Abstract
Objective:
Childhood obesity is a major public health concern worldwide while the current epidemic may be secondary to over consumption of high-fat, energy-rich foods. Purslane (Portulaca oleracea L.) has been traditionally used in medicine for several antioxidant and anti-atherogenic activities. In this study the anti-dyslipidemic effects of P.oleracea was evaluated in obese adolescents.
Methods:
In this triple-blinded randomized placebo-controlled clinical trial which was done from July 2011 to June 2012, obese adolescent patients whom were referred to the Isfahan Cardiovascular Research Institute (Iran) were randomly allocated to the two arms of cases and controls. The cases group was asked to take one capsule containing powdered P. oleracea seeds (500 milligrams) two times a day for one month, and the controls group were asked to take identical but placebo (lactose) capsules in the same way. Biochemical parameters including 12-hours fasting serum levels of total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were measured before the initiation and after the completion of the study protocol.
Findings:
Total cholesterol, LDL-C, and TG showed statistically significant changes over time (one month) in the P. oleracea group (p < 0.05). However, between-group analysis using general linear model (multivariate) test revealed that the differences in the mentioned parameters between two study groups were statistically significant just for LDL-C and TG, while others did not differ significantly.
Conclusion:
P. oleracea L. may have positive effects on serum lipids profile which may be attributed to its polyphenolic and antioxidant compounds. This herbal drug seems to be well-tolerated in adolescent population as well. Further studies are recommended.
Keywords: Portulaca oleracea, dyslipidemia, adolescent, obesity, clinical trial
1. INTRODUCTION
Obesity is a chronic metabolic disorder that is raised by multiple biological and environmental factors, a sedentary lifestyle, and a genetic predisposition. The increase in the prevalence of obesity and negative health outcomes are major public health problems throughout the world (1, 2). Obesity is also an important problem among children and adolescents age 2-19years, with alarmingly high prevalence rates of 16.9% being at or above the 95th percentile. The considerable notice would be the health consequences the obese child takes on into adulthood (3). Studies have shown that an elevated body mass index (BMI) in childhood and adolescence is correlated with an increase of adult coronary heart disease (CHD), cardiovascular disease (CVD), cancer, and premature mortality (4-7).
The current epidemic of childhood and adolescence obesity may be secondary to over-consumption of high-fat, energy-rich foods. Overweight adolescents should be encouraged to prevent further weight gain and manage obesity-related risk factors; however, it is also essential to provide optimal therapy for hypertension, dyslipidemia, and impaired fasting blood glucose, whenever indicated. Clinically, these patients may likely have high serum levels of low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG), as well as low serum levels of high-density lipoprotein cholesterol (HDL-C), all are documented to be risk factors for cardiovascular diseases.
The centerpiece of treatment for high blood cholesterol is a therapeutic lifestyle changes (TLC) plan consisting diet low in saturated fat and cholesterol, as well as weight reduction, keeping regular physical activity and smoking cessation. An adequate trial of TLC should be used in all patients; however, pharmacotherapy should be instituted concurrently in high-risk patients with low level of response to the mentioned modifications. The major limiting factor in the use of lipid-lowering drugs in children and adolescents is side effects. Definitive evidence does not yet exist regarding the safety and efficacy of long term use of medications in this age group and their effect on adult cardiovascular disease. Nonetheless, children and adolescents with important lipid abnormalities should be identified and treated (8).
An alternative dietary approach to lowering blood cholesterol is those dietary adjuncts. Daily adding dietary sources of fiber (e.g., vegetables, legumes, whole grains, fruits) to the diet will aid in lowering blood cholesterol levels. Having variety of clinically-effective components, herbal drugs may encompass many different pharmacological properties and likely fewer side effects compared to chemical drugs (9).
Purslane (Portulaca oleracea L.) as indicated by World Health Organization (WHO), is one of the mostly used medicinal plants, widespread throughout temperate and tropical areas of the world (10). As a traditional Chinese medicine, it has been used for several antioxidant, immunomodulatory and therapeutic effects in medical conditions including diabetes, atherosclerosis, vascular endothelial dysfunction, and urolithiasis (10-16).
