Abstract
The turlough form of Ranunculus repens is subjected to several months’ complete inundation with hard groundwater. Experimental flooding to the level of the soil surface had no effect on turlough or ruderal populations relative to drained controls. Experimental submergence resulted in direct tissue death of the ruderal population but did not affect the turlough population relative to drained controls. There was no detectable difference in the proportion of aerenchyma in drained, flooded and submerged roots of plants from either population. The proportion of aerenchyma increased with root age in the ruderal population. Up to twice the proportion of aerenchyma occurred in the lower third of the root in the turlough population relative to the middle and upper thirds. Submergence in artificially hardened tap water increased the amount of tissue death in the ruderal population, whereas it appeared to enhance the growth of plants from the turlough population relative to that of plants submerged in tap water. Only the ruderal population demonstrated a depth accommodation response in submerged conditions. Root concentrations of ethanol‐soluble carbohydrates were up to three times higher in a field‐ collected turlough population during winter and autumn months than those in a ruderal population. Low levels of ethanol‐insoluble carbohydrates were present in the turlough population but were absent from the ruderal population. Starch concentrations fluctuated greatly in the turlough population and were generally higher than those in the ruderal population. These results, together with those from previous investigations, suggest that the turlough population survives prolonged submergence by maintaining low levels of submerged photosynthesis, which may circulate oxygen within the plant tissues, and by utilizing storage carbohydrates for maintenance respiration.
Key words: Ranunculus repens L., creeping buttercup, turlough, flooding, submergence, storage carbohydrate, aerenchyma, depth accommodation
INTRODUCTION
Owing to the very low rate of diffusion of oxygen in water, the primary effect of soil flooding is to reduce aeration of the soil (Gambrell et al., 1991). The ability of herbaceous wetland species to tolerate flooded conditions is frequently associated with the production of well developed aerenchyma (Smirnoff and Crawford, 1983; Justin and Armstrong, 1987; Armstrong et al., 1991).
Complete submergence results in blockage of the diffusion pathway for O2 between the atmosphere and the root (Laan and Blom, 1990). Shoots of many amphibious plant species possess a ‘depth accommodation’ response (Sculthorpe, 1967; Ridge, 1987), which involves elongation of shoots or petioles enabling leaves to emerge from the water, photosynthesize aerially and provide a diffusive pathway for O2 to the roots (Maberly and Spence, 1989). If connection to the aerial environment is not facilitated, but there are adequate reserves for maintenance respiration and the resumption of growth, plants can remain dormant through periods of submergence or anoxia (Brändle, 1991). The ability to conserve reserves until environmental conditions are again suitable for growth is of paramount importance for the survival of many amphibious species (Barclay and Crawford, 1983).
Ranunculus repens (creeping buttercup) is a species that is generally found in relatively wet habitats (Harper, 1957), some of which are submerged for part of the year, e.g. turloughs, the temporary lakes of the west of Ireland. Morphologically distinct turlough populations of this species are subjected to periodic inundation with groundwater that has a high alkalinity (Lynn and Waldren, 2001a). These populations do not have the ability to utilize exogenous bicarbonate for the process of photosynthesis (Lynn and Waldren, 2002). It is not clear whether this species can tolerate waterlogged conditions (a) by providing oxygen to the roots via aerenchyma thus enabling aerobic respiration in root tissue; (b) by maintaining growth by utilizing reserves and remaining dormant for the period of submergence; or (c) by the elongation of petioles, which would reconnect their photosynthetic organs to the aerial environment.
The aim of this research was to investigate the effect of experimentally and naturally imposed flooded and submerged conditions on the physiology of populations of Ranunculus repens collected from habitats which vary widely in their susceptibility to soil flooding and inundation. The results will elucidate whether there are evolutionary processes acting on the populations which restrict them to particular habitats, or whether this species exhibits a high phenotypic flexibility, therefore possessing a broad tolerance to soil flooding and inundation resulting in the wide ecological amplitude of this species.
MATERIALS AND METHODS
Plant collection and propagation
Seeds of Ranunculus repens were collected from a ruderal habitat (Dodder, O 150 297) and a turlough basin (adjacent to Lough Gealáin, R 317 938) in Ireland (grid references relate to the Irish grid). Seeds were germinated in a growth room (14 h day, photosynthetically active radiation 60–100 µmol m–2 s–1, 25/22 °C day/night) on moist filter paper in glass Petri dishes. Five‐day‐old seedlings were transferred to compost (equal parts sand : peat : loam) enriched with 3·5 g l–1 Osmocote Plus (Sierra Chemical Company, Marysville, OH, USA) slow‐release fertilizer. Plants were cultivated in 500 cm3 pots, and were transferred to sand culture prior to the experimental treatments and fertilized with 30 ml full‐strength Hoagland’s solution twice weekly. All plants had more than eight leaves, which is considered to be the mature phase of growth for this species when grown from seed (Lynn and Waldren, 2001a).
The effect of flooding and submergence on growth and production of aerenchyma
Four replicates per population per treatment were either completely drained, flooded to the sand surface with tap water in domestic washing basins, or completely submerged with tap water in an aquarium (60 l–1 capacity) in growth room conditions. Populations were grouped together in all treatments as the ruderal population plants were considerably larger and might have shaded those of the turlough population. The water was changed three times a week in the submerged treatment, but was only topped up in the flooded treatment. No nutrients were added during the experimental period to reduce the proliferation of algae.
