Abstract
This paper reports first DNA C‐values for 28 angiosperm genera. These include first DNA C‐values for 25 families, of which 16 are monocots. Overall familial representation is 47·2 % for angiosperms, but is now much higher for monocots (75 %) and basal angiosperms (73·1 %) than for eudicots (38·7 %). Chromosome counts are reported for 22 taxa, including first records for six genera plus seven species. Unrepresented families will become increasingly enriched for monotypic taxa from obscure locations that are harder to access. Thus, completing familial representation for genome size for angiosperms may prove impossible in any short period, and progress towards this goal will become slower.
Key words: Nuclear DNA amounts, DNA C‐values, angiosperm families, chromosome numbers, monocots, genome size
INTRODUCTION
Within angiosperms, DNA C‐values (corresponding to the DNA amount in an unreplicated gametic nucleus) range about 1000‐fold from approx. 0·1 to 127·4 pg (Bennett et al., 2000). In recent years there has been increased interest in the causes and consequences of this huge range, with new research providing intriguing insights into the possible mechanisms that generate it (Vicient et al., 1999; Kirik et al., 2000; Shirasu et al., 2000; Bensasson et al., 2001; Petrov, 2001). However, available C‐value data for angiosperms (approx. 3500 species) still correspond to only approx. 1·4 % of an estimated 250 000 species.
The need to identify major gaps in C‐value data and to recommend targets for new work to fill them by international collaboration was confirmed at the Angiosperm Genome Size Workshop, held at the Royal Botanic Gardens, Kew (RBG, Kew) in 1997. C‐values were then still unavailable for 68 % of angiosperm families recognized by the Angiosperm Phylogeny Group (APG) classification (APG, 1998). Consequently, a goal of complete familial representation by 2002 was agreed. However, Bennett et al. (2000) noted that in a sixth supplementary list of C‐values for 691 species published or communicated since 1997, only 12 were also first estimates for families. As progress towards the goal of completing familial coverage was disappointing, new work to correct this was begun at RBG, Kew in 1999. Thus in 2001, Hanson et al. (2001a, b) reported first DNA C‐values for 50 angiosperm families determined in the new project, and another targeted study recently added first C‐values for a further five families in the basal angiosperms (Leitch and Hanson, 2002). Nevertheless, massive gaps still exist in our knowledge of C‐values, and over 50 % of angiosperm families still have no reported C‐value. The very size of this problem can be daunting and can act as a barrier to progress. In such cases it is useful to build on strengths and break the task into manageable parts. This approach was adopted when RBG, Kew recently elected to make monocots (which constitute 20 % of angiosperms) the key focus for work to complete a checklist for such species by 2007, and to link monocot species’ names to the wide range of information available in one seamless database. As part of this strategy, the general target of achieving full familial representation for angiosperms, set at the 1997 workshop, now has a particular goal of achieving this for monocots as our key focus. The prime aim of the present work was therefore to obtain first DNA C‐values for a further 25 families but with a particular emphasis on monocots.
MATERIALS AND METHODS
Plant material
Table 1 lists the 28 perennial species studied in the present work and gives their origin, source and reference data. Following the familial circumscriptions of the Angiosperm Phylogeny Group (APG II, 2003), 25 of the species are from families (Table 2) for which no DNA C‐values were previously included in the Angiosperm DNA C‐values database (Bennett and Leitch, 2001) or in Hanson et al. (2001a, b). The remaining three (Aphyllanthes monspeliensis, Triteleia laxa and Xanthorrhoea preisii) were also originally selected as representing families for which no published DNA C‐value was previously known, namely Aphyllanthaceae, Themidaceae and Xanthorrhoeaceae (APG, 1998). However, a new circumscription of families by the APG (APG II, 2003) has meant that Themidaceae and Aphyllanthaceae, which were previously recognized as separate families, are sunk together with five others into a newly circumscribed Asparagaceae. The circumscription of Xanthorrhoeaceae has also changed: in the classification of the APG (1998) the family contained just a single genus Xanthorrhoea, but recent phylogenetic studies have recognized that Xanthorrhoeaceae, together with Asphodelaceae and Hemerocallidaceae, form a strongly supported monophyletic group, so the new circumscription of Xanthorrhoeaceae also includes these other two families (APG II, 2003). As C‐value data were already available for genera in Asphodelaceae and Hemerocallidaceae, Xanthorrhoeaceae is no longer an unrepresented family. Nevertheless, these materials are included in Table 2 as they represent first DNA C‐value estimates for genera, if not for families.
Table 1.
