Abstract
Background
The clinical presentation and management of human metapneumovirus (hMPV) infections in immunocompromised children is not well understood.
Methods
We performed a retrospective evaluation of pediatric patients with laboratory-confirmed hMPV infections and underlying hematologic malignancy, solid tumors, solid organ transplant, rheumatologic disease, and/or receipt of chronic immunosuppressants. Data were analyzed using t tests and Fisher's exact tests.
Results
Overall, 55 patients (median age: 5 years; range: 5 months–19 years) with hMPV infection documented between 2006 and 2010 were identified, including 24 (44%) with hematologic malignancy, 9 (16%) undergoing hematopoietic stem cell transplant, 9 (16%) with solid tumors, and 8 (15%) with solid organ transplants. Three (5%) presented with fever alone, 35 (64%) presented with upper respiratory tract infections, and 16 (29%) presented with lower respiratory tract infections (LRTI). Twelve (23%) patients required intensive care unit admission and/or supplemental oxygen ≥28% FiO2. Those with severe disease were more likely to be neutropenic (P = .02), but otherwise did not differ by age (P = .27), hematopoietic stem cell transplant recipient status (P = .19), or presence of lymphopenia (P = .09). Nine (16%) patients received treatment with ribavirin, intravenous immunoglobulin, or both. Three children (5%) died of hMPV pneumonia.
Conclusions
Immunocompromised pediatric patients with hMPV infection have high rates of LRTI and mortality. The benefits of treatment with ribavirin and intravenous immunoglobulin in this patient population require further evaluation.
Keywords: human metapneumovirus, immunocompromised, pediatric, transplant, treatment
BACKGROUND
Human metapneumovirus (hMPV) is a paramyxovirus first identified in 2001 as a pathogen associated with upper and lower respiratory tract infections in children [1]. Seroprevalence studies show that almost all children acquire infection by 5 years of age, and that frequent reinfections occur through life [2, 3]. The prevalence of hMPV infection among hospitalized children with acute respiratory infection or fever is similar to that of other respiratory viruses. Clinical disease most closely resembles that associated with respiratory syncytial virus infection (RSV), a related paramyxovirus [4]. A prospective cohort study of hMPV revealed a 9.4% seroprevalence in children hospitalized with respiratory tract infections, with 63% of patients requiring supplemental oxygen and 3% requiring intensive care unit (ICU) admission [5]. Lower respiratory tract illnesses (LRTI) caused by hMPV includes bronchiolitis, pneumonia, and croup. hMPV is also associated with acute otitis media, as well as more rarely conjunctivitis, gastroenteritis, and rash [6, 7]. In adults with hematologic malignancy, hMPV is associated with high rates of progression from upper to lower respiratory tract disease and substantial mortality [8, 9].
The treatment of hMPV is mainly supportive. Animal data support the use of bronchodilators and corticosteroids, but no controlled trials have been done to assess their efficacy in human populations [10]. Ribavirin is a nucleoside analogue shown in in-vitro studies to have activity against hMPV [11]. Ribavirin is approved for use in treatment of respiratory syncytial virus (RSV), and is often used in combination with intravenous immunoglobulin (IVIG) in severely immunocompromised individuals. Few prior descriptions of the clinical management and treatment of hMPV respiratory tract infections in a pediatric immunocompromised population, including children with leukemia and solid organ transplant recipients, are available. We describe hMPV respiratory tract infections in 55 pediatric immunocompromised patients at Seattle Children's Hospital, reporting on their clinical presentation, management, and outcomes.
METHODS
Screening of all positive laboratory results for hMPV by both direct fluorescent antibody (DFA) and real-time reverse-transcriptase polymerase chain reaction (RT-qPCR) was performed to identify patients between the ages of newborn to 19 years who were diagnosed at Seattle Children's Hospital in Seattle, WA during the years 2006–2010. Of 368 total patients with positive hMPV laboratory results, we performed retrospective chart review to identify a subset of 55 patients with immunocompromised conditions. We defined an immunocompromised condition as presence of hematologic malignancy, solid tumor, rheumatologic disease receiving immunosuppressive therapy, solid organ transplant, primary immunodeficiency, receipt of chronic immunosuppressive therapy, or receipt of a hematopoietic stem cell transplant (HSCT). Using electronic chart review, we abstracted sociodemographic variables, symptoms, laboratory and radiologic values, clinical course, and treatment outcomes for these patients.