After 3 weeks of treatment a young and an older tap root of varying lengths were removed from each plant and cleaned thoroughly. Thin sections from within the top, middle and bottom third of these roots were taken and stored in a 1 : 1 solution of 70 % ethanol : 100 % glycerol. The sections were traced from images projected from an inverted microscope (Tech ‘A’; Kent‐A‐Vision Manufacturing Co., Raytown, MO, USA). The traces were scanned using a flat‐bed Apple Scanner and images were analysed using NIH Image (version 1.56, National Institute of Public Health, Bethesda, MD, USA) software which converted the scanned drawing to a bitmap image; this was then used to calculate the area of aerenchyma. Aerenchyma was expressed as a proportion of root cross‐sectional area. The non‐parametric Kruskall–Wallis test was employed, using SPSS 6.1 (Chicago, IL, USA), to compare the proportion of aerenchyma between populations, treatments, roots of varying age and in sections from various positions along the root.
The experimental plants were partitioned into roots, petioles, leaves and stolons. Leaf area was determined using a Delta‐T leaf area meter (Cambridge, UK). The plant parts were dried for 48 h at 80 °C, cooled in a dessicator and then weighed. Production of daughter ramets was negligible so the weight of any ramet leaves, petioles or roots produced during the experiment was assigned to the respective mother plant category, e.g. ramet leaves were pooled with mother leaves etc.; stolon weights were included as part of the total plant weight. The ratio of dry weight of dead parts to dry weight of live parts was calculated. Any brown or necrotic plant material was classified as dead. Epiphytic algae were removed by wiping with a soft damp tissue during media changes.
Comparisons between populations subjected to the different water regimes were analysed by two‐way factorial ANOVA using Data Desk 6.0 (Data Description Inc., Ithaca, NY, USA). A Scheffe post hoc test was used to analyse any population × treatment interactions.
The effect of submergence in artificially hardened water on growth
Aquaria in growth room conditions were filled with 60 l of either tap water, or tap water artificially hardened using the recipe outlined in the American Association of Public Health (1992). Five replicates per treatment per population were assigned to separate aquaria. Plants were randomized within the aquaria, and re‐randomized following a water change. The media were changed three times a week. Alkalinity and pH were examined in the freshly changed solution and in the same solution 2 d later using the Gran titration method outlined in Makereth et al. (1978). After 4 weeks, plants were partitioned for dry weight determination. Petiole extension was measured as the difference between the length of the longest petiole at the start of the experiment and at the end of the experiment.
Comparisons between populations subjected to the different water treatments were analysed using a two‐way factorial ANOVA. A Scheffe post hoc test was used to analyse any population × treatment interactions.
Changes in total non‐structural carbohydrates
Ten plants were collected from both a turlough basin population (Cooloorta R 337 979) and a damp ruderal population (Kilkorkan, R 350 941) bimonthly for a year. Root samples were taken from each of the plants and cleaned thoroughly of soil within 24 h of collection. Ethanol‐soluble and ethanol‐insoluble carbohydrates and starch fractions were extracted using the methods outlined in Farrar (1980). Carbohydrate concentrations were expressed as hexose units g–1 f. wt.
Plants collected and analysed in November were retained and cultivated in freely drained compost (equal parts sand : peat : loam) in 500 cm3 pots in heated glasshouse conditions (minimum 12 °C). Carbohydrate concentrations were analysed after 4 months in cultivation to determine whether differences between populations were environmentally induced.
Comparisons between populations at the different time periods were analysed using a two‐way factorial ANOVA. Interactions were analysed using Scheffe or Bonferrroni post hoc tests, the former test being used when sample sizes were equal.
RESULTS
The effect of flooding and submergence on growth and production of aerenchyma
Flooding did not induce any obvious visual injuries in either population. Submergence had a deleterious effect on the ruderal population, evidenced by leaf decay and blackening of root tissue. The ruderal population displayed considerable petiole elongation in the submerged treatment, although this was not quantified in this experiment. There was no noticeable extension in the turlough population in the submerged treatment, and no obvious decay of plant parts, although a certain amount of yellowing of leaves did occur.
Root, leaf and total dry weights and leaf area were significantly higher in the ruderal population than in the turlough population (Fig. 1A–D; Table 1). Flooding and submergence had no effect on these parameters in the turlough population relative to the drained treatment, but in the ruderal population the submerged treatment resulted in significantly lower parameters than the drained and flooded treatments (P < 0·05 Scheffe post hoc test). Petiole dry weight was significantly higher in the ruderal population than in the turlough population (Fig. 1E; Table 1). There was no significant effect of flooding or submergence on this growth parameter.
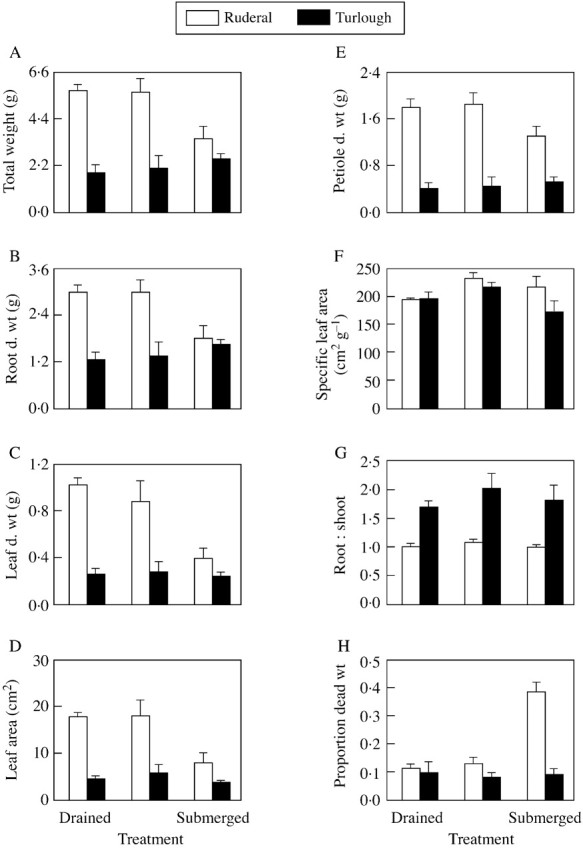
Fig. 1. The effect of 3 weeks’ flooding or submergence on the growth parameters of 6‐month‐old turlough and ruderal populations of Ranunculus repens.