Geographical region of origin, source of experimental material, RBG Kew identity number (ID no.), cytology number (Cyt. no.) and identification status for the 28 species studied in the present work
| Entry no. | Taxon | Origin | Source of material‡ | ID no. | Cyt. no. | Identification status† |
| Monocots | ||||||
| Alismatales | ||||||
| 1 | Zostera marina L. | Europe, Australia, New Guinea | RBG,K | – | 01‐136 | b |
| Asparagales | ||||||
| 2 | Aphyllanthes monspeliensis L. | Portugal to Italy, N. Africa | RBG,K | 1990‐2610 | 01‐145 | a |
| 3 | Triteleia laxa Benth. | West and North America | RBG,K | 1960‐67034 | 01‐146 | a |
| 4 | Astelia fragrans Colenso | Mascarenes, New Guinea, Australia, New Zealand, Polynesia to Hawaii and Chile | RBG,K | 1989‐2690 | 01‐176 | c |
| 5 | Blandfordia punicea Sweet. | E. Australia | RBG,K | 2000‐3965 | 01‐121 | c |
| 6 | Doryanthes palmeri W. Hill ex Benth. | E. Australia | RBG,K | 1948‐60704 | 01‐175 | a |
| 7 | Ixiolirion ledebourii Fisch. & Mey. | West and central Asia | RBG,K | 1989‐3153 | 01‐126 | c |
| 8 | Odontostomum hartwegii Torr. | California | RBG,K | 1987‐8225 | 01‐135 | d |
| 9 | Xanthorrhoea preisii Endl. | Australia | RBG,K | 1990‐2825 | 01‐127 | c |
| 10 | Xeronema callistemon W. R. B. Oliv. | New Caledonia and north New Zealand | RBG,K | 1974‐1783 | 01‐150 | c |
| Dioscoreales | ||||||
| 11 | Narthecium ossifragum Huds. | Northern temperate Europe | JSB | 2001‐4165 | 01‐161 | b* |
| Liliales | ||||||
| 12 | Lapageria rosea Ruiz & Pav. | Chile and Argentina | RBG,K | – | 01‐169 | c |
| 13 | Ripogonum papuanum C. T. White | New Guinea, Australia, New Zealand | RBG,K | 1987‐8058 | 01‐216 | c |
| Commelinoids | ||||||
| 14 | Dasypogon hookeri Drumm. | SW Australia | RBG,K | – | 01‐134 | c |
| 15 | Hanguana malayana Merrill | Sri Lanka, SE Asia and Malaysia | RBG,K | 1998‐1475 | 00‐23 | c |
| Poales | ||||||
| 16 | Eriocaulon aquaticum Druce | Tropical and warm areas | RBG,K | 1998‐3616 | 01‐151 | d |
| 17 | Flagellaria guineensis Schum. | Old world tropics | CBG | 2002‐747 | 02‐73 | c |
| 18 | Rhodocoma gigantea (Kunth) H. P. Linder | SW and East Cape | RBG,K | 1996‐2437 | 01‐167 | c |
| 19 | Xyris gracilis R.Br. ssp. gracilis§ | Australia and Africa | RBG,K | 1984‐2761 | 01‐147 | b* |
| Core eudicots | ||||||
| 20 | Buxus sempervirens L. | W. Europe, Mediterranean to S. Africa, temp. E. Asia, W. Indies and Central America | SP | – | 02‐14 | b* |
| 21 | Trochodendron aralioides Siebold & Zucc. | Korea and Japan to Taiwan | RBG,K | 2000‐100 | 02‐82 | c |
| Higher eudicots | ||||||
| Ericales | ||||||
| 22 | Myrsine africana L. | Azores, Africa, Asia | SS | – | 01‐130 | b* |
| 23 | Planchonella eerwah (F. M. Bailey) van Royen | Trop. America, Asia to Pacific and Africa | RBG,K | 1986‐2961 | 01‐139 | c |
| 24 | Pterostyrax psilophylla Diels ex Perkins | Burma to Japan | RBG,K | 1999‐4201 | 01‐154 | c |
| Euasterid I | ||||||
| 25 | Merrilliodendron megacarpum (Hemsl.) Sleum. | Philippines and W. Pacific | RBG,K | 1990‐1136 | 02‐15 | c |
| Garryales | ||||||
| 26 | Garrya fremontii Torr. | Washington to Panama and W. Indies | RBG,K | 1998‐2069 | 02‐74 | c |
| Solanales | ||||||
| 27 | Montinia caryophyllacea Thunb. | S. Africa | SS | – | 01‐132 | b* |
| Euasterid II | ||||||
| 28 | Escallonia rubra Pers. | S. America, especially around the Andes | RBG,K | 2000‐2609 | 01‐158 | c |
† Identification information: a, taxonomically verified and herbarium voucher prepared for species; b, no herbarium voucher, but species has been taxonomically verified; b*, species taxonomically verified, and is currently being grown on at RBG, Kew to prepare a herbarium voucher; c, species not taxonomically verified, but is currently being grown on at RBG, Kew to prepare a herbarium voucher; d, herbarium voucher prepared, but species has not been taxonomically verified.
‡ Plant material obtained from RBG, Kew (RBG,K), Cambridge Botanic Gardens (CBG), Silverhill Seeds, S. Africa (SS), Syon Park Garden Centre (SP) or John Shipton Bulbs, Wales (JSB).
§ Authority of species not known or unclear to present authors.
Table 2.