The uniqueness of purslane as the richest vegetable source of omega-3 and poly-unsaturated fatty acids (PUFA) is well documented. Purslane also contains high amounts of vitamins E and C, beta carotene, and different flavonoids, known to have antiatherogenic activities (10, 11). Despite having such favorable effects, the studies indicating its therapeutic properties were mainly conducted on animal samples; and very few human studies have been methodologically designed as a clinical trial on it. It seems that there exist convincing prerequisite data having to design a placebo-controlled clinical trial focusing on anti-hyperlipidemic effects of Portulaca oleracea in obese adolescents, as a vulnerable population for adverse consequences of dyslipidemia, which may strongly take benefits of non-pharmacological anti-hyperlipidemic modalities.
2. MATERIALS AND METHODS
This triple-blinded randomized placebo-controlled clinical trial was registered in the Iranian Registry of Clinical Trials (No. IRCT201109122306N4). It was conducted from July 2011 to June 2012in Isfahan Cardiovascular Research Institute, affiliated with Isfahan University of Medical Sciences, Isfahan, Iran. Ethical issues of the study protocol was in accordance with the Declaration of Helsinki (17) and was ethically approved by the board of human studies at Isfahan University of Medical Sciences (Registration code:388592). Oral assent and written consent was obtained from participants and their parents, respectively, after providing detailed oral information about the study objectives and protocol.
Regarding the study objectives, inclusion criteria were considered the followings: non-smoker adolescents defining as being aged between 12 to 18 years old, with documented dyslipidemia, and having a body mass index (BMI) equal or more than the age- and gender-specific 95th percentile, which is confirmed to be appropriate for the Iranian children and adolescents (18). Dyslipidemia was defined as serum total cholesterol(Total-C) or low-density lipoprotein cholesterol (LDL-C) or triglycerides (TG) equal or more than the age- and gender-specific 95th percentile, or high-density lipoprotein cholesterol (HDL-C) lower than 5th percentile (18).
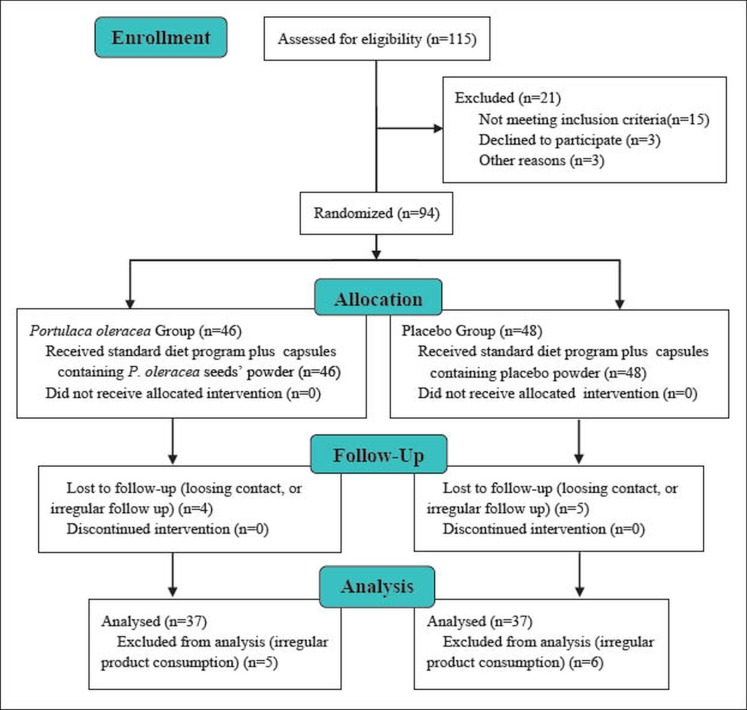
Eligible patients who met all the inclusion criteria were selected using convenient sampling method. After receiving standard recommendations for healthy eating habits and regular physical activity (19), they were randomly assigned to two study groups using simple randomization sampling method. The cases group were asked to use one capsule containing powdered P. oleracea seeds (500 milligrams), two times a day for one month, and the placebo group were asked to take one placebo capsule containing equal amounts of a placebo powder (lactose) twice a day in identical packages twice a day for the same time period. Having any other chronic diseases, using any medication assuming to effect on lipid profile, and irregular use of the administered drug (containing either the case or the placebo ingredients) were considered as exclusion criteria. The recruitment protocol is schematically shown in CONSORT diagram of the study (Figure 1).
