Abstract
Introduction:
Age-related macular degeneration (AMD) is a leading cause of irreversible serious vision damage in persons over 50 years of age. In treating AMD many medicaments are applied such as inhibitors of vascular endothelial growth factor (VEGF), have been very carefully included over the last few years after a series of study research.
Aims:
To analyze the past methods of treatment, discuss emerging therapies which could advance the treatment of exudative AMD. The past anti-VEGF therapies require frequent repetitions of administration, with uncertain visual acuity recovery, as not all patients react to anti-VEGF therapy. Consequently, there is a need to find out additional therapies which could improve the treatment of exudative AMD. The real aim in the treating of AMD is to prevent CNV development.
Methods:
A survey of the current clinical research and results in the field of the present and future treatments of exudative AMD.
Results:
There are many areas of research into new methods of the exudative AMD treatment.
Conclusion:
The future therapies for exudative AMD treatment have a potential not only to reduce the frequency of administration and follow-up visits, but also to improve effects of treatment by targeting additional ways of CNV development, increasing the aptitude of target binding and extending durability of treatment.
Keywords: age-related macular degeneration, therapy, anti-VEGF
1. INTRODUCTION
From the aspect of pharmaco-ophthalmology, currently the most important area of interest is the study of new medications in the therapy for age-related macular degeneration (AMD); so, we are witnessing emergence of many new therapeutic products being used in the treatment of the disease that are being very carefully included through a series of study research. Today AMD is a leading cause of severe, irreversible loss of vision after 50 years of age, and a reason for more than 46% of cases of severe vision loss, with visual acuity under 0.1. It is manifested in two basic forms: dry (atrophic, drusenoid, non exudative) and wet (exudative).
The aim of this study is to analyze the past methods of treatment, discuss emerging therapies which could advance the treatment of exudative AMD. The past anti-VEGF therapies require frequent repetitions of administration, with uncertain visual acuity recovery, as not all patients react to anti-VEGF therapy. Consequently, there is a need to find out additional therapies which could improve the treatment of exudative AMD. The real aim in the treating of AMD is to prevent CNV development.
2. THERAPEUTIC OPTIONS FOR DRY AMD
For almost two decades laser photocoagulation (LPC) was the only clinically proved way of treatment of exudative AMD prior to the emergence of photo-dynamic therapy (1, 2). LPC application is limited to extra foveal lesions and juxta-foveal CNV in which the scar should not affect the foveal avascular zone (FAZ) after treatment (3). Results were not good enough for the patients treated with LPC, damage was often considerably bigger than benefit; the efficient treatment by this method was attained in only 15% of cases, with irreversible loss of central vision and increase in scotoma of the central visual field as an adverse effect, not as a treatment complication (4).
Development of verteporfin photo-dynamic therapy (PDT-V) aroused a new hope in the treatment of exudative AMD (5, 6). The treatment was approved by the Food and Drug Administration (FDA) – American food and drug agency in April 2000. In PDT-V, the cold laser of a specific wave length of 689 mm affects verteporfin injected intravenously 15 minutes before (Visudyne®), photosensitive colour which prefers to be dispersed in capillary endothelial cells of CNV membranes by delivery of energy of 50 J/cm2 intensity 600 mW/cm2 lasting 83 s, with a spot size of 1000 µm more than the largest CNV diameter. When the laser is activated, abnormal blood vessels are destroyed, while the healthy ones remain undamaged. Today, PDT treatment is significantly less used, in a combination with intravitreal triamcinolone (7, 8), or combined with inhibitors of vascular endothelial growth factor (VEGF), which improve treatment efficacy (9, 10). Additionally, as the frequency of predominantly classic membranes is 22% of total CNV number, that was another reason to look for a better medicine for the treatment of exudative AMD (11, 12).
After several dozens of studies, the medicaments from the VEGF inhibitor group were separated as particularly efficient compared to all other treatments. VEGF are promoters of neovascularisation and vascular permeability.