Chromosome number (2n), ploidy level (x), replicated genome size and nuclear DNA contents, calibration standard species and method used to estimate DNA C‐values in 28 species from 25 families unrepresented in the Angiosperm DNA C‐values database
| Entry no. | Taxon | Family | 2n† | Ploidy level (x) | Genome size: 4C DNA amount/ploidy level (pg) | 4C DNA amount ± s.d. (pg) | 1C DNA amount (Mbp)§ | Calibration standard species‡ | Method¶ |
| Monocots | |||||||||
| Alismatales | |||||||||
| 1 | Zostera marina | Zosteraceae | 12* | 2 | 0·63 | 1·26 ± 0·08 | 309 | Vigna | Fe |
| Asparagales | |||||||||
| 2 | Aphyllanthes monspeliensis | Asparagaceae | ∼32 | ? | – | 2·59 ± 0·04 | 635 | Vigna | FC |
| 3 | Triteleia laxa | Asparagaceae | 28* | 4 | 10·65 | 42·59 ± 0·42 | 10 435 | Allium | FC |
| 4 | Astelia fragrans | Asteliaceae | ∼60 | 8 | 0·63 | 5·06 ± 0·02 | 1240 | Oryza | FC |
| 5 | Blandfordia punicea | Blandfordiaceae | 68 | 4 | 8·13 | 32·53 ± 1·57 | 7970 | Pisum | Fe |
| 6 | Doryanthes palmeri | Doryanthaceae | 48 | ? | – | 13·22 ± 0·04 | 3239 | Pisum | FC |
| 7 | Ixiolirion ledebourii | Ixioliriaceae | ∼24 | 2 | 2·03 | 4·06 ± 0·31 | 995 | Vigna | Fe |
| 8 | Odontostomum hartwegii | Techophilaeaceae | 20 | 2 | 5·12 | 10·23 ± 0·09 | 2506 | Pisum | FC |
| 9 | Xanthorrhoea preissii | Xanthorrhoeaceae | 22 | 2 | 2·07 | 4·14 ± 0·31 | 1014 | Vigna | Fe |
| 10 | Xeronema callistemon | Xeronemataceae | 34 | 2 or 4 | – | 13·10 ± 0·06 | 3210 | Pisum | FC |
| Dioscoreales | |||||||||
| 11 | Narthecium ossifragum | Nartheciaceae | 26* | 2 | 0·83 | 1·65 ± 0·03 | 404 | Vigna | FC |
| Liliales | |||||||||
| 12 | Lapageria rosea | Philesiaceae | 30 + 1B | 2 | 13·56 | 27·12 ± 1·95 | 6644 | Pisum | Fe |
| 13 | Ripogonum papuanum | Ripogonaceae | 30 | 2 | 22·29 | 44·58 ± 2·87 | 10 922 | Pisum | Fe |
| Commelinoids | |||||||||
| 14 | Dasypogon hookeri | Dasypogonaceae | 14 | 2 | 0·87 | 1·74 ± 0·01 | 426 | Vigna | FC |
| 15 | Hanguana malayana | Hanguanaceae | ∼170 | ? | – | 6·58 ± 0·65 | 1612 | Hordeum | Fe |
| Poales | |||||||||
| 16 | Eriocaulon aquaticum | Eriocaulaceae | 32 | 4 | 4·19 | 16·74 ± 0·14 | 4101 | Pisum | FC |
| 17 | Flagellaria guineensis | Flagellariaceae | 38* | 2 | 1·80 | 3·59 ± 0·03 | 880 | Oryza | FC |
| 18 | Rhodocoma gigantea | Restionaceae | – | – | – | 2·97 ± 0·04 | 728 | Vigna | FC |
| 19 | Xyris gracilis ssp. gracilis | Xyridaceae | 26* | 2 | 14·02 | 28·03 ± 0·44 | 6867 | Allium | FC |
| Core eudicots | |||||||||
| 20 | Buxus sempervirens | Buxaceae | 28 | 2 or 4 | – | 3·24 ± 0·01 | 794 | Oryza | FC |
| 21 | Trochodendron aralioides | Trochodendraceae | 38 | 2 | 3·82 | 7·64 ± 0·02 | 1872 | Solanum | FC |
| Higher eudicots | |||||||||
| Ericales | |||||||||
| 22 | Myrsine africana | Myrsinaceae | 46 | ? | – | 4·92 ± 0·25 | 1205 | Vigna | Fe |
| 23 | Planchonella eerwah | Sapotaceae | ∼24 | 2 | 1·08 | 2·15 ± 0·15 | 527 | Vigna | Fe |
| 24 | Pterostyrax psilophylla | Styracaceae | 24 | 2 | 1·77 | 3·54 ± 0·32 | 867 | Vigna | Fe |
| Euasterid I | |||||||||
| 25 | Merrilliodendron megacarpum | Icacinaceae | 30 | 2 | 2·19 | 4·37 ± 0·07 | 1071 | Oryza | FC |
| Garryales | |||||||||
| 26 | Garrya fremontii | Garryaceae | ∼20 | 2 | 3·04 | 6·08 ± 0·05 | 1490 | Solanum | FC |
| Solanales | |||||||||
| 27 | Montinia caryophyllacea | Montiniaceae | 24 | 2 | 1·13 | 2·26 ± 0·18 | 554 | Vigna | Fe |
| Euasterid II | |||||||||
| 28 | Escallonia rubra | Escalloniaceae | 24 | 2 | 0·85 | 1·69 ± 0·22 | 414 | Vigna | Fe |
† Chromosome numbers labelled with an asterisk were taken from literature, all others were determined for the present work.
‡ Calibration standard used: Oryza, Oryza sativa IR36, 4C = 2·02 pg; Vigna, Vigna radiata ‘Berken’, 4C = 2·12 pg; Solanum, Solanum lycopsersicum ‘Gardener’s Delight’, 4C = 4·00 pg; Pisum, Pisum sativum ‘Minerva Maple’, 4C = 19·46 pg; Hordeum, Hordeum vulgare ‘Sultan’, 4C = 22·24 pg; Allium, Allium cepa ‘Ailsa Craig’, 4C = 67·1 pg.
§ 1 pg = 980 Mbp.
¶ Fe, Feulgen microdensitometry; FC, flow cytometry.