Figure 1.
CONSORT diagram of the study
Sample Size Calculation
Considering to have a 90% chance of detecting a difference (at least 10 mg/dl) in mean cholesterol change at 5% level of significance, and assuming the standard deviation of total cholesterol as 15.47mg/dl obtained from the data analysis of a previous similar study (20), the sample size was calculated to be 35 in each case and control groups.
Dosage Form Preparation for Purslane
Potulaca oleracea seeds were purchased from a local medicinal plants market in Isfahan province, Iran, and were taxonomically confirmed by two academic herbal botanists. Standardization was done based on determining the amount of total phenolic components in purslane seeds, using Folin-Ciocalteu colorimetric method (21). Placebo capsules were filled with lactose, which has not any effect on human lipid profile. Gallic acid, Folin–Ciocalteu reagent, sodium carbonate powder and lactose powder were purchased from Merck chemical company (Germany). Figure 2 shows the practical steps for determining the amount of phenolic components in 500 mg P. oleracea seeds.
Figure 2.
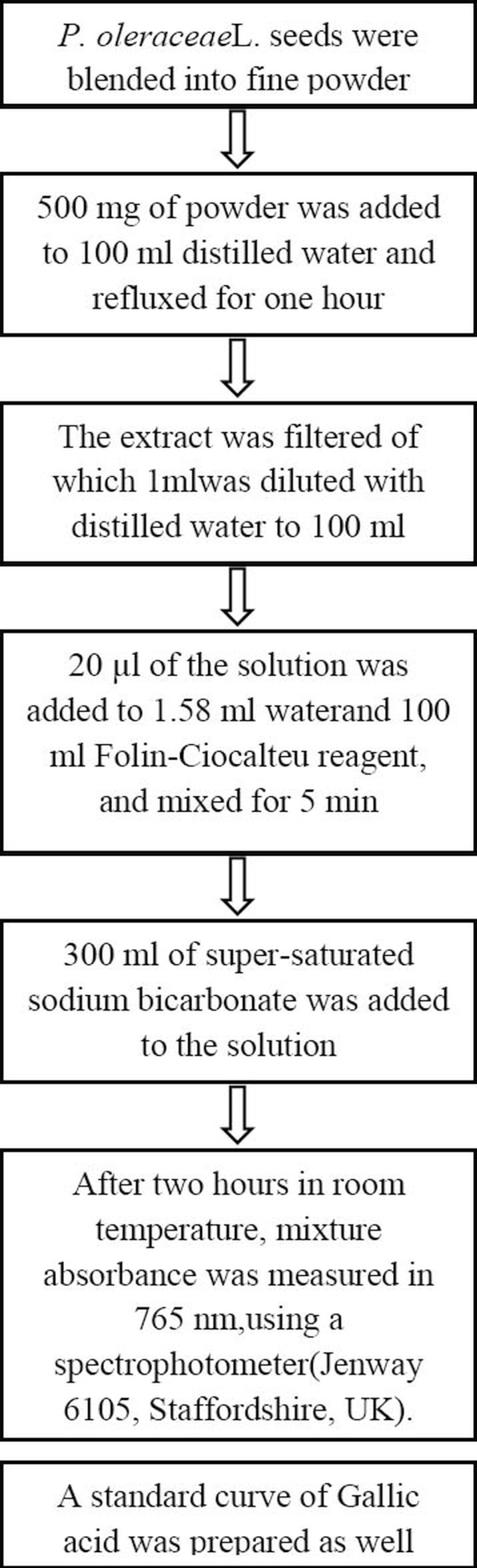
Practical steps for determining the amount of phenolic components in 500 mg powder of Portulaca oleracea seeds
Data Collection and Follow-up
Parents were asked to contact our clinic two weeks after the study initiation and to report the compliance of the children for using the medication, as well as any side effects of the medication used. Also, children’s compliances were followed the by regular phone calls and confirmed by interviewing them before sampling. The findings of those participants who did not regularly take the medications (missing more than four days of drug consumption) were excluded from the statistical analysis (Figure 1). Biochemical parameters including 12-hrs fasting serum levels of Total-C, LDL-C, HDL-C, and TG were measured before the initiation and after the completion of the study protocol.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) software (Chicago, IL, USA) version 16.0. Descriptive statistics are presented for the baseline characteristics of the participants. Normal distribution of any quantitative measures was assessed by Kolmogorov-Smirnov test. Independent-samples t-test analysis was performed to verify any significant differences in the baseline demographic (age, BMI) and biochemical parameters between study subjects in the case and control groups. The changes of the mentioned parameters after study completion in each case and control group was evaluated by paired-samples t-test. General linear model (multivariate) test was used to compare the over-time changes in the investigated parameters between the two study groups. The significance level was set at P-value < 0.05 for all comparisons.