Respiratory specimens were obtained by nasal washes for patients with suspected respiratory viral infections by attending physicians, or from bronchoalveolar lavage fluid (BAL) when this was performed. DFA was performed using virus-specific mouse monoclonal antibodies (Chemicon, Temecula, CA), and RT-qPCR was performed using previously published methods at the University of Washington Virology Laboratories [12]. Viral load values were obtained on a subset of patients whose samples were tested by RT-qPCR. Estimated viral load data were calculated from cycle threshold values on RT-qPCR analysis using stored standard curve data for hMPV. Upper respiratory tract infection (URTI) was defined as hMPV documented in an upper respiratory tract specimen in a patient with compatible symptoms in the absence of radiographic or clinical evidence of pneumonia. LRTI was defined as a new pulmonary infiltrate or presence of lower respiratory tract symptoms (wheezing or hypoxia) in association with a positive lower respiratory tract specimen or a positive upper respiratory tract specimen if the patient did not undergo BAL. Neutropenia was defined as absolute neutrophil count <1000 cells/mL. Lymphopenia was defined as lymphocyte count <300 cells/mL (not age-adjusted), and severe lymphopenia as lymphocyte count <100 cells/mL.
Infections were defined as nosocomial if the patient was hospitalized within 7 days of diagnosis, and otherwise classified as community acquired. Severe disease was defined as requiring ICU stay and/or use of supplemental oxygen ≥FiO2 0.28. Mortality attributable to hMPV was defined as death due to respiratory failure from pneumonia during the hospitalization where hMPV was diagnosed, and hMPV was considered a contributor to lung injury. Data were entered into a password-protected Excel spreadsheet, and analyzed using Stata 11.0 (STATA Corp, College Station, TX). Comparison of clinical characteristics was performed using Fisher's exact tests for categorical variables and t tests with unequal variance for continuous variables. This study was approved by the Seattle Children's Hospital IRB.
RESULTS
Clinical Characteristics
Overall, 55 hMPV-infected immunocompromised patients were identified from May 29, 2006 to March 26, 2010. The characteristics of these patients are listed in Table 1. The median age was 5 years (range, 5 months–19 years), and the majority had a hematologic malignancy as an underlying condition. Of these, most had acute lymphoblastic leukemia (ALL) (n = 21; 88%). The nine solid tumors included Wilms tumor (n = 3; 33%), osteosarcoma (n = 1; 11%), and ovarian cancer (n = 1; 11%). Nine (16%) patients were undergoing HSCT. Indications for transplant included aplastic anemia (n = 4; 44%) and severe combined immunodeficiency (SCID) (n = 2; 22%). Eight (15%) patients were solid organ transplant recipients, and all were receiving immunosuppressive therapy. Eight (15%) hMPV infections were acquired nosocomially, while 46 (85%) were community acquired. Of these 46 cases, 24 (52%) patients were hospitalized for evaluation of their hMPV infection.
Table 1.
Characteristics of Immunocompromised Children With Human Metapneumovirus (HMPV) Infection. Data Are Shown as Number (n [%]) Unless Otherwise Indicated
| Characteristics | n = 5 |
|---|---|
| Age in years, median (range) | 5 (0.4–19) |
| Female sex | 26 (47) |
| Underlying condition | |
| Hematologic malignancy | 24 (44) |
| ALL | 21 (88) |
| HSCT recipient | 9 (16) |
| Pre-transplant | 3 (33) |
| Post-transplant | 6 (67) |
| Solid organ transplant | 8 (15) |
| Heart | 4 (50) |
| Kidney | 1 (13) |
| Liver | 3 (38) |
| Solid tumors | 9 (16) |
| Primary immunodeficiency | 3 (5) |
| Other | 3 (5) |
| Chemotherapy recipienta (n = 29) | 29 (100) |
| Steroid use | 12 (22) |
| Season of infection | |
| Winter (Dec–Feb) | 19 (35) |
| Spring (March–May) | 32 (58) |
| Community acquiredb | 46 (85) |
| Nosocomial | 8 (15) |
aIn patients with hematologic malignancy or solid tumors.
bDefined as outpatients at the time of hMPV diagnosis.