Table 1.
ANOVA summary (F ratio) of the growth parameters of a turlough and ruderal population of Ranunculus repens flooded, submerged or freely drained for 3 weeks (expt 1) and submerged for 4 weeks in either tap water or artificially hardened tap water (expt 2)
| Experiment 1 | Experiment 2 | |||||
| Character | Population | Treatment | Interaction | Population | Treatment | Interaction |
| Total dry weight | 50·85*** | 2·04 | 5·55* | 2·13 | 1·40 | 7.06* |
| Root dry weight | 29·77*** | 1·79 | 5·65* | 0·01 | 2·23 | 8.10* |
| Petiole dry weight | 95·43*** | 1·16 | 2·80 | 12·98** | 1·19 | 6.9/9/* |
| Leaf dry weight | 42·05*** | 6·45** | 5·54* | 0·14 | 0·49 | 6·42* |
| Leaf area | 40·59*** | 5·58** | 3·31* | 7.21* | 1·02 | 2·61 |
| SLA | 1·43 | 2·47 | 0·54 | 15·66** | 0·85 | 9·66* |
| Root: shoot ratio | 34·61*** | 0·77 | 0·24 | 34·11*** | 9.17* | 4·23 |
| Proportion of dead weight | 39·13*** | 21·80*** | 20·81*** | 55·11*** | 11·32* | 11·9/7* |
| Petiole extension | 20.08*** | 0·18 | 0·98 | |||
| Leaf loss | 0·21 | 2·40 | 1·40 | |||
*** P < 0·001; ** P < 0·01; * P < 0·05.
There was no significant difference in specific leaf area between populations or between treatments (Fig. 1F; Table 1). The turlough population had a significantly higher root : shoot ratio than the ruderal population (Fig. 1G; Table 1) although there was no effect of flooding or submergence on this ratio (Table 1). The ratio of dead to total plant dry weight varied between populations depending on the treatment imposed. Flooding or submergence did not change this ratio in the turlough population (Table 1; P > 0·05 Scheffe post hoc test). However, the proportion of dead weight was significantly higher in the submerged plants of the ruderal population compared with plants in the other treatments, and also considerably higher than that in the submerged turlough plants (Fig. 1H; P < 0·05 Scheffe post hoc test).
There was no difference in the proportion of aerenchyma produced by plants in the drained, flooded or submerged treatments (Fig. 2; P > 0·05). There was also no difference in the proportion of aerenchyma produced by either population (P > 0·05). The proportion of aerenchyma produced in the top, middle or bottom third of the root did not differ in the ruderal population (P > 0·05), but there was a significantly higher proportion of aerenchyma in the lower third of the root of turlough plants than in the middle and upper thirds (P < 0·05). Older roots generally had a greater proportion of aerenchyma than did newer roots, although this was only significant in the ruderal population (P < 0·05). However, due to the subjective sampling of old and young roots, a potential range of root age was sampled, rather than discrete age categories; care must therefore be taken when interpreting these results.
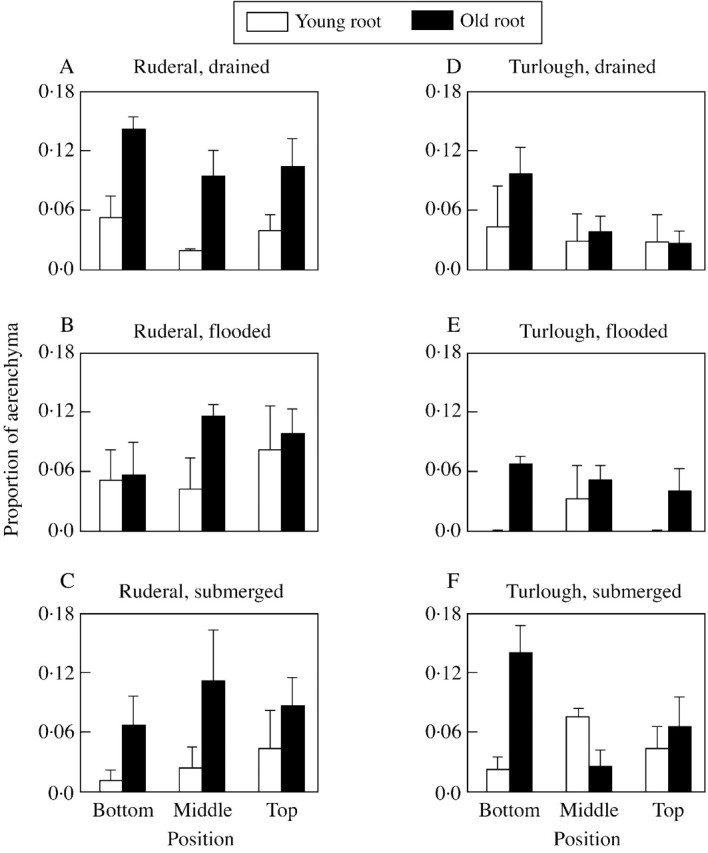
Fig. 2. The effect of 3 weeks’ flooding or submergence on the proportion of root aerenchyma in a ruderal and turlough population of Ranunculus repens. Sections were taken from the top, middle and bottom thirds of the tap root of old or young roots (categorized subjectively).