Back in 1997 when Kliffen et al. reported increased VEGF expression in wet AMD, that aroused special interest in new therapeutic procedures for AMD treatment (13). The first studies of this diffusible factor produced by the retina date back to 1948 when Michaelson published first papers related to X factor which participates in neovascularisation in the retina and iris in proliferative diabetic retinopathy and the central retinal vein occlusion (14).
Today we know that VEGF is of key importance in the pathogenesis of wet age-related macular degeneration and that it is responsible for increase in vascular permeability and neovascularisation simulation. Increased VEGF production is a consequence of oxidative cell stress, which is a defensive response of the body so that there is a proliferation of newly-formed blood vessels and formation of neovascular membrane. These newly-formed blood vessels are very fragile so that they easily bleed and let exudates through, which causes damage to the retinal tissues, especially to photo receptors. Exactly for this reason, one of possible strategies of prevention and treatment of the subretinal neovascular membrane is the VEGF inhibition and reduction in vascular permeability. A large multi centric study of inhibitors of angiogenesis in eye diseases accompanied with neovascularisation (VISION) (15) proved an unquestionable VEGF role in the development of subretinal neovascularisation and of VEGF inhibitors in the suppression of neovascularisation. VEGF inhibitors are medications that inhibit the formation and growth of newly-formed blood vessels by binding to VEGF-protein necessary for the formation and growth of the neovascular membrane.
Anti VEGF medicaments changed significantly paradigm in the treatment of AMD, enabling the essential opening of the door to a targeted therapy. At the very beginning of introduction in AMD therapy, they demonstrated clear effects as improvement of visual functions compared with other therapies, and thus became the front line of defense in the treatment of neovascular AMD (Table 1). Still today anti-VEGF therapy is the mainstay and gold standard in the treatment of exudative AMD ((16). Efficient treatment of neovascular AMD began as of the first application of anti-VEGF with FDA’s approval of pegaptanib sodium (Macugen®, Eyetech Pharmaceuticals, Inc-Pfizer Inc.) in December 2004, and ranibizumab (Lucentis®, Genentech, Inc., South San Francisco, CA) in June 2006.
Table 1.
Review of intravitreally applied antiVEGF treatment modalities for wet AMD
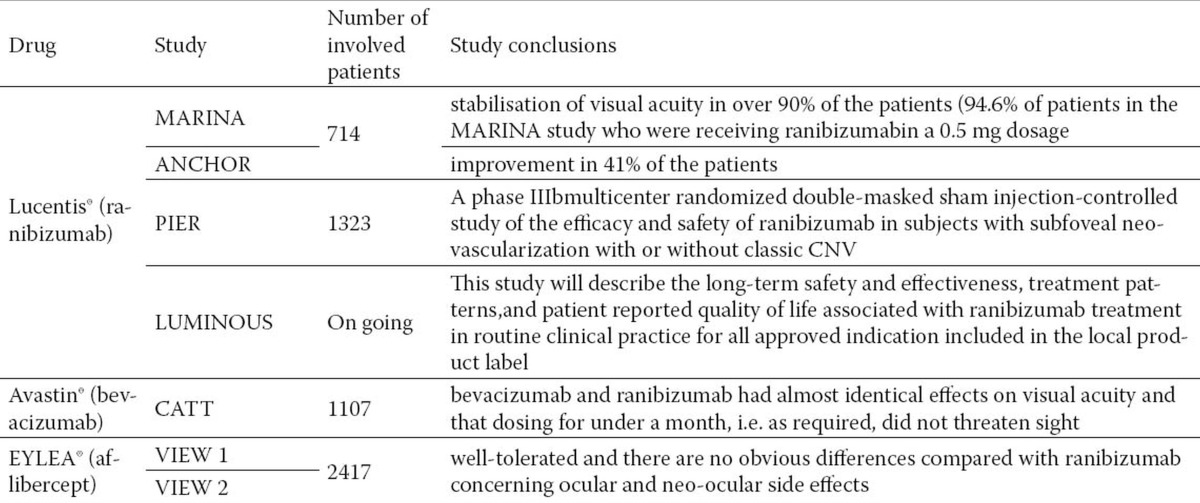
Macugen® (pegaptanib sodium) was the first antiangiogenetic therapeutic product approved by FDA and the first medicine clearly taking effect on the basic pathological substrate in wet AMD (17). It proved efficient in the stabilization of visual acuity in 70% of cases, but that was not sufficient as it did not show statistical significance in terms of recovery of visual acuity. Namely, only in 20% of patients discovered in the early stage of exudative AMD, visual acuity was better after the treatment with pegaptanib. Macugen® may be used for all kinds of exudative AMD membranes by intravitreal injections of 0.3 mg every six weeks, usually over a period of at least two years.