3. RESULTS
The total amount of phenolic components of 500 mg of the prepared P. oleracea L. Seeds (provided as fine powder in drug-filled capsules) after triple measurement by Folin–Ciocalteu method was identified to be approximately equivalent to 1.8 mg Gallic acid.
Seventy four volunteers completed the study. Distribution of age, sex and baseline body mass index (BMI) in two study groups is presented in table 1. There were no significant differences in the mean age and BMI (table 1), as well as baseline lipid profile measures (as indicated in table 2) between the case and control groups. According to the Kolmogorov-Smirnov analysis, all quantitative parameters distributed normally in the two study groups (p > 0.05).
Table 1.
Distribution of age, sex and baseline body mass index (BMI) in two study groups. Data presented as Number, or Mean ± SD, where applicable. BMI: body mass index, calculated by dividing weight by the height squared (kg/m2); SD: standard deviation. * Between-group analysis with Independent-samples t-test.
Table 2.
Biochemical parameters before initiation and after completion of the study protocol in two study groups Data presented as Number, or Mean ± SD, where applicable. HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TG: triglyceride; Total-C: total cholesterol; SD: standard deviation. * Within-group analysis with Paired-samples t-test; **Between-group analysis with General linear model (multivariate) test.
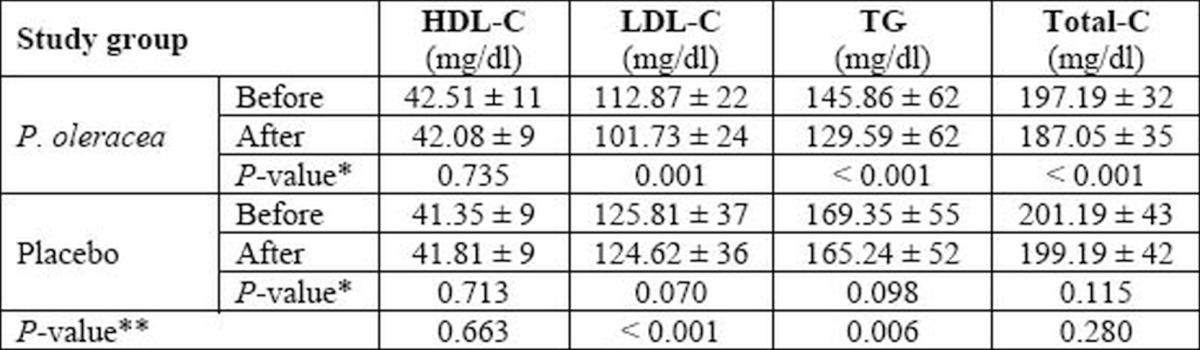
Biochemical measures of serum lipid profilein two study groups are presented in table 2. As evident, the amount of Total-C, LDL-C, and TG bear significant changes over time (one month) in the P. oleracea group; while there are no significant changes in all investigated parameters between the beginning and the end measures in control group. However, between-group analysis using General linear model (multivariate) test reveals that the differences in the mentioned parameters between two study groups are statistically significant just for LDL-C and TG, while others do not differ significantly. No significant side effects were reported by participants of both groups.