The present sample, whilst highly diverse, is focused on four groups that are of particular current interest to RBG, Kew, but notably on monocots. Whereas monocots constitute approx. 20 % of angiosperm species, they form 68 % of the present sample (19 species), and provide 64 % (16 species) of the first values for 25 angiosperm families. A second focus is geographical, with over a third (42 %) of the species being from south‐east Asia and Australasia (mainly Australia). The third focus is on species of economic utility in a broad sense: wood of Buxus sempervirens (common box) is used to make rulers, musical instruments and croquet balls; dried leaves of Zostera marina are used for matting; stems of Flagellaria guineensis are used for basketry and fish traps; resin of Xanthorrhoea preissii is used to varnish or lacquer metals; and Narthecium ossifragum (bog asphodel) has been used as a substitute for saffron in Scotland. Cultivated garden ornamentals include Astelia fragrans, Blandfordia punicea, Triteleia laxa, Xyris gracilis, Myrsine africana, Pterostyrax psilophylla and Trochodendron aralioides. Other species of horticultural interest include the popular garden plants Buxus sempervirens and Escallonia rubra that are used as hedge plants. A fourth focus concerns conservation status: the Queensland rainforest tree Planchonella eerwah, which was not seen from the time of its naming in 1894 until its rediscovery in 1980, is endangered; Pterostyrax psilophylla is thought to be vulnerable in the wild; and Blandfordia punicea (whose flowers were found in the gut of the first emu shot in Australia in 1788) is protected in the wild (Mabberley, 1997). These foci are not mutually exclusive, and many materials were chosen to contribute to more than one. However, their prime interest is as previously unrepresented families and genera to provide useful further additions to the Angiosperm DNA C‐values database.
Growth of plants
Actively growing root tips or young leaves were collected from established plants that were either potted or grown in beds (all sources listed in Table 1). Myrsine africana and Montinia caryophyllacea were grown from seed. Prior to germination, seeds were sterilized, stratified and scarified as necessary, and placed on 1 % agar in a Petri dish.
Estimating total nuclear DNA C‐values
DNA C‐values of the test species were estimated using either flow cytometry or Feulgen microdensitometry. The method used for each taxon is shown in Table 2 together with the calibration standard used. Several different calibration standards were used to cover the range of 4C‐values encountered. The 4C‐values used to convert arbitrary units into absolute values were taken from Bennett and Leitch (1995) except for Solanum lycopersicum L. ‘Gardener’s Delight’ (4C = 4·00 pg), which was determined by Obermayer et al. (2002).
Flow cytometry.
Young healthy leaf tissue was collected from the test species and calibration standard, and was co‐chopped in isolation buffer. The solution was filtered through a 30‐µm nylon mesh, then digested with RNase and stained with the non‐base specific DNA stain propidium iodide, as described in Obermayer and Greilhuber (1999). Samples for 16 test species were analysed on a Partec PA II flow cytometer with a 100 W high pressure mercury lamp, a ×40 gel objective and a high‐quality red sensitive photo‐multiplier. The optical bench set‐up and filter types are described in Obermayer et al. (2002). The linearity of the machine was checked on a regular basis using chicken red blood cells. For each test species, three preparations of unknown and standard material were usually made and each was analysed five times (5000 nuclei per run). Coefficients of variation (CVs) were usually less than 3 %, otherwise the number of preparations was increased. Absolute 4C DNA values were calculated using the following formula: mean peak ratio × 4C‐value of calibration standard used.
Feulgen microdensitometry.
DNA C‐values for 12 test species were estimated using a Vickers M85a microdensitometer using the methods described in Hanson et al. (2001a).
Chromosome counts
Chromosome counts were obtained using a standard root tip squash technique as previously described in Hanson et al. (2001a). Photographs of metaphase cells were taken on a Zeiss Photomicroscope III using Pan F film. Microscope preparations and photographs are stored at the Jodrell Laboratory, RBG, Kew. In five instances when it was not possible to obtain chromosome counts from living material, chromosome counts were taken either from Fedorov (1969) or from the ‘Indexes to plant chromosome numbers’ series published by the Missouri Botanical Garden (Goldblatt and Johnson, 2002).
RESULTS
Nuclear DNA amounts
Table 2 gives 4C DNA amounts estimated for the 28 taxa studied; these ranged from 1·26 pg in Zostera marina (Zosteraceae) to 44·58 pg in Ripogonum papuanum (Ripogonaceae). Thus, these 4C values differed 35‐fold, which is a narrow range restricted to the lowest 9 % of the approx. 1000‐fold variation known for angiosperms as a whole. Moreover, the mean 4C DNA amount of the present sample (10·63 pg) is also low compared with that for the 3543 species (24·97 pg) in the Angiosperm DNA C‐values database (Bennett and Leitch, 2001; Hanson et al., 2001a, b). The mean 4C value for 17 diploids (9·07 pg) was significantly lower (P = 0·05) than that for four polyploids (24·23 pg) (NB seven species were excluded from this comparison as their ploidy level was unknown or unclear). Further analysis showed that replicated mean genome size (calculated as 4C DNA value/ploidy level) in 17 diploids (4·54 pg) was smaller than that in four polyploids (5·90 pg), but not significantly so (P = 0·68).
The prime focus of the present work was monocots. The mean 4C‐value for the 19 monocot species (13·78 pg) was significantly larger (P = 0·01) than that for nine eudicot species (3·99 pg). Similarly, replicated mean genome size (2C) in 14 monocots (6·20 pg) was larger than that in seven eudicots (1·98 pg), and significantly so (P = 0·04). Further analysis showed that the overall difference between monocots and eudicots was also seen in diploids alone. Thus, the mean 4C value for ten diploid monocots (12·64 pg) was larger than that for seven diploid eudicots (3·96 pg), but not significantly so (P = 0·10). As the present sample did not include polyploid eudicots, a similar comparison for polyploids alone was not possible.