Root cortical cells were arranged hexagonally. Aeren chyma production appeared to be restricted to the mid‐cortex region and the production resulted from lysis of cells.
The effect of submergence in artificially hardened water on growth
Total, root, petiole and leaf dry weight data were significantly lower in plants submerged in hardened water than those submerged in tap water in the ruderal population (Fig. 3A–D; P < 0·05 Scheffe post hoc test). The turlough population had a significantly lower total plant dry weight and leaf dry weight than the ruderal population in the tap water treatment (Fig. 3A and D, respectively; P < 0·05 Scheffe post hoc test); however, there was no significant difference in root dry weight and petiole dry weight between the populations (Fig. 3B and C, respectively; P > 0·05 Scheffe post hoc test). In the hardened water treatment there was no significant difference in total, root, petiole or leaf dry weights between the ruderal and the turlough population (Fig. 3A, B and D, respectively; P > 0·05 Scheffe post hoc test).
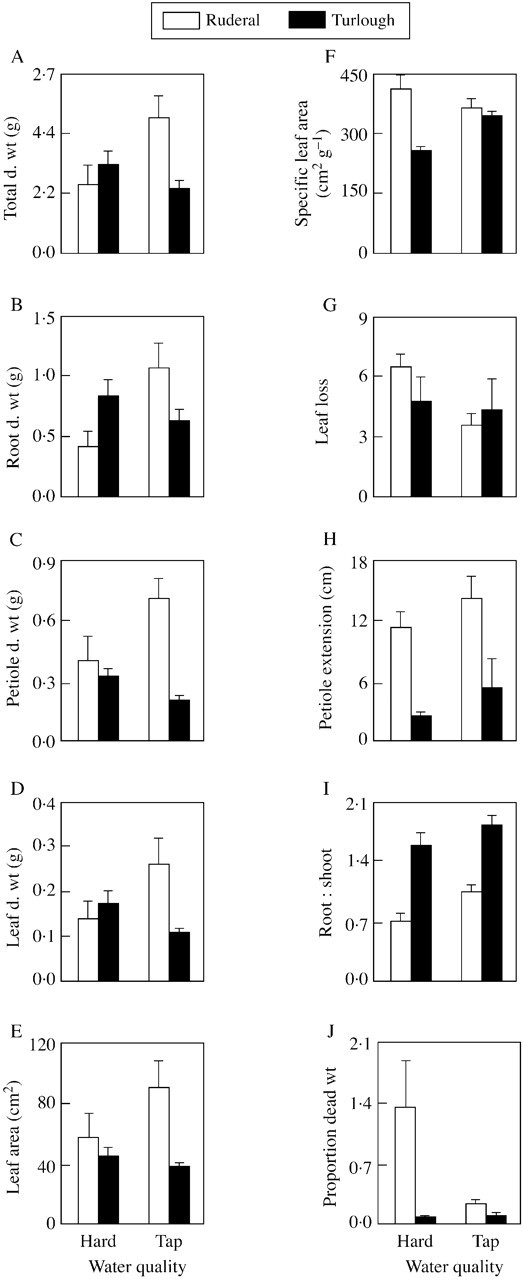
Fig. 3. The effect of 4 weeks’ submergence in tap water or artificially hardened tap water on the growth parameters of 5‐month‐old turlough and ruderal populations of Ranunculus repens.
The leaf area of ruderal population plants was significantly higher than that of the turlough population plants in both treatments (Fig. 3E). There was no effect of treatment on leaf area for either population (Table 1). Specific leaf area was significantly higher in the ruderal population than the turlough population in the hardened water treatment, but there was no significant difference between the populations in the tap water treatment (Fig. 3F; P > 0·05 Scheffe post hoc test). Specific leaf area was significantly higher in the turlough population in the tap water treatment than in the hardened water treatment; there was no difference between treatments for the ruderal population (Fig. 3F; P < 0·05 Scheffe post hoc test). There was no significant difference in leaf loss between populations or treatments (Fig. 3G; Table 1).
The petioles of the ruderal population extended significantly further than those of the turlough population in both treatments (Fig. 3H; Table 1). Petiole extension was not affected differently by the tap water or hardened water treatment in either population (Table 2).
Table 2.
Change in alkalinity and pH measurements recorded for experimental water (tap water and artificially hardened tap water) before submergence of plants from turlough and ruderal populations and following 3 d of submergence
| Population | Treatment | Δ Alkalinity | Δ pH |
| Turlough | Tap water | –0·11 | 0·08 |
| Hard water | 0·57 | 0·06 | |
| Ruderal | Tap water | –0·67 | –0·04 |
| Hard water | 0·03 | –0·01 |
Experimental conditions had been imposed for 18 d prior to measure ments.
The turlough population had a significantly higher root:shoot ratio in both treatments than the ruderal population. This ratio was significantly higher in the tap water treatment in both populations (Fig. 3I; Table 1).
The ruderal population had a significantly higher proportion of dead weight than the turlough population in both treatments (Fig. 3J; Table 1). The proportion of dead weight was significantly higher in the hardened water treatment for the ruderal population; there was no difference between treatments for the turlough population (Fig. 3J; P > 0·05 Scheffe post hoc test).
The alkalinity of the water changed very little between the water changes (Table 2), and there was no apparent consistency to the change with treatment or population, suggesting that differences may be due to experimental error. The pH increased in the turlough population in both treatments and decreased slightly in the ruderal population.
Changes in total non‐structural carbohydrates
Both populations exhibited the same pattern of change of ethanol‐soluble carbohydrates: a drop during the spring and early summer months, and an increase during the late summer and winter months (Fig. 4A); this trend was more pronounced in the turlough population. Differences between the populations occurred in July, September and November, with the turlough population having a higher concentration of this carbohydrate fraction (Table 3; P < 0·05 Bonferroni post hoc test). The increase within the turlough population during this time frame was not detected statistically, although the decrease between January and March was significant (P < 0·05 Bonferroni post hoc test). Con centrations in the ruderal population did not change significantly throughout the year, apart from a significant increase between September and November (P < 0·05 Bonferroni post‐hoc test).