Lucentis® (ranibizumab) was approved in 2006 for intravitreal administration in the treatment of all subtypes of neovascular AMD, based on the results of three large-scale double-masked randomized controlled trials. Lucentis® works on the principle of competitive VEGF inhibition in extracellular space owing to penetration into all retinal layers and affinity to all VEGF isoforms. Ranibizumab is a fragment of recombinant humanized monoclonal antibody targeted against human vascular endothelial growth factor A (VEGF-A). It binds with high affinity to VEGF-A isoforms (e.g. VEGF110, VEGF121 i VEGF165), thus preventing binding VEGF-A to its receptors VEGFR-1 and VEGFR-2. VEGF-A binding to its receptors leads to endothelial cell proliferation and neovascularisation, as well as to the increased vascular leakage, with a consequential exudative AMD progression. Lucentis® (48 kDa) is a Fab fragment of murine MAb Anti-VEGF-A(~150 kDa), which achieves 5-20 times higher affinity to VEGF compared to the antibody from which it derived. Penetration into all retinal layers increased, cytotoxicity and inflammatory potential decreased, while the shortening of half-life minimized system effects of this medication. Clinical safety and efficacy of Ranibizumab medicament were estimated through three randomized, double-masked, placebo or actively controlled studies during 24 months with patients having wet age-related macular degeneration (MARINA - Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration) (18), ANCHOR (Anti-VEGF antibody for the treatment of predominantly classic choroidal neovascularisation in AMD) (19) and PIER (20) (A phase IIIb multi centric randomized double-masked sham injection-controlled study of the efficacy and safety of ranibizumab in subjects with subfoveal neovascularization with or without classic CNV). These studies covered a total of 1,323 patients (879 active and 444 control ones); it was concluded that the medicament was efficient and could be applied for all wet AMD types. The studies showed that ranibizumab led to stabilization of visual acuity in over 90% of the patients (94.6% of patients in the MARINA study who were receiving ranibizumab in a 0.5 mg dosage), and to improvement in 41% of the patients (in the ANCHOR study). Effect of the therapy is better if wet AMD is established earlier and therapy administered according to the proposed protocol with at least three intravitreal injections of ranibizumab 0.5 mg (0.05 mL) in a four-week interval. Further therapy is administered monthly (about eight injections annually) with monitoring and continuation of the therapy according to the retinologist’s suggestion in the course of the second year of treatment (based on fluorescein angiography–FA and optical coherent tomography - OCT). It is usually necessary to administer eight injections in the first year of treatment and six in the second.
Intravitreal administration of ranibizumab had very promising results worldwide, with the absence of retinal toxicity, as well as nonexistence of serious system and local ophthalmological adverse effects related to the intravitreal administration of the medicament. Complications that can occur during the ranibizumab administration are rare, and they are locally related only to complications which can happen in all intravitreally administered medicaments, such as suffusion, anterior uveitis, briefly increased intra ocular pressure, cataract, and less frequently retinal detachment, haemophthalmos, and very rarely endophthfalmitis. System complications are very serious, but fortunately rare (21). Described have been cardiovascular complications, acute myocardial infarction with a possibility of lethal outcome, cerebrovascular accidents ranging from mild transient ischemic attack to serious cerebrovascular insult (22, 23).