Chromosome counts
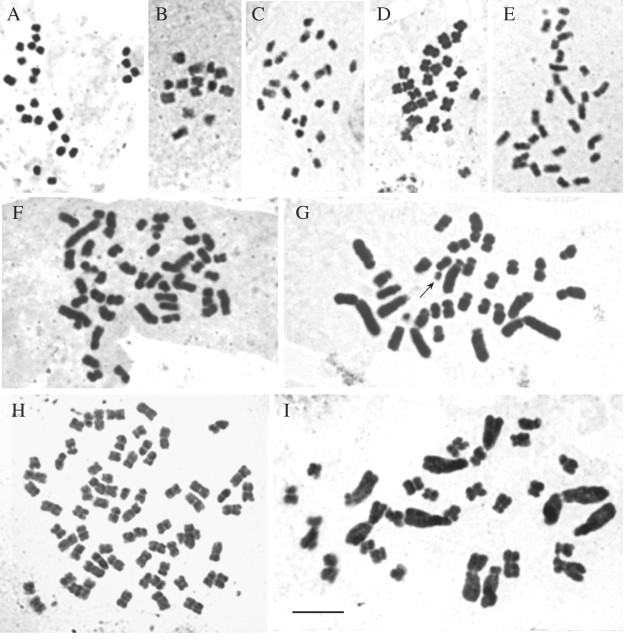
Table 2 gives a count (or in six cases, an estimate) of chromosome number for 22 of the 28 listed materials studied, and counts for the five remaining taxa taken from the literature. The chromosome number for Rhodocoma gigantea is unknown. Table 2 also gives ploidy levels for 21 of the 28 listed materials, based on a comparison of the count for the present material and all counts reported for related material(s) in the same genus, or by analysing karyotypes. Chromosome preparations for nine of the species are shown in Fig. 1, arranged in order of increasing DNA amount from Escallonia rubra (4C = 1·69 pg; Fig. 1A) to Ripogonum papuanum (4C = 44·58 pg; Fig. 1I).
Fig. 1. Somatic chromosomes arranged (A–I) in ascending order of 4C DNA amount. A, Escallonia rubra, 2n = 24, 4C = 1·69 pg. B, Dasypogon hookeri, 2n = 14, 4C = 1·74 pg. C, Montinia caryophyllacea, 2n = 24, 4C = 2·26 pg. D, Pterostyrax psilophylla, 2n = 24, 4C = 3·54 pg. E, Merrilliodendron megacarpum, 2n = 30, 4C = 4·37 pg. F, Doryanthes palmeri, 2n = 48, 4C = 13·22 pg. G, Lapageria rosea, 2n = 30 + 1B (arrow), 4C = 27·12 pg. H, Blandfordia punicea, 2n = 68, 4C = 32·53 pg. I, Ripogonum papuanum, 2n = 30, 4C = 44·58 pg. Scale bar = 5 µm.
A search through Fedorov (1969) and the ‘Indexes to plant chromosome numbers’ (Goldblatt and Johnson, 2002) showed that there were six new generic records: (1) Dasypogon hookeri (Dasypogonaceae; Fig. 1B); (2) Montinia caryophyllacea (Montiniaceae; Fig. 1C); (3) Merrilliodendron megacarpum (Icacinaceae; Fig. 1E); (4) Hanguana malayana (Hanguanaceae); (5) Lapageria rosea (Philesiaceae; Fig. 1G); and (6) Ripogonum papuanum (Ripogonaceae; Fig. 1I). Lapageria rosea had 2n = 30 + 1B; this is probably the first record of B chromosomes in Philesiaceae, in which this genus is placed (APG II, 2003), although B chromosomes occur frequently in genera belonging to the order Liliales (Jones and Rees, 1982), which includes Philesiaceae.
There are also seven new species records: (1) Blandfordia punicea was counted with 2n = 68 (Fig. 1H). While no previous count of 68 has been reported for other species of Blandfordia, a count of 2n = 34 obtained for several species suggests that the present material is tetraploid. (2) Eriocaulon aquaticum: numerous counts have been reported for Eriocaulon species, ranging from 2n = 24 to approx. 110. A first count of 2n = 32 for E. aquaticum is reported here, agreeing with published counts for E. septangulare With. and E. pellucidum Michaux by Moldenke (1969) and Löve and Löve (1982), respectively. (3) The first count of 2n = 24 for Pterostyrax psilophylla (Fig. 1D) agrees with one other published count for P. corymbosa by Manshard (1936). (4) Astelia fragrans: although there are counts for 11 other species of Astelia, with 2n = 16, 60, 64, approx. 80 and 210 (Scottsberg, 1955; Wheeler, 1966), the count of 2n = approx. 60 is the first for A. fragrans. (5) Ixiolirion ledebourii: previous work has recorded 2n = 24 for I. tataricum and I. montanum and 2n = 72 in I. tataricum, so the first count of 2n = approx. 24 for I. ledebourii reported here agrees with previous counts for other species. (6) Garrya fremontii: a first count of 2n = approx. 20 is reported for Garrya fremontii. Counts previously reported for G. elliptica Dougl. (Meurman, 1930) and G. lindheimeri Torr. (Turner, 1960) had 2n = 22. (7) The count of 2n = approx. 24 for Planchonella eerwah is related to the only other count for a Planchonella species, i.e. P. sandvicensis by Scottsberg (1955), which was tetraploid with 2n = 48. The counts obtained for the nine remaining species all agreed with previously published counts including that for Doryanthes palmeri (2n = 48; Fig. 1F).