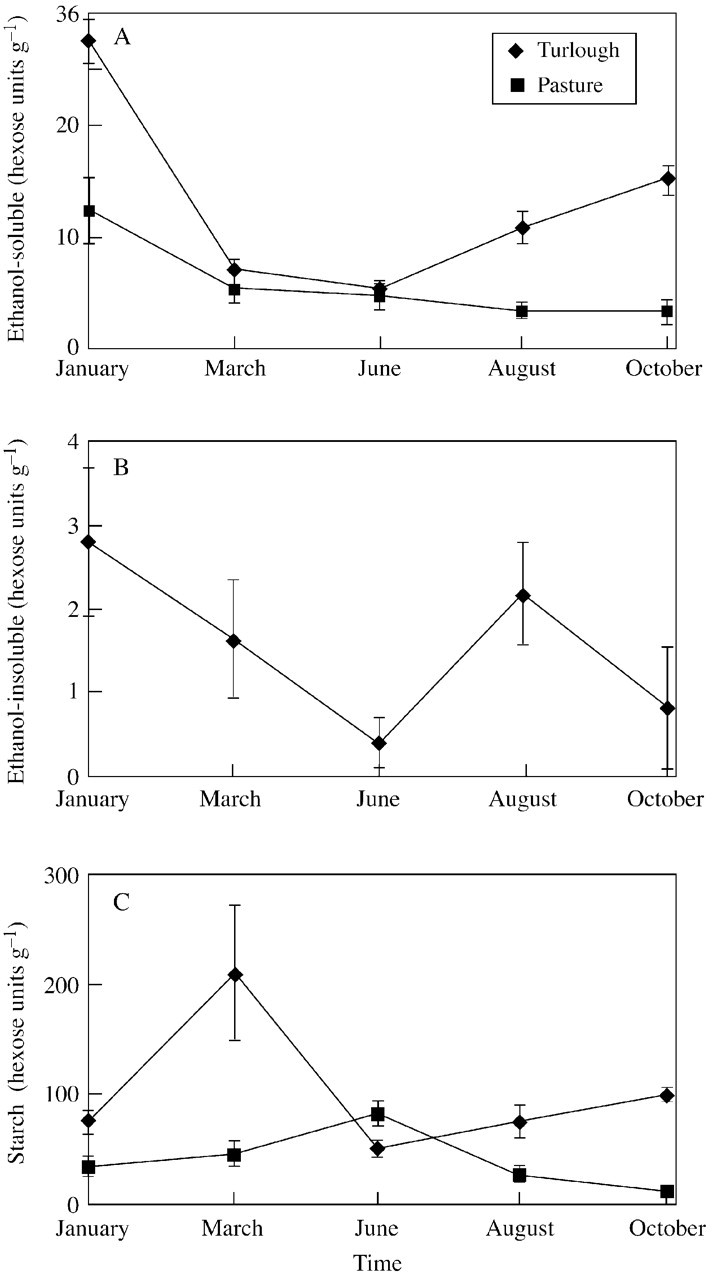
Fig. 4. Changes in total non‐structural carbohydrates (A, ethanol‐soluble; B, ethanol‐insoluble; C, starch) across 1 year in the fresh roots of turlough and ruderal populations of Ranunculus repens.
Table 3.
ANOVA (F ratio) summary for different proportions of non‐structural carbohydrate (mg g–1 hexose units) concentrations in fresh roots of turlough and ruderal populations of Ranunculus repens collected bimonthly from the field over a 1‐year period (expt 1), and comparisons between field‐collected and cultivated stock (expt 2).
| Source of variation | Ethanol‐soluble | Ethanol‐insoluble | Starch | |
| Experiment 1 | Population | 61.99*** | 39.77*** | |
| Time | 14.94*** | 2·28 | ||
| Population × Time | 3.22** | 8.25*** | ||
| Experiment 2 | Population | 1·85 | 1·53 | 0·09 |
| Time | 12.68** | 0·31 | 14.41*** | |
| Population × Time | 29.19*** | 11.66** | 0·72 |
*** P < 0·001; ** P < 0·01; * P < 0·05.
As the concentration of ethanol‐insoluble carbohydrate reached a detectable level only in November in the ruderal population, no statistical comparisons were made between populations. A low concentration of this carbohydrate fraction was present throughout the year in the turlough population in fluctuating, but not in significantly different, amounts (P < 0·05; Fig. 4B).
Concentrations of starch did not change significantly over the time period in the ruderal population, although there appeared to be a peak in concentration in May (Fig. 4C). Starch concentrations changed differently over time in the two populations (Table 3). Two peaks in starch concentration occurred in the turlough population, in March and in September; in these months, concentrations were significantly higher in the turlough population than those in the ruderal population (P < 0·05 Bonferroni post hoc test). Only the March concentration was a significant peak for the turlough population (P < 0·05 Bonferroni post hoc test).
Following the period in cultivation the ethanol‐soluble fraction of the ruderal plants increased significantly (P < 0·05 Bonferroni post hoc test), whereas there was no change in that of the turlough plants (Table 3; P > 0·05 Bonferroni post hoc test). The increase of this fraction following cultivation in the ruderal population resulted in concentrations similar to those in the turlough population (Fig. 5A).