The overall characteristics of the hMPV illness episodes are listed in Table 2. The majority of patients presented with fever (n = 44; 80%) and/or cough (n = 42; 82%). Lymphopenia was present in 7 (13%) patients at diagnosis, and neutropenia was present in 19 (35%). Of the 35 patients who presented with URTI symptoms alone, only 2 (6%) progressed to LRTI. As compared to those presenting with fever or URTI, children presenting with LRTIs did not differ by age (P = .80) or presence of fever (P = .70), cough (P = 1.00), neutropenia (P = .21), or lymphopenia (P = .09).
Table 2.
Characteristics of Human Metapneumovirus Illness Episodes
| Number of Infections | 55 |
|---|---|
| Severe diseasea | 8 (15) |
| Neutropenia (ANC < 1000) | 19 (35) |
| Lymphopenia (ALC < 300) [n = 54] | 7 (13) |
| Severe lymphopenia (ALC < 100) [n = 54] | 5 (9) |
| Clinical symptoms | |
| Cough [n = 51] | 42 (86) |
| Fever | 44 (80) |
| Stage at presentation | |
| Asymptomatic | 1 (2) |
| Fever | 3 (5) |
| Upper respiratory tract infection | 35 (64) |
| Lower respiratory tract infection | 16 (29) |
| Abnormal chest imaging [n = 36] | 16 (44) |
| Median initial viral load in log10 copies/mL (range) [n = 6] | 6.96 (2.75–7.22) |
| Diagnostic modality | |
| Direct fluorescent antibody | 54 (98) |
| Polymerase chain reaction | 6 (11) |
| Co-infections | 12 (22) |
| Adenovirus | 3 (5) |
| Cytomegalovirus | 3 (5) |
| Epstein Barr virus | 1 (2) |
| Influenza B | 1 (2) |
| Parainfluenza | 1(2) |
| Human herpes virus | 81 (2) |
| Rhinovirus | 1 (2) |
| Staphylococcus | 3 (7) |
| Other | 3 (7) |
| Hospitalization required [n = 46]a | 24 (52) |
| Treatment received | |
| Ribavirin and intravenous immunoglobulin | 5 (9) |
| Ribavirin alone | 2 (4) |
| Intravenous immunoglobulin alone | 2 (4) |
| Receipt of antibiotics | 22 (40) |
| Intensive care unit stay [n = 52] | 6 (12) |
| Receipt of supplemental oxygen [n = 54] | 9 (17) |
| Severe disease [n = 52]b | 12 (23) |
| Death attributed to human metapneumovirus infection | 3 (5) |
aOf the 46 patients who were outpatient at the time of diagnosis.
bSevere disease is defined as intensive care unit stay and/or use of supplemental oxygen (FiO2 ≥ 0.28).
Samples tested included nasal wash (n = 43; 91%), BAL (n = 1; 2%), or both (n = 3; 6%). Diagnosis of hMPV was made by DFA alone in 49 (89%) patients, by PCR alone in 1 patient (2%), and by DFA and PCR in 5 (9%) patients. Twelve (23%) patients met criteria for severe disease, 6 (50%) of whom were admitted to the intensive care unit (ICU). Those with severe disease were more likely to be neutropenic (P = .02), but otherwise did not differ by age (P = .27), HSCT recipient status (P = .19), or presence of lymphopenia (P = .09). ICU stay alone was not associated with age (P = .59) or presence of neutropenia (P = .38), or lymphopenia (P = .54), though HSCT recipients were more likely to require ICU stay (P < .01).