A chromosome count is available for only about 25 % of angiosperm species (Bennett, 1998). Although the basic technique is well established, obtaining chromosome counts for a previously unstudied taxon is neither trivial nor certain within a short period, especially if the material is rare, growth is strongly seasonal or tissue that is suitable for cytological work is in limited supply for conservation or other reasons. Clearly, obtaining a chromosome count remains highly desirable for the six species studied in this work for which no exact chromosome count could be made (see Table 2), and this remains our aim (see recommendations of the Angiosperm Genome size meeting at http://www.rbgkew.org.uk/cval/conference.htmloutline). However, the Convention on Biological Diversity (Programme UNE, 1992) noted the need to make biodiversity data available, despite imperfections; a view that we support. Consequently, we are determined to make C‐value estimates for the taxa still lacking counts available quickly, whilst attempting to obtain and publish counts for difficult materials as they become available. By the same token, publishing good estimates of chromosome number (as has been done for five species) seems highly worthwhile since this information is potentially useful until the exact number is determined or confirmed.
DISCUSSION
Survey of available DNA C‐values
As part of an ongoing programme to increase familial representation in the Angiosperm DNA C‐values database (Bennett and Leitch, 2001), the present paper reports C‐values for a further 25 previously unrepresented families, with 16 (64 %) belonging to the monocots. The overall 4C‐values differed 35‐fold, with both the smallest and largest species (1·26 pg in Zostera marina and 44·58 pg in Ripogonum papuanum) being represented by monocots.
Previous work has noted that monocots may have a C‐value profile very different from that of dicots, with a clear tendency for monocots to have a significantly larger DNA amount than dicots (Bennett and Leitch, 2000). The realization that angiosperms are now divided into three main groups (i.e. basal angiosperms, monocots and eudicots; APG, 1998) has not changed this trend as an analysis shows that monocots have the largest mean, mode and range of 4C‐values of these major subdivisions (Table 3). Thus, while both monocots and eudicots contain species at the lowest end of the range, the largest‐known monocot 4C‐value (509·6 pg) is nearly 40 % bigger than the largest‐known eudicot value (4C = 317·3 pg). This trend is reflected within the sample of 25 families reported here, with monocots being characterized by a significantly larger mean and range of 4C‐values than eudicots. Thus, the mean for 19 monocots (mean 4C = 13·78 pg, range 1·26–44·58 pg) is nearly three times larger than that for nine eudicots (mean 4C = 3·99 pg, range 1·69–7·64 pg). Even if replicated genome size is analysed rather than 4C‐values, the mean for 14 monocots (6·20 pg) is still significantly larger than that for seven eudicots (1·98 pg). Interestingly, the nine highest C‐values in Table 2 are for monocot families belonging to Liliales, Asparagales or commelinoids. These three orders of monocots were shown to be the only ones that contained species with very large C‐values (defined as 4C ≥ 140 pg; Leitch et al., 1998).
Recent research is progressing our understanding of how genome size may increase or decrease. Together with polyploidy, the role of retrotransposition (SanMiguel et al., 1996) is now recognized to be a major factor leading to increases in DNA C‐value. Bennetzen and Kellogg (1997) suggested that these processes, if left unchecked, could lead to ever‐expanding ‘obese’ genomes. Other mechanisms are being elucidated that can bring about a reduction in DNA amount. In the work of Petrov et al. (2000), the rate at which DNA was lost from grasshopper genomes was slower in species with larger genomes compared with that in species having smaller genomes. Similarly, work on the repair of experimentally induced double‐stranded breaks in DNA showed that in the small genome of arabidopsis, double‐stranded breaks were more often repaired with larger deletions and fewer insertions than those in tobacco, which has a genome nearly 60 times larger (Kirik et al., 2000). Thus, genome size itself may play a role in determining the rate of DNA loss from a genome. It is tempting to speculate that beyond a critical genome size (which may vary depending on the species), it becomes increasingly difficult for the genome to ‘go on a diet’ and lose DNA, as the mass of DNA itself progressively prevents/inhibits the action of the mechanisms causing DNA loss. Left unchecked, this could be one way in which the truly ‘obese’ genomes in some monocots have evolved.
How many angiosperm families are there?
As noted previously (Hanson et al., 2001a), authorities differ as to how many angiosperm families they recognize, ranging from 200 to 533 for the eight different systems of classification listed by Brummitt (1992). Moreover, the number of families recognized can also vary with time, as new families are created and previously recognized families are split or sunk on the basis of new data (APG, 1998; APG II, 2003). Even during the course of the current work, the circumscriptions of the families Themidaceae, Xanthor rhoeaceae and Aphyllanthaceae have changed (as noted in Materials and Methods). Such changes complicate the endeavour of tracking how many, and what proportion (%) of families are represented in the database, as previously noted (Hanson et al., 2001a). The present work follows the names and circumscriptions of the APG II (2003), which recognizes 453 families comprising 27 basal angiosperms, 81 monocots and 345 eudicots. While this revised classification broadly agrees with the 1998 publication of the Angiosperm Phylogeny Group (APG, 1998) used in Hanson et al. (2001a, b), the overall trend of their work has been to reduce the total number of recognized families from 462 in 1998 to 453 in 2002.