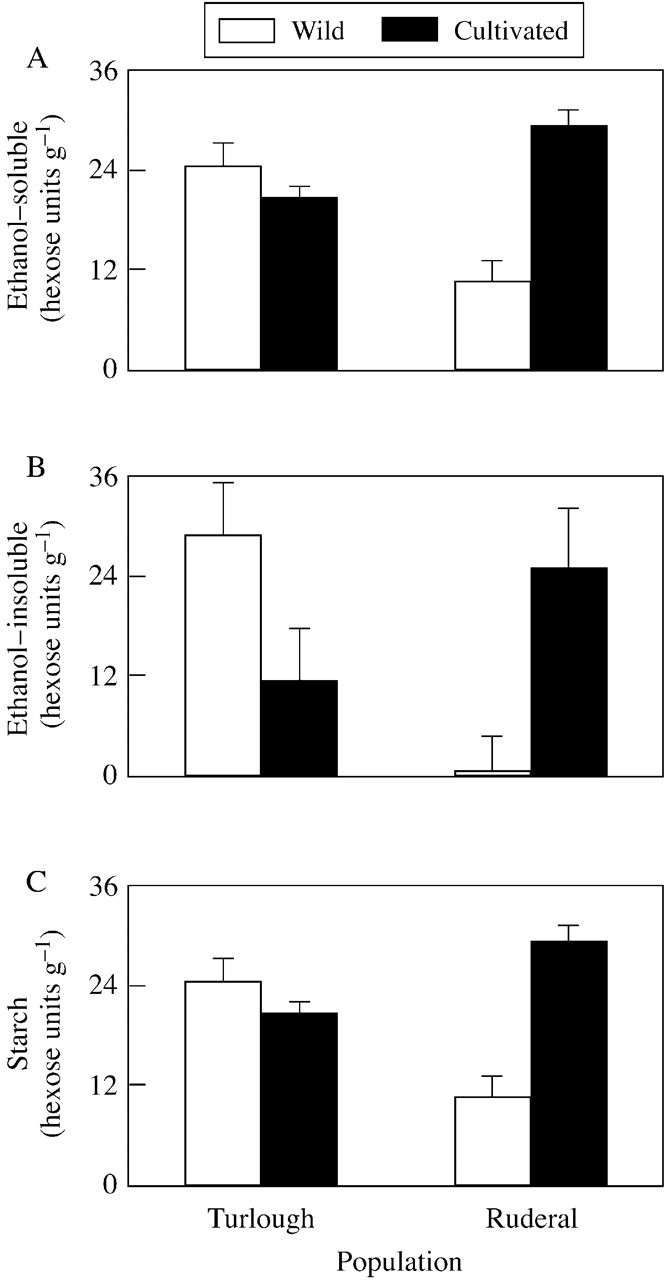
Fig. 5. Changes in total non‐structural carbohydrates (A, ethanol‐soluble; B, ethanol‐insoluble; C, starch) in the fresh roots of turlough and ruderal populations of Ranunculus repens collected in November and cultivated for 2 months.
The ethanol‐insoluble fraction decreased significantly following cultivation in the turlough population and increased significantly in the ruderal population (Table 3; P < 0·05 Bonferroni post hoc test). Starch increased by the same proportion in both populations following cultivation (Table 3), and concentrations were similar in both populations (Fig. 5C).
DISCUSSION
The lack of visual evidence of flooding injury suggests a broad tolerance of this species to flooded conditions, as the ruderal population would never have encountered waterlogged soils in its natural habitat. In no instance did flooding reduce the biomass of any plant parameter in either of the populations relative to that of the control, which suggests that soil saturation does not impair optimal growth. Flooding is reported to enhance growth significantly in many species (e.g. Davies and Singh, 1983; Justin and Armstrong, 1987). Continued growth in a waterlogged environment is often associated with increased root porosity (Smirnoff and Crawford, 1983; Justin and Armstrong, 1987; Laan et al., 1989). In the present study aerenchyma in the tap root were produced lysogenously under drained, flooded and submerged conditions. Other wetland species also produce aerenchyma in drained conditions (e.g. Smirnoff and Crawford, 1983; Jackson et al., 1985; Justin and Armstrong, 1987). The proportion of aerenchyma produced was highly variable, with no apparent sequence of production down through the root. As only percentage cavity formation was recorded and not total porosity (which would also include intercellular space), the actual porosity of the root was likely to have been underestimated. However, as the cortical cells were arranged hexagonally this underestimation was not as great as it would have been were the cells arranged cubically. Hexagonal packing is not as conducive to the production of aerenchyma as cubically arranged cortical cells; it does, however, increase the strength of the root (Justin and Armstrong, 1987). Ranunculus repens typically occurs in wet, heavy soils (Harper, 1957), which are often susceptible to poaching (Sarukhán and Harper, 1973); a strong root system is therefore likely to be essential to survive trampling.
The proportion of aerenchyma was apparently adequate to combat any problems associated with the anoxic conditions induced by flooding as complete anoxia imposed for 7 d resulted in the death of Ranunculus repens (Barclay and Crawford, 1983); thus, if the root system had been denied oxygen for more than 1 week it would have succumbed. The fact that there were no major differences in biomass production by plants subjected to the flooded treatment or the drained treatment suggests that oxygen had been transported to the root system.
Relative to the drained and flooded treatments, submergence had a huge deleterious effect on all the plant growth parameters measured in the ruderal population. The effect of submergence appeared to be due to the direct death of plant parts. On average, more plant tissue died than remained functional when this population was submerged in hardened water. Submergence had no deleterious effect on the growth of the turlough population.