Avastin®(bevacizumab) has a similar effect as ranibizumab, humanized monoclonal antibody targeted at VEFG. It was designed for intravenous application and approved by FDA for the treatment of colon cancer. It was obtained from the same murine antibody as ranibizumab. The basic difference is molecular at the level of the chain length: ranibizumab is a 48-kD Fab fragment, while bevacizumab 149-kD is an antibody. The difference results in various possibilities of the medicament to reach the targeted part and stay longer in the eye after injection. Another difference is a low price of bevacizumab in comparison with ranibizumab (1mg intravitreal dose is about $5.5) (24). First results of Avastin administration to treat CNV in AMD were published in August 2005 (25, 26), while still waiting for FDA’s approval of Lucentis®. Very shortly after, many researchers all over the world started examining possibilities of intravitreal administration of the medicament. The original purpose of bevacizumab was to treat patients with metastatic colon and lung cancers in combination with the standard chemotherapy; in 2008 FDA approved it also for the treatment of patients with metastatic breast cancer. All past studies have shown that it is safe and efficient in the CNV treatment of exudative AMD, which is no less efficient than ranibizumab. However, perfectly clear results were obtained through a large randomized multi centric study financed by the American National Eye Institute of the National Institute of Health. The study implied comparative study of safety and efficacy of ranibizumab (Lucentis®) and bevacizumab (Avastin®) in the treatment of AMD. The study was entitled Comparison of Age-Related Macular Degeneration Trials (CATT Study) and covered 1,107 patients with newly diagnosed exudative AMD, who were monitored for two years, randomly classified into one of the four groups in which bevacizumab or ranibizumab were administered every four weeks in the first year, and then in a variable dosage depending on findings, or one of the two tested medicaments was administered in varying doses over a two-year period. In May 2011, the authors submitted a report on one-year results ((27). This randomized clinical trial showed that bevacizumab and ranibizumab had almost identical effects on visual acuity and that dosing for under a month, i.e. as required, did not threaten sight. Both medicaments dramatically reduced the quantity of retinal and subretinal fluid, but it was ranibizumab which more often eliminated the fluid. Although there were no differences between the medicaments in mortality rates and occurrences of arterial thrombosis, there were some more serious adverse effects in the patients treated with bevacizumab (risk ratio 1.29). As none of these medicaments eliminated neovascularisation, treatment for the largest number of patients was continued over an undefined period of time. Thus, it is very important to establish long-term effects of these medicaments as well as the most appropriate dosage regimen. At the end of the two-year period of monitoring, it was concluded that both ranibizumab and bevacizumab had similar effects on visual acuity but higher rate of serious adverse effects in the patients treated with bevacizumab.