4. DISCUSSION
Cardiovascular disorders, especially atherosclerosis is predicted to become the most important disease in 2020, having dyslipidemia as a its major risk factor. A causal relationship between elevated serum lipids and the development of atherosclerotic plaques has been previously well established (22).
During the past three decades, epidemiologic studies have established a direct relationship between blood cholesterol concentrations in a population and the incidence of CHD events (23-25). Since the early 1990s, the results of numerous major clinical trials have conclusively demonstrated the value of lipid-modifying therapy to prevent CHD events (26-30).
In addition to different chemical drugs to modify dyslipidemia, non-pharmacological treatments also can significantly reduce morbidity from CHD. Being most widely used, edible plants are considered as one of the main resources of anti-atherosclerosis components, especially favorable in more susceptible populations such as pediatrics and adolescents.
This trial, which to the best of our knowledge is the initiative one in the adolescent age group, revealed that daily consumption of 1 g. P.oleracea seeds for one month may decrease the serum levels of Total cholesterol, LDL-C, and TG in dyslipidemic adolescents.
Nearly all the therapeutic values of purslane (P. oleracea) are attributed to the presence of many biologically active compounds including flavonoids, Alkaloids, Coumarins, and high content of ω-3 fatty acids with considerable beneficial in preventing heart attacks and strengthening the immune system (10, 31). Predominantly containing poly-unsaturated (omega-3) fatty acids, make purslane a noteworthy medicinal plant to have favorable effects on cholesterol and triglyceride levels. Consumption of foods rich in omega-3 fatty acids for several times per week has been associated with a reduced risk of heart disease, and they are recommended as part of a low-fat diet (32, 33). Several mechanisms have been proposed to explain how PUFA might beneficially affect risk factors implicated in the pathogenesis of atherosclerosis; these include improving vascular reactivity, decreasing platelet aggregation, lowering serum triglycerides, decreasing blood pressure, preventing arrhythmias and reducing endothelial inflammation (34).
Some studies on the cholesterol lowering effect of P.oleracea L. have previously been conducted on animals and humans (13, 35-38). The only human study on the anti-hyperlipidemic effects of purslane was conducted on 93 Iranian patients with LDL-C more than 100 mg/dl referred to an internal clinic in Shahrekord, Iran, in which lipid profile of patients was assessed before and 45 days after taking 50-60 g purslane leaves or lovastatin 20 mg, daily. The serum levels of Total-C, LDL-C, and TG decreased significantly in both study groups, however, only TG decreasing effect of purslane was significantly more evident than that seen in lovastatin group (38).
Patients compliance issues maybe considered as the main limitation for this study. Moreover, considering more than one-month duration for future studies maybe a reasonable approach to get even more favorable changes in other serum lipids, namely HDL-C, as well as inflammatory biochemical factors.
5. CONCLUSION
P.oleracea L. may have positive effects on serum lipids profile of human which can be attributed to its polyphenolic and antioxidant compounds. Besides, being well-tolerated in adolescent population, it can presumably be considered for future long-term studies on prevention and treatment of dyslipidemia and atherosclerotic disorders in this population.