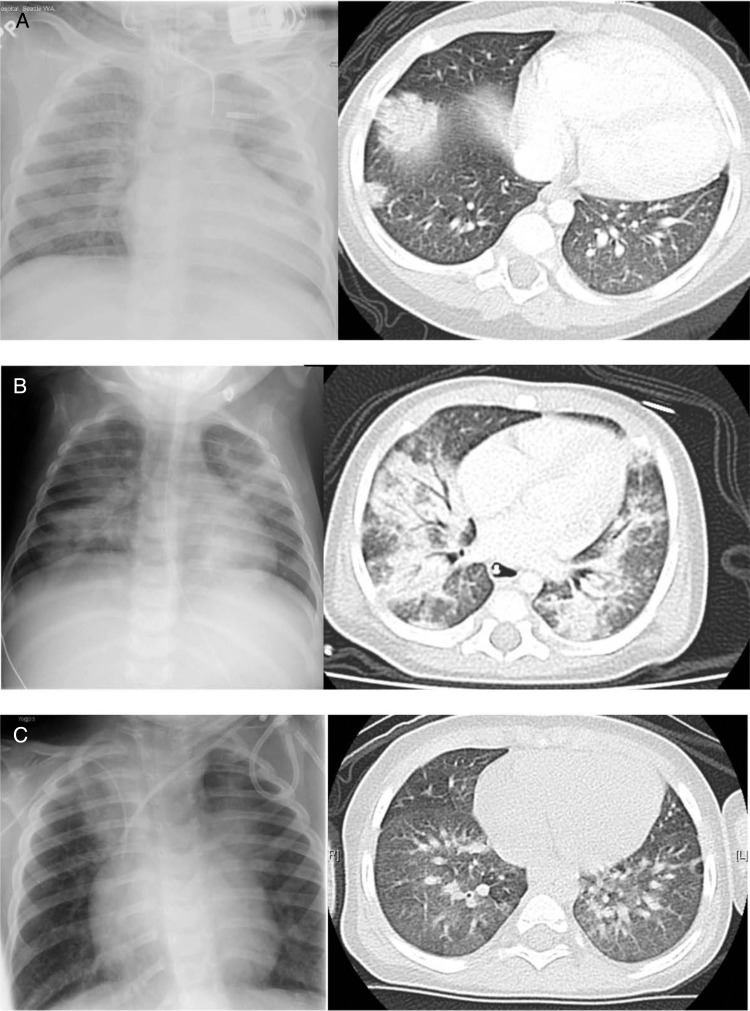
Nine (16%) patients were HSCT recipients; indications for transplant included aplastic anemia (n = 4, 44%), SCID (n = 2, 22%), Ewing sarcoma (n = 1, 11%), acute lymphoblastic leukemia (ALL) (n = 1, 11%), and osteopetrosis (n = 1, 11%). In this subset, 4 (50%) had abnormal chest imaging, and 1 (11%) required supplemental oxygen. The chest radiograph and computed tomography imaging of 3 representative patients are illustrated in Figure 1 (Patients 1, 2, and 3). Three (33%) of these patients had a diagnosis of hMPV prior to transplant, including 2 children with SCID and 1 with aplastic anemia. In general, the policy at our institution is to delay transplant if patients have respiratory tract infections; however, in these 3 cases, it was felt that due to their underlying disease and lack of functioning immune system, these patients would not be able to clear their viral infection without HSCT. Two of these transplant recipients subsequently died due to hMPV infection.
Figure 1.
Chest imaging findings of 3 patients. (A) Patient 1 is an 11-month-old boy with severe combined immunodeficiency (SCID) who survived and cleared human metapneumovirus (hMPV) 511 days after diagnosis. His chest computed tomography (CT) scan shows right lower lobe consolidation. (B) Patient 2 is a 5-month-old girl with SCID who died of respiratory failure secondary to hMPV pneumonia. She has bilateral lower lobe consolidation on chest CT. (C) Patient 3 is a 2-year-old boy with aplastic anemia who died of respiratory failure secondary to hMPV pneumonia. His chest CT shows airspace consolidation with air bronchograms.
Eight (15%) patients were solid organ transplant recipients. The median time from transplant to hMPV infection was 12 months (range, 5–57 months). None required ICU stay or supplemental oxygen. Three (50%) of the 6 who had chest imaging performed had abnormalities on chest radiograph. None received treatment with ribavirin or IVIG, and though there was 1 death, it was not attributed to hMPV infection. As compared to other immunocompromised patients, solid organ transplant recipients were no more likely to have fever (P = .19), cough (P = .62), abnormal chest imaging (P = 1.00), or to be classified as LRTI disease on initial clinical presentation (P = .35).
