies CL, Abrams KR, Lambert PC, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007; 334: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ebrahim S, Taylor F, Ward K, et al. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database Syst Rev 2011; 1. [DOI] [PubMed] [Google Scholar]
- 46. Pignone M, Phillips C, Mulrow C. Use of lipid lowering drugs for primary prevention of coronary heart disease: meta-analysis of randomised trials. BMJ 2000; 321: 983–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.