Acknowledgments:
This study was a Pharm.D thesis project financially supported by the Vice- chancellery for research and technology at Isfahan University of Medical Sciences. Authors would like to acknowledge the staff in laboratory department of Isfahan Cardiovascular Research Institute for their assistance. We also thank Dr. Iraj Mehregan for his kind help in taxonomically confirmation of the prepared Portulaca oleracea seeds.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults 1999-2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Masic I, Rahimic M, Dilic M, Kadribasic R, Toromanovic S. Socio- medical characteristics of coronary disease in Bosnia and Herzegovina and the world. Mater Sociomed. 2011;23(3):171–183. doi: 10.5455/msm.2011.23.171-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents 2007-2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 4.James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263(4):336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 5.Biro FM, Wien M. Childhood obesity and adult morbidities. Am J ClinNutr. 2010;91(5):1499S–1505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Dam RM, Willett WC, Manson JE, Hu FB. The relationship between overweight in adolescence and premature death in women. Ann Intern Med. 2006;145(2):91–97. doi: 10.7326/0003-4819-145-2-200607180-00006. [DOI] [PubMed] [Google Scholar]
- 7.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Longterm morbidity and mortality of overweight adolescents. A follow- up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327(19):1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 8.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 9.Benzie IF, Wachtel-Galor S. 2nd ed. Boca Raton (FL): CRC Press; 2011. Herbal Medicine: Biomolecular and Clinical Aspects. [PubMed] [Google Scholar]
- 10.Alam MA, Juraimi AS, Rafii MY, Abdul Hamid A, Aslani F, Hasan MM, et al. Evaluation of Antioxidant Compounds, Antioxidant Activities, and Mineral Composition of 13 Collected Purslane (Portulacaoleracea L.) Accessions. BioMed Research International. 2014 doi: 10.1155/2014/296063. Article ID 296063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barakat LA, Mahmoud RH. The antiatherogenic, renal protective and immune-modulatory effects of purslane, pumpkin andflax seeds on hypercholesterolemic rats. North American Journal of Medical Sciences. 2011;3(9):351–357. doi: 10.4297/najms.2011.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao D, Li Q, Fan Y. Hypoglycemic effects and mechanisms of Portulacaoleracea L. in alloxan-induced diabetic rats. Journal of Medicinal Plants Research. 2010;4(19):1996–2003. [Google Scholar]
- 13.Lee AS, Lee YJ, Lee SM, Yoon JJ, Kim JS, Kang DG, et al. Portulaca oleracea ameliorates diabetic vascular inflammation and endothelial dysfunction in db/db mice. Evidence-Based Complementary and Alternative Medicine. 2012 doi: 10.1155/2012/741824. Article ID 741824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F, Li Q, Gao D, Peng Y, Feng C. Preparation and antidiabetic activity of polysaccharide from Portulaca oleracea L. African Journal of Biotechnology. 2009;8(4):569–573. [Google Scholar]
- 15.Chen Y, Shen Z, Chen X. Evaluation of free radicals scavenging and immunity-modulatory activities of Purslane polysaccharides. J Food Composit Anal. 2007;22:303–306. [Google Scholar]
- 16.Kishore DV, Moosavi F, Varma RK. Effect of ethanolic extract of Portulaca oleracea Linn. On ethylene glycol and ammonium chloride induced urolithiasis. International Journal of Pharmacy and Pharmaceutical Sciences. 2013;5(2):134–140. [Google Scholar]
- 17.WMA Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Subjects. [Last accessed: April 2014]. Available at: http://www.wma.net/en/30publications/10policies/b3/
- 18.Kelishadi R, Hashemipour M, Sarraf-Zadegan N, Amiri M. Trend of atherosclerosis risk factors in children of Isfahan. Asian Cardiovasc Thorac. 2001;9:36–40. [Google Scholar]
- 19.Behrman R, Kliegman R, Jenson H. 19th ed. Philadelphia: Elsevier Saunders; 2011. Nelson textbook of pediatrics. [Google Scholar]