Lynn and Waldren (2002) demonstrated that neither the turlough nor the ruderal population was able to utilize bicarbonate as a source of CO2 to any great extent in solutions of increasing alkalinity. However, photosynthesis in the turlough population increased to an apparently greater extent at higher alkalinities than that in the ruderal population, suggesting that the turlough population has a greater ability to utilize bicarbonate. Allen and Spence (1981) demonstrated that there was no discrete bicarbonate ‘user’ and ‘non‐user’ category, but that at a given alkalinity there was a gradation of bicarbonate use. The lower capacity for submerged photosynthesis in the ruderal population was reflected by the lack of any increase in the pH in the aquaria solution. An increase in pH would suggest a shift in the equilibrium of the various carbonate species within the media. The artificially hardened water had a pH of over 8, indicating that the exogenous carbon was present in the bicarbonate form (Stumm and Morgan, 1981). Any uptake of bicarbonate for photosynthetic purposes would result in the extrusion of OH– ions and thus an increase in pH (Walker and Smith, 1977). An increase in pH was observed in the solution surrounding the turlough population suggesting a greater photosynthetic activity; however, results must be interpreted with caution due to the algal contamination and the volume of water in question. A higher rate of oxygen evolution, together with the fact that the turlough population is smaller in stature, may be important in reducing tissue damage which could potentially be induced by increasing O2 deficiency in an unstirred media.
The two‐ to three‐times‐greater petiole elongation observed in the ruderal population compared with that in the turlough population was unlikely to be due simply to the increased mechanical uptake of water without any involvement of growth regulators to enhance extensibility; clearly, further work is required to elucidate the effects of ethylene on petiole elongation in different populations of R. repens. No extreme ‘depth accommodation’ responses have been observed in turlough populations in their natural surroundings (Lynn, 1998). The light attenuation, particularly in the deeper turloughs, would reduce tissue ethylene concentrations since production is inhibited by the attenuation of far‐red light (Spence, 1981). However, the lack of any major elongation response in the relatively shallow conditions imposed by experimental submergence in aquaria suggests that the relatively low elongation response is likely to be a genetically controlled adaptive feature in these turlough populations. Turlough populations must endure submergence in their natural habitat, often in several metres of water, and any attempt of ‘depth accommodation’ would be mechanically unfeasible.
It is possible that if the petiole extension observed in the ruderal population was allowed to reach the surface then the effects of submergence may have been ameliorated. Elongation growth is reported to compete for carbohydrate reserves essential for maintenance processes (Setter et al., 1997), which may explain, in part, the deleterious effect of submergence on the ruderal population. Removal of the media resulted in collapse of the petioles in the ruderal population. This was also observed for Rumex maritimus in situations where the water was too deep for emergence: chlorosis of older leaves resulted in weakening and collapse when the water levels receded (Van der Sman et al., 1993). Ranunculus spp. have been shown to differ in the amount and distribution of strengthening tissue depending on the habitat in which the species was derived (Usherwood et al., 1997): more aquatic species had less strengthening tissue as an adaptation to flowing water. It is possible that the amount of strengthening tissue can be modified in R. repens depending on the external conditions.
Starch was the major carbohydrate reserve in the roots of both the turlough and ruderal population. Carbohydrate concentrations are determined by the balance between consumption in respiration and growth, access to stored reserves and production of carbohydrates in photosynthesis (Setter et al., 1987). The large fluctuations observed in the turlough population may reflect fluctuations in the water level. For example, the turlough was empty in May and July, thus starch concentrations may have fallen due to the initiation of growth. The increase in September may have been a result of the rise in water levels and a corresponding impairment of growth (Setter et al., 1987), or an accumulation of reserves following the reduction of growth that occurs late in the growing season (Lynn and Waldren, 2001b). The subsequent decline in November may have been due to the utilization of reserves for maintenance respiration if the low rates of photosynthesis observed in submerged conditions failed to cover these costs. The peak in March is more difficult to interpret although it may be explained simply by a drop in the water level previous to the sampling date. The relatively constant concentrations of starch observed in the ruderal populations may simply indicate that increases in growth were very closely correlated with increases in photosynthesis, therefore there was no need to rely on reserves.
The increased concentrations of ethanol‐soluble carbohydrates in the turlough population compared with those in the ruderal population, particularly in the autumn and winter months, may reflect the mobilization of starch reserves for maintenance respiration.
The ethanol‐insoluble carbohydrate fraction was present in low concentrations throughout the year in the turlough population, and only barely reached the level of detection in the ruderal population. It is possible that this fraction contains compounds such as fructan (Gonzalez et al., 1989) that serve as osmoregulators during times of ‘stress’ (Albrecht et al., 1997; Avigad and Dey, 1997) and which may serve to ameliorate problems associated with submergence; however, the low concentrations recorded may not be sufficient to have any osmotic impact.
The increase in all carbohydrate fractions in the ruderal population following cultivation suggests more optimal growth conditions in cultivation relative to winter conditions in the field. However, for the turlough population only the starch fraction increased significantly in cultivation. The lack of change in the ethanol‐soluble fraction in the turlough population in cultivation may be due to the use of this fraction for maintenance respiration in the winter field conditions as a result of reduced levels of photosynthesis, and the use of this fraction for increased growth in cultivation. Root carbohydrate fractions have been reported to fluctuate as a result of varying metabolic demands that are induced developmentally (Hendrix et al., 1994) or seasonally (Wells, 2002; Oleksyn et al., 2000). The decrease in the concentration of ethanol‐insoluble carbohydrate may simply have been part of the overall fluctuations that occur in this fraction across the year. The increase in this fraction in the ruderal population was interesting: as it was detected only once at a negligible concentration in the field, it may not have the same carbohydrate constituents as the ethanol‐insoluble fraction found in the turlough plant root. Clearly, these results cannot be interpreted further without determining the carbohydrates present in this fraction.