EYLEA®(aflibercept), known as “VEGF trap-eye”, is a fusion protein for VEGF which prevents VEGF binding to its foreseen aims on receptors. It was developed by Regeneron Pharmaceuticals and Bayer HealthCare firms and approved by FDA in November 2011, and by European Medicines Agency (EMA) in November 2012 for the treatment of neovascular AMD. Two large-scale random sample multi centric studies lasting 96 weeks – VEIW 1 and VIEW 2 (VEGF Trap: Investigation of Efficacy and Safety in Wet AMD) confirmed safety and efficacy of aflibercept in the treatment of exudative AMD compared with the standard treatment with ranibizumab. These studies showed that comparatively less intensive treatment regimen with aflibercept was as safe and efficient with AMD as the more intensive regimens, and as relatively intensive treatment regimen with ranibizumab (28). These trials included 2,417 patients in two separate multi centric studies conducted parallel at the centers of North America (VIEW 1 study) and at the centers of South America, Europe, Asia, and Australia (VIEW 2 study) (29). Aflibercept is a human fusion protein binding to all VEGF-A types and placental growth factor. Its administration in a 2mg dosage every four weeks during the first three months, and then every eight weeks, enables less intensive dosage regimen, less frequent intravitreal administration, and thus a smaller number of hospital admissions as its basic advantage. On the other hand, non-responders to ranibizumab and/or bevacizumab with polipoidal choroidopathy or RPE detachment - pigment epithelial detachment (PED) within exudative AMD may find their chance in aflibercept, as the medicament works on these forms of AMD as well (30). Aflibercept is generally well-tolerated and there are no obvious differences compared with ranibizumab concerning ocular and neo-ocular side effects. The most frequent ocular effects of aflibercept did not differ from other anti-VEGF medicaments, and the most common serious system side effects were typical of those which would be expected in senior population; they included lung inflammation, myocardial infarction and atrial fibrillation. Dosage intervals are based on the VIEW study regimen applying monthly units so that aflibercept is administered once per month in three dosages, and then once every two months until 12 months have elapsed. After that, it is expected to administer aflibercept when clinically indicated, i.e. as required, with a frequency from once per month to once in three months.
3. TELESCOPIC INTRA OCULAR IMPLANTS
In July 2010, FDA approved the intra ocular implant increasing the retinal image and thus improving central vision in serious AMD or Stargardt’s macular dystrophy. That enabled vision recovery even in patients with end-stage AMD which cannot be treated with any other treatment methods. It is a small, telescopic implant 4mm in size, which is implanted in the capsular bag and directs image from the damaged macula to paramacular area of the healthy retina. It was created by VisionCare Ophthalmic Technologies and it provides an increased image (2.2 x or 3 x) in the eye in which it is implanted, thus enabling close viewing, reading. The other eye is left without an implant to enable peripheral vision and orientation in space, especially important for participants in traffic. This monovision is rather difficult for patients to adapt to, but it does not limit them too much. In principle, the patients with end-stage AMD are very motivated for any possibility of vision recovery so that the skill they have to master in alternate viewing with the eye in which they have a telescopic implant and viewing with the eye without an implant is not so difficult for them; but, they do need a certain process of adaptation which individually takes days, weeks or months (31). To build-in miniature telescopic implants, the following criteria must be met: both eyes must have end-stage AMD, without a possibility to recover, mainly with already developed fibrovascular complex or geographic atrophy; they did not undergo a cataract surgery on the eye in which a telescopic implant will be built-in, but there is grade 2 cataract, or above; they have good peripheral vision, without changes on the retinal periphery; they are fully trained for the use of outer telescopic systems and they underwent complete training by a sub-specialist in low-vision; they achieve improvement of at least five letters by ETDRS scale with the support of outer telescopic systems; they have fully transparent, healthy retina, with preserved sufficient endothelial cell density, visual acuity 0.025-0.125; and they are over 75 years of age. Such cataract surgical intervention with implantation of the telescopic system in the capsular bag costs about £13,000 (32).
A combination of anti-VEGF therapy and ionizing radiation offers another option to reduce treatment frequency. Ionizing radiation causes powerful inhibitory effects on the new blood vessels, inducing interruptions in double-stranded DNA structure, which results in antiangiogenetic, antiinflammatory, and antifibrotic effects (33). Additionally, oncology trials have shown synergetic antiangiogenic effect when both radiation therapy and anti-VEGF agents are used. Although radiation was also used earlier for the treatment of AMD, it was never widely applied as it did not produce a significant effect on visual acuity, while difficulties in delivering targeted dosages led to the development of many complications in some patients.