Progress towards completing familial representation of DNA C‐values
Combining the data in the Angiosperm DNA C‐values database (Bennett and Leitch, 2001) with that in Hanson et al. (2001a, b) and Leitch and Hanson (2002) brings the total number of families with C‐value data to 214, corresponding to 47·2 % of angiosperm families recognized by APG II (2003; Table 4). Given the specific focus on monocots, progress in this group is also assessed.
Table 4.
Cumulative proportion of angiosperm families with C‐value data represented in the Angiosperm DNA C‐values database, Hanson et al. (2001a, b), Leitch and Hanson (2002), and the present work
| Source of data | Cumulative number of families represented | Cumulative proportion of all 453 families represented (%) | Cumulative proportion of 27 basal families represented (%) | Cumulative proportion of 345 eudicot families represented (%) | Cumulative proportion of 81 monocot families represented (%) |
| Angiosperm DNA C‐values database (release 3·0, Sept. 2001) | 135 | 29·8 | 51·8 | 24·0 | 46·9 |
| Hanson et al. (2001a) | 160 | 35·3 | 51·8 | 30·7 | 49·4 |
| Hanson et al. (2001b)* | 184 | 40·6 | 51·8 | 36·2 | 55·6 |
| Leitch and Hanson (2002) | 189 | 41·9 | 70·3 | 36·2 | 55·6 |
| Current paper | 214 | 47·2 | 70·3 | 38·8 | 75·3 |
* Although Hanson et al. (2001b) listed C‐values for 25 families, the new familial circumscription of Asparagaceae by APG II (2003) now encompasses Haemerocallidaceae (listed in Table 3 of Hanson et al., 2001b), so this family is excluded from the above table.
APG II (2003) recognizes 81 monocot families. A survey of available C‐value data shows that prior to the current highly targeted approach of increasing familial representation in the C‐values database (i.e. Hanson et al., 2001a, b; this paper), less that half of the moncot families (38 in total) were represented. The present paper with first C‐values for 16 unrepresented monocot families, together with the work of Hanson et al. (2001a, b), has increased this number to 61. Thus, 75 % of all monocot families now have at least one known C‐value (Table 4). Representation for monocot families is currently much higher than that for eudicots, which comprise 345 families with data available for only 38·7 % of these. The only other group of angiosperms where familial representation approaches that in monocots is the basal angiosperms, where there is at least one DNA C‐value estimate for 19 of the 27 families (70·3 %) (Leitch and Hanson, 2002). Both these high familial representations are the result of specific targeted studies, demonstrating, once again, the value of this approach for improving familial representation.
The future
We plan to estimate first DNA C‐values for a further 50 angiosperm families in 2002. Thus, work carried out at RBG, Kew should have resulted in first estimates of DNA C‐values for over 100 angiosperm families since 1999, increasing familial representation to over 58 %. Together, these data represent significant progress towards achieving the goal set in 1997. However, it is important to realise that working towards completing familial representation will become progressively more difficult, as the remaining unrepresented families increasingly comprise monotypic taxa from obscure locations that are difficult to access. Hitherto, obtaining materials of unrepresented families was not difficult, but it has recently become noticeably harder to access such materials for new DNA C‐value estimations. For this reason, completing familial representation for angiosperms in general, and monocots in particular, may prove impossible in any short period, and progress towards this goal will become slower. Whilst collect ing material of at least ten of the 20 remain ing unrepresented monocot families seems feasible, many of the remaining families may be difficult to obtain including, for example, Triuridaceae (Pandanales), all members of which are achlorophyllous mycotrophic parasites, and Burmanniaceae (Dioscoreales), which are very difficult to grow in culture as they depend on adequate fungus partners which themselves are mycorrhizal partners of other green plants. It will also be difficult to collect other monocot families that are not widely in cultivation [e.g. Mayacaceae (Poales), Cymodoceaceae (Alismatales), Posidoniaceae (Alismatales)]. It will therefore be important to set realistic goals that allow for the law of diminishing returns in proportion to the effort expended. Be that as it may, progress is still likely to increase familial representation to approx. 60 % of angiosperm families by 2002. Whilst this falls far short of the target set in 1997, the achievements of the last 5 years will, nevertheless, have equalled those of the previous 40.
Table 3.