- 20.Kelishadi R, Hashemi M, Mohammadifard N, Khavarian N. Association of changes in oxidative and proinflammatory states with changes in vascular function after a lifestyle modification trial among obese children. Clinical chemistry. 2008;54(1):147–153. doi: 10.1373/clinchem.2007.089953. [DOI] [PubMed] [Google Scholar]
- 21.Waterman PG, Mole S. Oxford: Blackwell Scientific Publication; 1994. Analysis of phenolic plant metabolites. [Google Scholar]
- 22.Jain KS, Kathiravan MK, Somani RS, Shishoo CJ. The biology and chemistry of hyperlipidemia. Bio organic and medicinal Chemistry. 2007;15:4674–4699. doi: 10.1016/j.bmc.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 23.Neaton JD, Blackburn H, Jacobs D, Kuller L, Lee DJ, Sherwin R, et al. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor InterventionTrial. Multiple Risk Factor Intervention Trial ResearchGroup. Arch Intern Med. 1992;152:1490–500. [PubMed] [Google Scholar]
- 24.Jacobs D, Blackburn H, Higgins M, Reed D, Iso H, McMillan G, et al. Report of the Conference on Low BloodCholesterol: mortality associations. Circulation. 1992;86:1046–1060. doi: 10.1161/01.cir.86.3.1046. [DOI] [PubMed] [Google Scholar]
- 25.Castelli WP. Epidemiology of coronary heart disease: the Framingham study. Am J Med. 1984;76:4–12. doi: 10.1016/0002-9343(84)90952-5. [DOI] [PubMed] [Google Scholar]
- 26.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, et al. High-dose atorvastatin vs. usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial [published correction appears in JAMA 2005; 294: 3092. JAMA. 2005;294:2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 28.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes [published correction appears in N Engl J Med. 2006; 354: 778] N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 29.Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcome Trial-Lipid Lowering Arm (ASCOT-LLA): a multicenter randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 30.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicenter randomized placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 31.Okafor IA, Ezejindu DN. Phytochemical studies on Portulaca oleracea (purslane) plant. G.J.B.A.H.S. 2014;3(1):132–136. [Google Scholar]
- 32.Dietary supplementation with omega-3polyunsaturated fatty acids and vitamin E after myocardialinfarction: results of the GISSI-Prevenzione trial. GruppoItaliano per lo Studio della Sopravvivenzanell’ In fartomiocardico [published corrections appear in Lancet. 2007; 369: 106; Lancet. 2001; 357:642] Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 33.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid onmajor coronary events in hypercholesterolaemic patients (JELIS):a randomized open-label, blinded endpoint analysis [published correction appears in Lancet. 2007;370:220] Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 34.Micallef MA, Garg M L. Anti-inflammatory and cardioprotective effect of n-3 polyunsaturated fatty acids and plant sterols in hyperlipidemic individuals. Atherosclerosis. 2008;204:476–482. doi: 10.1016/j.atherosclerosis.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 35.Akila G, Djamil K, Saadia B. Portulacaoleracea extract increases lecithin: cholesterolacyl-transferase and paraoxonase 1 activities and enhances reverse cholesterol transport in streptozotocin- induced diabetic rat. Pharmacognosy journal. 2014;6(3):1–9. [Google Scholar]
- 36.Changizi-Ashtiyani S, Zarei A, Taheri S, Rasekh F, Ramazani M. The Effects of Portulaca oleracea alcoholic extract on induced hypercholesteroleomia in rats. Zahedan Journal of Research in Medical Sciences. 2013;15(6):34–39. [Google Scholar]
- 37.Barakat LA, Mahmoud RH. The antiatherogenic, renal protective and immunomodulatory effects of purslane, pumpkin and flax seeds on hypercholesterolemic rats. North American Journal of Medical Sciences. 2011;3(9):351–357. doi: 10.4297/najms.2011.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gatreh-Samani K, Farrokhi E, Khalili B, Rafieian M, Moradi MT. Purslane and lovastatin effects on serum araoxanase1 activity. J ShahrekordUniv Med Sci. 2011;13(1):9–15. Article in Persian. [Google Scholar]