However, new options, such as epimacular brachytherapy and precise robotic stereotactic radiation therapy, enable a safe targeted delivery of the most appropriate dosage, minimizing damage to the surrounding structures and improving results. Epimacular brachytherapy delivers targeted β radiation to CNV via 20 gauge sclerotomy after pars plana vitrectomy. In another trial covering 24 patients who knew nothing about the treatment, the subjects were treated with only one dosage of 24-Gy brachytherapy and two ranibizumab injections, followed by ranibizumab injections as required. After 12 months, great improvement of visual acuity was achieved, without any subsequent need for additional anti-VEGF therapy in as many as 70% of patients (34). However, results of the third phase of this study of safety and efficacy of epimacular brachytherapy in the treatment of neovascular AMD did not confirm superiority of this method over ranibizumab monotherapy.
4. CONCLUSION
Recent years have been the most exciting years for retinology and the treatment of neovascular AMD, with revolutionary discoveries which have completely changed the paradigm o neovascular AMD treatment. Nevertheless, despite extraordinary progress made in the treatment of neovascular AMD with anti-VAGF therapy, patients still need many injections and many follow-up visits. In the future, tendency will be to improve the efficacy of AMD treatment through orientation to additional ways of CNV development that would be at the cellular and molecular levels, which would enable durability of treatment.
An ideal medicament for the treatment of AMD should: give the best possible visual acuity; be used through the least frequent administration, preferably once and possibly topically, in which case it should have excellent penetration to the last pole; have excellent bio-usability, no side or adverse effects; be inexpensive, easily available, simple to administer in any health institution. We are still rather far away from creating all these characteristics in only one medicament, but there are many preclinical and clinical studies underway which are well on the way to find out the “ideal one”.
Abbreviations: AMD – age-related macular degeneration, VEGF - vascular endothelial growth factor, CNV - choroidal neovascularisation, AREDS - Age-Related Eye Disease Study, RPE – retinal pigment epithelium, LPC - laser photocoagulation, FDA - Food and Drug Administration, PED - pigment epithelial detachment
Footnotes
CONFLICT OF INTEREST: NONE DECLARED
REFERENCES
- 1.Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy. Five-year results from randomized clinical trials. Arch Ophthalmol. 1991;109:1109–1114. [PubMed] [Google Scholar]
- 2.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular egeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1991;109:1220–1231. doi: 10.1001/archopht.1991.01080090044025. [DOI] [PubMed] [Google Scholar]
- 3.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Arch Ophthalmol. 1993;111:1200–1209. doi: 10.1001/archopht.1993.01090090052019. [DOI] [PubMed] [Google Scholar]
- 4.Macular Photocoagulation Study Group. Laser photocoagulation for juxta-foveal choroidal neovascularisation. Five-year results from randomized clinical trials. Arch Ophthalmol. 1994;112:500–509. [PubMed] [Google Scholar]
- 5.Verteporfin in Photo-dynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization--verteporfin in photo-dynamic therapy report 2. Am J Ophthalmol. 2001;131:541–560. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- 6.Gragoudas ES, Adamis AP, Cunningham ET, Jr, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Erfurth U, Michels S, Augustin A. Perspectives on verteporfin therapy combined with intravitreal corticosteroids. Arch Ophthalmol. 2006;124:561–563. doi: 10.1001/archopht.124.4.561. [DOI] [PubMed] [Google Scholar]
- 8.Zarbin M. Should corticosteroids be considered as part of the standard care with photo-dynamic therapy? Arch Ophthalmol. 2006;124:563–571. doi: 10.1001/archopht.124.4.563. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser PK, Boyer DS, Cruess AF, Slakter JS, Pilz S, Weisberger A DENALI Study Group. Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: twelve-month results of the DENALI study. Ophthalmology. 2012;119(5):1001–1010. doi: 10.1016/j.ophtha.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Larsen M, Schmidt-Erfurth U, Lanzetta P, Wolf S, Simader C, Tokaji E, et al. MONT BLANC Study Group. Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: twelve-month MONT BLANC study results. Ophthalmology. 2012;119(5):992–1000. doi: 10.1016/j.ophtha.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T ANCHOR Study Group. Ranibizumab versus verteporfin photo-dynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Augustin AJ, Schmidt-Erfurth U. Verteporfin therapy and triamcinolone acetonide: convergent modes of action for treatment of neovascular age-related macular degeneration. Eur J Ophthalmol. 2006;16(6):824–834. doi: 10.1177/112067210601600607. [DOI] [PubMed] [Google Scholar]