Minimum (min.), maximum (max.), mean, mode and range of 4C DNA values in the major subdivisions of angiosperms
| No. of species with C‐values | Min. (pg) | Max. (pg) | Mean (pg) | Mode (pg) | Range | |
| Basal angiosperms | 67 | 1·7 | 35·4 | 7·97 | 3·20 | 20·8 |
| Monocots | 1498 | 0·6 | 509·6 | 41·73 | 3·80 | 849·3 |
| Eudicots | 1978 | ∼0·4 | 317·3 | 12·85 | 2·80 | 793·3 |
| All angiosperms | 3543 | ∼0·4 | 509·6 | 24·97 | 2·20 | 1274·0 |
Data taken from the Angiosperm DNA C‐values database (Bennett and Leitch, 2001) and Hanson et al. (2001a, b)
Supplementary Material
Received: 22 August 2002; Returned for revision: 19 September 2002; Accepted: 25 September 2002 Published electronically: 31 October 2002
References
- APG.1998. An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden 85: 531–553. [Google Scholar]
- APG II.2003. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Botanical Journal of the Linnean Society (in press). [Google Scholar]
- BennettMD.1998. Plant genome values: How much do we know? Proceedings of the National Academy of Sciences of the USA 95: 2011–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BennettMD, Leitch IJ.1995. Nuclear DNA amounts in angiosperms. Annals of Botany 76: 113–176. [Google Scholar]
- BennettMD, Leitch IJ.2000. Variation in nuclear DNA amount (C‐value) in monocots and its significance. In: Wilson KL, Morrison DA, eds. Monocots: systematics and evolution Melbourne: CSIRO, 137–146. [Google Scholar]
- BennettMD, Leitch IJ.2001. Angiosperm DNA C‐values database (release 3.1, Sept. 2001). http://www.rbgkew.org.uk/cval/homepage.html [Google Scholar]
- BennettMD, Bhandol P, Leitch IJ.2000. Nuclear DNA amounts in angiosperms and their modern uses—807 new estimates. Annals of Botany 86: 859–909. [Google Scholar]
- BennetzenJL, Kellogg EA.1997. Do plants have a one‐way ticket to genomic obesity? Plant Cell 9: 1509–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BensassonD, Petrov DA, Zhang DX, Hartl DL, Hewitt GM.2001. Genomic gigantism: DNA loss is slow in mountain grasshoppers. Molecular Biology and Evolution 18: 246–253. [DOI] [PubMed] [Google Scholar]
- BrummittRG.1992. Vascular plant families and genera. Kew: Royal Botanic Gardens, Kew. [Google Scholar]
- FedorovA.1969. Chromosome numbers of flowering plants. Leningrad: Nauka Publishers. [Google Scholar]
- GoldblattP, Johnson DE.2002. Index to plant chromosome numbers. http://mobot.mobot.org/W3T/Search/ipcn.html [Google Scholar]
- HansonL, McMahon KA, Johnson MAT, Bennett MD.2001a First nuclear DNA C‐values for 25 angiosperm families. Annals of Botany 87: 251–258. [DOI] [PubMed] [Google Scholar]
- HansonL, McMahon KA, Johnson MAT, Bennett MD.2001b First nuclear DNA C‐values for another 25 angiosperm families. Annals of Botany 88: 851–858. [DOI] [PubMed] [Google Scholar]
- JonesRN, Rees H.1982. B chromosomes. London: Academic Press. [Google Scholar]
- KirikA, Salomon S, Puchta H.2000. Species‐specific double‐strand break repair and genome evolution in plants. EMBO Journal 19: 5562–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeitchIJ, Hanson L.2002. DNA C‐values in seven families fill phylogenetic gaps in the basal angiosperms. Botanical Journal of the Linnean Society 140: 175–179. [Google Scholar]
- LeitchIJ, Chase MW, Bennett MD.1998. Phylogenetic analysis of DNA C‐values provides evidence for a small ancestral genome size in flowering plants. Annals of Botany 82: 85–94. [Google Scholar]
- LöveA, Löve D.1982. IOPB chromosome number reports LXXVII. Taxon 31: 766–768. [Google Scholar]
- MabberleyDJ.1997. The plant book. Cambridge: Cambridge University Press. [Google Scholar]
- ManshardE.1936. Embryologische Untersuchungen an Styrax obassia Seib. et Zucc. Planta 25: 364–386. [Google Scholar]
- MeurmanO.1930. Chromosome numbers in the family Cornaceae. Memoranda Societatis Fauna et Flora Fennica 6: 95–100. [Google Scholar]
- MoldenkeHN.1969. Additional notes on the Eriocaulaceae XXII. Phytologia 18: 344–396. [Google Scholar]
- ObermayerR, Greilhuber J.1999. Genome size in Chinese soybean accessions—stable or variable? Annals of Botany 84: 259–262. [Google Scholar]
- ObermayerR, Leitch IJ, Hanson L, Bennett MD.2002. Nuclear DNA C‐values in 30 species double the familial representation in pteridophytes. Annals of Botany 90: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PetrovDA.2001. Evolution of genome size: new approaches to an old problem. Trends in Genetics 17: 23–28. [DOI] [PubMed] [Google Scholar]
- PetrovDA, Sangster TA, Johnston JS, Hartl DL, Shaw KL.2000. Evidence for DNA loss as a determinant of genome size. Science 287: 1060–1062. [DOI] [PubMed] [Google Scholar]
- Programme UNE.1992. Convention on Biological Diversity. United Nations Environment Programme, Nairobi, Kenya. [Google Scholar]
- SanMiguelPet al.1996. Nested retrotransposons in the intergenic regions of the maize genome. Science 274: 765–768. [DOI] [PubMed] [Google Scholar]
- ScottsbergC.1955. Chromosome numbers in Hawaiian flowering plants. Arkiv fur Botanik 3: 63–70. [Google Scholar]
- ShirasuK, Schulman AH, Lahaye T, Schulze‐Lefert P.2000. A contiguous 66‐kb barley DNA sequence provides evidence for reversible genome expansion. Genome Research 10: 908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TurnerBL.1960. Meiotic chromosome numbers in Texas species of the genus Coreopsis (Compositae – Heliantheae). Southwestern Naturalist 5: 12–15. [Google Scholar]
- VicientCM, Suoniemi A, Anamthawat‐Jonsson K, Tanskanen J, Beharav A, Nevo E, Schulman AH.1999. Retrotransposon BARE‐1 and its role in genome evolution in the genus Hordeum Plant Cell 11: 1769–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WheelerJM.1966. Cytotaxonomy of the large Asteliads (Liliaceae) of the North Island of New Zealand. New Zealand Journal of Botany 4: 95–113. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.