Virologic Characteristics of hMPV Episodes
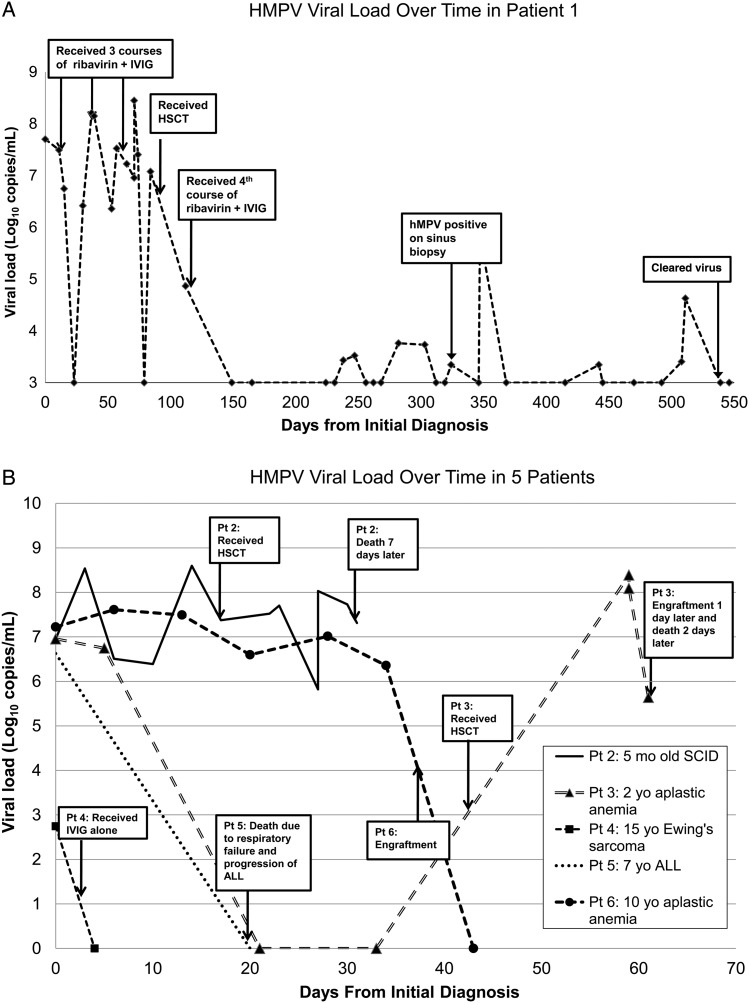
Six patients had respiratory samples collected at multiple time points. The median number of samples collected was 7 (range, 2–44 samples), and the median duration of follow-up was 37 days (range, 4–562 days). Types of samples were primarily nasal wash samples, but also included two BAL, one sinus biopsy, and one lung biopsy sample in Patient 1, as well as 1 BAL sample in Patient 2. Median initial viral load was estimated as 6.96 log10 copies/mL (range, 2.75–7.22 log10 copies/mL). The viral load data for all 6 patients are illustrated in Figures 2A (Patient 1) and 2B (Patients 2–6). In the 3 patients who died, the median time between initial and final sample was 7 days (range, 2–20 days), and the median change in viral load was 1.31 log10 copies/mL (range, −6.23 to 0.36 log10 copies/mL). For the 3 patients who survived, the median time to viral clearance was 43 days (range, 4–511 days).
Figure 2.
(A) Viral loads in Patient 1 (11-month-old with severe combined immunodeficiency) over time. IVIG, intravenous immunoglobulin; HSCT, hematopoietic stem cell transplant; hMPV, human metapneumovirus. (B) Viral loads in Patients 2 through 6 over time. ALL, acute lymphoblastic leukemia; mo, month; SCID, severe combined immunodeficiency syndrome; yo, year old.
Treatment of hMPV
Nine patients were treated for their hMPV infections (median age 9 years; range, 5 months–19 years). These patients included 5 HSCT recipients, 2 patients with hematologic malignancy, and 2 patients with solid tumors. Indications for HSCT included aplastic anemia (n = 2, 22%), SCID (n = 2, 22%), and Ewing sarcoma (n = 1, 11%).
Eight (89%) of these treated patients had fever, 6 (75%) had cough, and 7 (78%) had abnormal chest imaging. Five (56%) had a copathogen identified. Five (56%) were hospitalized in the ICU, and 5 (56%) received supplemental oxygen. Five (56%) patients received both ribavirin and IVIG, 2 (22%) received ribavirin alone, and 2 (22%) received IVIG alone.
Of the 5 HSCT recipients, 3 (60%) had hMPV detected prior to transplant (median time prior to transplant: 43 days; range, 93–21 days), and 1 detected 11 days after transplant. Treatment with ribavirin and IVIG was initiated a median of 5 days after hMPV diagnosis (range, 2–46 days), and several patients received multiple courses of ribavirin and IVIG. Treatment with ribavirin alone (n = 2) was initiated 2 and 4 days after diagnosis, respectively. Of the 7 patients who received ribavirin, 6 (86%) received inhaled ribavirin at a dose of 2 g 3 times daily for a 5-day course, and 1 (14%) received an 11-day course of intravenous ribavirin.