- 13.Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, De Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. Br. J. Ophthalmol. 1997;81(2):154–162. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michaelson IC. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal disorders. Trans Ophthalmol Soc UK 68. 1948:137–180. [Google Scholar]
- 15.Singerman LJ, Masonson H, Patel M, Adamis AP, Buggage R, Cunningham E, et al. Pegaptanib sodium for neovascular age-related macular degeneration: third-year safety results of the VEGF Inhibition Study in Ocular Neovascularisation (VISION) trial. Br J Ophthalmol. 2008;92(12):1606–1611. doi: 10.1136/bjo.2007.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 17.Ng EW, Shima DT, Calias P, Cunningham ET, Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5(2):123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 19.Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 20.Regillo C, Brown D, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145:239–248. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Bocco MCA, Glacet-Bernard A, Zourdani A, Coscas G, Soubrane G. Intravitreous injection: retrospective study on 2028 injections and their side effects. J Fr Ophtalmol. 2008;31(7):693–698. doi: 10.1016/s0181-5512(08)74383-3. [DOI] [PubMed] [Google Scholar]
- 22.Bressler NM, Boyer DS, Williams DF, Butler S, Francom SF, Brown B, et al. Cerebrovascular accidents in patients treated for choroidal neovascularization withranibizumab in randomized controlled trials. Retina. 2012;32(9):1821–1828. doi: 10.1097/IAE.0b013e31825db6ba. [DOI] [PubMed] [Google Scholar]
- 23.Singer MA, Awh CC, Sadda S, Freeman WR, Antoszyk AN, Wong P, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119(6):1175–1183. doi: 10.1016/j.ophtha.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Rosenfeld PJ. Intravitreal Avastin: the low cost alternative to Lucentis? American Journal of Ophthalmology. 2006 Jul;142:141–143. doi: 10.1016/j.ajo.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 25.Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg lasers Imaging. 2005;36:336–339. [PubMed] [Google Scholar]
- 26.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–335. [PubMed] [Google Scholar]
- 27.CATT Research Group. Ranibizumab i bevacizumab for neovascular age-realted macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Stewart MW. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br J Ophthalmol. 2012;96:1157–1158. doi: 10.1136/bjophthalmol-2011-300654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho H, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013;97(8):1032–1035. doi: 10.1136/bjophthalmol-2013-303344. [DOI] [PubMed] [Google Scholar]
- 31.Singer MA, Amir N, Herro A, Porbandarwalla SS, Pollard J. Improving quality of life in patients with end-stage age-related macular degeneration: focus on miniature ocular implants. Clin Ophthalmol. 2012;6:33–39. doi: 10.2147/OPTH.S15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown GC, Brown MM, Lieske HB, Lieske PA, Brown KS, Lane SS. Comparative effectiveness and cost-effectiveness of the implantable miniature telescope. Ophthalmology. 2011;118(9):1834–1843. doi: 10.1016/j.ophtha.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan L, Krishnan EC, Jewell WR. Immediate effect of irradiation on microvasculature. Int J Radiat Oncol Biol Phys. 1988;15(1):147–150. doi: 10.1016/0360-3016(88)90359-8. [DOI] [PubMed] [Google Scholar]
- 34.Avila MP, Farah ME, Santos A, Carla L, Fuji G, Rossi J, et al. Three-year safety and visual acuity results of epimacular 90 strontium/90 yttrium brachytherapy with bevacizumab for the treatment of subfoveal choroidal neovascularization secondary to age-related macular degeneration. Retina. 2012;32(1):10–18. doi: 10.1097/IAE.0b013e31822528fc. [DOI] [PubMed] [Google Scholar]