Mortality Attributed to hMPV
Three patients (5%) died of respiratory failure related to hMPV pneumonia. Two of these deaths occurred in HSCT recipients who were diagnosed with hMPV prior to transplant (Figures 1 and 2B: Patients 2 and 3). One HSCT recipient had not engrafted at time of death, while the other engrafted the day prior to death. Both were treated with ribavirin and IVIG. The third death occurred in a patient with ALL and secondary acute myelogenous leukemia on salvage chemotherapy who did not receive hMPV-specific treatment (Figure 2B: Patient 5). HMPV was considered to be a contributing factor to her death, though the primary cause of death was thought to be progression of ALL. The median time to death was 37 days (range, 37–64 days).
CONCLUSIONS
Human metapneumovirus infections in our immunocompromised patient population was associated with high rates of LRTI at presentation, and an attributable mortality of 5%, rates substantially higher than that in the general population where hMPV is most commonly a self-limited URTI. In our study, 12 (23%) of immunocompromised children with hMPV were classified as having severe disease, and neutropenia was identified as a significant risk factor for disease severity. This rate is comparable or even higher than previous rates reported in seriously immunocompromised adult patients [13].
Similar retrospective studies performed in pediatric cancer patients have looked at morbidity and mortality related to other respiratory viruses. Parainfluenza virus was associated with LRTI in 20–36% of patients with a mortality rate of 0–7% in a mixed population of pediatric cancer and transplant patients [14, 15]. Similarly, respiratory syncytial virus (RSV) has been associated with LRTI in 28% and mortality in 8% of a mixed group of pediatric and transplant patients [16]. More recently, pandemic H1N1 and seasonal influenza A were associated with LRTI rates of 20–42% and 4–27% and mortality rates of 10–12% and 2–4%, respectively, despite treatment of the majority of patients with antiviral drugs [17, 18]. An additional study performed in a larger group of patients described a lower rate of LRTI (16%) associated with pandemic H1N1 with only 1 severe case, with no related mortality [19]. Prospective studies of hMPV in adults with hematologic malignancies demonstrated that 9% of respiratory tract infections were attributed to hMPV, and that the majority of these were in HSCT recipients [13]. Among HSCT recipients, hMPV was associated with 19% mortality.
Our study is one of the first to describe the presentation and management of symptomatic hMPV infection in immunocompromised children. The presentation of hMPV in pediatric solid organ transplant recipients was shown to be similar to patients with other immunocompromising conditions, with fever and cough as the most common symptoms. We also show that the majority of immunocompromised patients diagnosed with hMPV are subsequently admitted to the hospital for further evaluation, but that only 2% of those initially presenting with URTI symptoms go on to develop LRTI. However, there were no clear differences in the presentation of patients with URTI or LRTI in terms of age, symptoms (fever or cough), laboratory abnormalities, or underlying condition that would clearly identify risk factors for LRTI disease and guide decision-making. Interestingly, 2 of the 3 patient deaths were in patients with hMPV pneumonia diagnosed prior to HSCT. Both of these patients, however, had underlying diseases (aplastic anemia and SCID), which made it extremely unlikely that they would be able to clear their viral infection without reconstitution of their immune system through HSCT. These patients both underwent HSCT, and subsequently died of hMPV pneumonia despite use of ribavirin and IVIG and supportive care including mechanical ventilation and ICU stay. These 2 patients continued to have detectable virus within days of death.
At our institution as well as other transplant centers, all pediatric HSCT recipients are screened by respiratory viral PCR prior to transplant. Pretransplant screening for RSV and delay of transplant in adults is effective in reducing rates of pneumonia [20]. Our policy in pediatric patients is to delay transplant if patients have evidence of infection with RSV, influenza, adenovirus, or hMPV, even if patients are asymptomatic. Transplantation is not generally delayed for asymptomatic shedding of rhinovirus, bocavirus, or coronavirus. This study further enforces the risks associated with transplantation during documented hMPV viral shedding or disease. Because infection may result in such serious disease, potential modalities to treat or at least stabilize patients while white blood cells and/or immune function can return are considered and utilized in these patients. Treatment with IVIG and ribavirin is currently being utilized at our institution for severely immunocompromised individuals [21, 22]. Among the patients who received treatment, we demonstrated a 22% mortality rate as compared to a 2% mortality rate in untreated individuals. This, however, is almost certainly a reflection of clinical decision-making to initiate treatment in individuals with more severe disease.
In this retrospective study, we identified hMPV-infected immunocompromised children through screening of laboratory results from our virology laboratory. We did not screen asymptomatic children and may not have captured complete data on children who were evaluated solely as outpatients at other private clinics or hospitalized in other institutions. As the regional pediatric referral center, most immunocompromised patients are seen and followed at our institution but because of our large geographic referral area, it is possible that some children were seen elsewhere. Therefore, our study could be biased towards detection of more severe hMPV disease. Also, it is possible that a subset of earlier hMPV-positive cases were missed through use of DFA as compared to PCR, a more sensitive detection technique [12]. Furthermore, we were unable to capture all data at the time of clinical presentation, or to obtain full information regarding the clinical decision-making for the administration of ribavirin and/or IVIG. Therefore, the comparison of outcomes in patients who did and did not receive treatment is problematic, and the potential benefits of therapy are not readily evaluable. Unlike a previous hMPV study conducted in adult HSCT recipient patients in Italy [23], we did not assess hMPV shedding in asymptomatic patients and unfortunately cannot comment on rates of symptomatic versus asymptomatic shedding of hMPV in our patients. Finally, we were limited in our ability to classify disease severity based on hypoxemia, as some patients did not have oxygen saturations charted. We suspect that a higher proportion of our patients would be classified as having severe disease using criteria such as that used by Papenburg et al [5]. Although only 8 (15%) of our patients were identified as having acquired their hMPV infection nosocomially, the vast majority of the patients were seen frequently on an outpatient basis in clinic for blood draws, interventions, and chemotherapy, making it difficult to differentiate true community-acquired disease from that acquired in the hospital setting.
A prospective randomized study of ribavirin and IVIG administration, or of newer therapeutic agents under development for treatment of paramyxovirus infections [24, 25], would be helpful given the high morbidity associated with hMPV in the immunocompromised population. In particular, a study of the use of novel agents, including fusion inhibitors [26], could be studied in this population given their potential to serve as postexposure prophylaxis in high-risk individuals. The documentation of severe hMPV disease in immunocompromised children supports previous data documenting severe and fatal hMPV disease reported in adult HSCT recipients [9, 13] and highlights the need for treatment options in this population.
Acknowledgments
Financial support. This work was supported by the National Institutes of Health (1K23AI103105 to H.Y.C.).
Potential conflicts of interest. J.A.E. has received research support from Roche, Gilead, GlaxoSmithKline, and Chimerix and served as a consultant to GlaxoSmithKline. All other authors declare no conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. C.R. and J.A.E. jointly conceived the study. E.F., H.Y.C., and C.R. performed the chart review. J.K. provided the virologic data. H.Y.C. performed the data analysis and wrote the manuscript. All authors discussed the results and implications and commented on the manuscript at all stages.
Prior presentation of results. These results have been presented in part at the International Respiratory Syncytial Virus Symposium in Santa Fe, New Mexico in 2012.
References
- 1.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nature Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebihara T, Endo R, Kikuta H, Ishiguro N, Ishiko H, Kobayashi K. Comparison of the seroprevalence of human metapneumovirus and human respiratory syncytial virus. J Med Virol. 2004;72:304–6. doi: 10.1002/jmv.10572. [DOI] [PubMed] [Google Scholar]
- 3.Williams JV, Wang CK, Yang CF, et al. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387–95. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams JV, Edwards KM, Weinberg GA, et al. Population based incidence of human metapneumovirus infection among hospitalized children. J Infect Dis. 2010;201:1890–8. doi: 10.1086/652782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papenburg J, Hamelin ME, Ouhoummane N, et al. Comparison of risk factors for human metapneumovirus and respiratory syncytial virus disease severity in young children. J Infect Dis. 2012;206:178–89. doi: 10.1093/infdis/jis333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams JV, Tollefson SJ, Nair S, Chonmaitree T. Association of human metapneumovirus with acute otitis media. Int J Pediatr Otorhinolaryngol. 2006;70:1189–93. doi: 10.1016/j.ijporl.2005.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Hoogen BG, Osterhaus DM, Fouchier RA. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J. 2004;23:S25–32. doi: 10.1097/01.inf.0000108190.09824.e8. [DOI] [PubMed] [Google Scholar]
- 8.Renaud C, Campbell AP. Changing epidemiology of respiratory viral infections in hematopoietic cell transplant recipients and solid organ transplant recipients. Curr Opin Infect Dis. 2011;24:333–43. doi: 10.1097/QCO.0b013e3283480440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144:344–9. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 10.Hamelin ME, Prince GA, Boivin G. Effect of ribavirin and glucocorticoid treatment in a mouse model of human metapneumovirus infection. Antimicrob Agents Chemother. 2006;50:774–7. doi: 10.1128/AAC.50.2.774-777.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wyde PR, Chetty SN, Jewell AM, Boivin G, Piedra PA. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antiviral Res. 2003;60:51–9. doi: 10.1016/s0166-3542(03)00153-0. [DOI] [PubMed] [Google Scholar]
- 12.Kuypers J, Wright N, Ferrenberg J, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44:2382–8. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams JV, Martino R, Rabella N, et al. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis. 2005;192:1061–5. doi: 10.1086/432732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeng SH, Yoo HS, Choi SH, et al. Impact of parainfluenza virus infection in pediatric cancer patients. Pediatr Blood Cancer. 2012;59:708–10. doi: 10.1002/pbc.23390. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan A, Wang C, Yang J, et al. Parainfluenza virus infections in children with hematologic malignancies. Pediatr Infect Dis J. 2011;30:855–9. doi: 10.1097/INF.0b013e31821d190f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Saleeby CM, Somes GW, DeVincenzo JP, Gaur AH. Risk factors for severe respiratory syncytial virus disease in children with cancer: the importance of lymphopenia and young age. Pediatrics. 2008;121:235–43. doi: 10.1542/peds.2007-1102. [DOI] [PubMed] [Google Scholar]
- 17.Paganini H, Parra A, Ruvinsky S, et al. Clinical features and outcome of 2009 influenza A (H1N1) virus infections in children with malignant diseases: a casecontrol study. J Pediatr Hematol Oncol. 2011;33:e5–8. doi: 10.1097/MPH.0b013e3181f73f68. [DOI] [PubMed] [Google Scholar]
- 18.Shah DP, El Taoum KK, Shah JN, et al. Characteristics and outcomes of pandemic 2009/H1N1 versus seasonal influenza in children with cancer. Pediatr Infect Dis J. 2012;31:373–8. doi: 10.1097/INF.0b013e3182481ef8. [DOI] [PubMed] [Google Scholar]
- 19.Caselli D, Carraro F, Castagnola E, et al. Morbidity of pandemic H1N1 influenza in children with cancer. Pediatr Blood Cancer. 2010;55:226–8. doi: 10.1002/pbc.22619. [DOI] [PubMed] [Google Scholar]
- 20.Peck AJ, Corey L, Boeckh M. Pretransplantation respiratory syncytial virus infection: impact of a strategy to delay transplantation. Clin Infect Dis. 2004;39:673–80. doi: 10.1086/422994. [DOI] [PubMed] [Google Scholar]
- 21.Kitanovski L, Kopriva S, Pokorn M, et al. Treatment of severe human metapneumovirus (hMPV) pneumonia in an immunocompromised child with oral ribavirin and IVIG. J Pediatr Hematol Oncol. 2013;35:e311–3. doi: 10.1097/MPH.0b013e3182915d2d. [DOI] [PubMed] [Google Scholar]
- 22.Shahda S, Carlos WG, Kiel PJ, Khan BA, Hage CA. The human metapneumovirus: a case series and review of the literature. Transpl Infect Dis. 2011;13:324–8. doi: 10.1111/j.1399-3062.2010.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Debiaggi M, Canducci F, Sampaolo M, et al. Persistent symptomless human metapneumovirus infection in hematopoietic stem cell transplant recipients. J Infect Dis. 2006;194:474–8. doi: 10.1086/505881. [DOI] [PubMed] [Google Scholar]
- 24.Empey KM, Peebles RS, Jr, Kolls JK. Pharmacologic advances in the treatment and prevention of respiratory syncytial virus. Clin Infect Dis. 2010;50:1258–67. doi: 10.1086/651603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuster JE, Cox RG, Hastings AK, et al. A broadly neutralizing human monoclonal antibody exhibits in vivo efficacy against both human metapneumovirus and respiratory syncytial virus. J Infect Dis. 2014 doi: 10.1093/infdis/jiu307. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feuillet F, Lina B, Rosa-Calatrava M, Boivin G. Ten years of human metapneumovirus research. J Clin Virol. 2012;53:97–105. doi: 10.1016/j.jcv.2011.10.002. [DOI] [PubMed] [Google Scholar]