Physiological adaptations to amphibious conditions have also been documented by Linhart and Baker (1973) who hypothesized that the disruptive selection caused by fluctuating water levels in a temporary pond was responsible for differences in response to waterlogging by populations of Veronica peregrina. The turlough populations endure several months of complete submergence in soils with a redox potential that indicates the absence of oxygen (Lynn, 1998). These populations appear to be able to survive these prolonged periods of submergence by maintaining a low level of photosynthesis, which may circulate oxygen within plant tissues, and also by reducing metabolic processes and relying, to a certain extent, on reserve material for maintenance respiration.
ACKNOWLEDGEMENTS
Thanks to Drs Marjan Jongen and Mike Williams for their technical assistance with carbohydrate extraction. This research was funded by the National Heritage Council of Ireland. D.E.L. was in receipt of a Forbairt research studentship.
Supplementary Material
Received: 10 June 2002; Returned for revision: 8 August 2002; Accepted: 16 October 2002
References
- AlbrechtG, Biemelt S, Baumgartner S.1997. Accumulation of fructans following oxygen deficiency stress in related plant species with different flooding tolerances. New Phytologist 136: 137–144. [Google Scholar]
- AllenED, Spence DHN.1981. The differential ability of aquatic plants to utilise the inorganic carbon supply in fresh waters. New Phytologist 87: 269–283. [Google Scholar]
- American Association of Public Health.1992. Toxicity test methods. In: Clesceri CS, Greenberg AE, Eaton AD, Franson MAH, eds. Standard methods for the examination of water and waste water. Washington: American Association of Public Health, 16. [Google Scholar]
- ArmstrongW, Justin SHFW, Beckett PM, Lythe SM.1991. Root adaptation to waterlogging. Aquatic Botany 39: 57–73. [Google Scholar]
- AvigadG, Dey PM.1997. Carbohydrate metabolism: storage carbo hydrates. In: Dey PM, Harborne JB, eds. Plant biochemistry London: Academic Press, 143–204. [Google Scholar]
- BarclayAM, Crawford RMM.1983. The effect of anaerobiosis on carbohydrate levels in storage tissues of wetland plants. Annals of Botany 51: 255–259. [Google Scholar]
- BrändleRA.1991. Flooding resistance of rhizomatous amphibious plants. In: Jackson MB, Davies DD, eds. Plant life under O2deprivation The Hague: Academic Publishers, 35–46. [Google Scholar]
- DaviesMS, Singh AK.1983. Population differentiation in Festuca rubra L. and Agrostis stolonifera L. in response to waterlogging. New Phytologist 94: 573–583. [Google Scholar]
- DrewMC.1983. Plant injury to oxygen deficiency in the root environment: a review. Plant and Soil 75: 179–199. [Google Scholar]
- FarrarJF.1980. Allocation of carbon to growth, storage and respiration in the vegetative barley plant. Plant Cell and Environment 3: 97–105. [Google Scholar]
- GambrellRP, Delaune RD, Patrick WH.1991. Redox processes in soils following oxygen depletion. In: Jackson MB, Davies DD, eds. Plant life under O2deprivation The Hague: Academic Publishers, 101–107. [Google Scholar]
- GonzalezB, Boucaud J, Langlois J.1989. Comparative estimation of non‐structural carbohydrate contents in perennial ryegrass by enzymatic and high performance chromatography. Journal of Plant Physiology 134: 251–253. [Google Scholar]
- HarperJL.1957. Biological flora of the British Isles: Ranunculus acris L., R. repens L. and R. bulbosus L. Journal of Ecology 45: 289–342. [Google Scholar]
- HendrixDL, Mauney JR, Kimball BA, Lewin K, Nagy J, Hendrey GR.1994. Influence of elevated CO2 and mild water‐stress on non‐structural carbohydrates in field‐grown cotton tissues. Agricultural and Forest Meteorology 70: 153–162. [Google Scholar]
- JacksonMB, Fenning TM, Jenkins W.1985. Aerenchyma (gas‐space) formation in adventitious roots of rice (Oryza sativa L.) is not controlled by ethylene or small partial pressures of oxygen. Journal of Experimental Botany 36: 1566–1572. [Google Scholar]
- JustinSHFW, Armstrong W.1987. The anatomical characteristics of roots and plant response to soil flooding. New Phytologist 106: 465–495. [Google Scholar]
- LaanP, Blom CWPM.1990. Growth and survival responses of Rumex species to flooded and submerged conditions: the importance of shoot elongation, underwater photosynthesis and reserve carbo hydrates. Journal of Experimental Botany 41: 775–783. [Google Scholar]
- LaanP, Berrevoets MJ, Lythe SM, Armstrong W, Blom CWPM.1989. Root morphology and aerenchyma formation as indicators of the flood‐tolerance of Rumex spp. Journal of Ecology 77: 693–705. [Google Scholar]
- LinhartYB, Baker G.1973. Intra‐population differentiation of physiological response to flooding in a population of Veronica peregrina Nature 242: 275–276. [Google Scholar]
- LynnDE.1998. Morphological and physiological variation in the turlough form of Ranunculus repens. PhD Thesis, University of Dublin, Ireland. [Google Scholar]
- LynnDE, Waldren S.2001a Morphological variation in populations of Ranunculus repens from the temporary limestone lakes (turloughs) in the West of Ireland. Annals of Botany 87: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LynnDE, Waldren S.2001b Variation in life history characteristics between clones of Ranunculus repens grown in experimental garden conditions. Weed Research 41: 421–432. [Google Scholar]
- LynnDE, Waldren S.2002. Physiological variation in populations of Ranunculus repens from the temporary limestone lakes (turloughs) in the West of Ireland. Annals of Botany 89: 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MaberlySC, Spence DHN.1983. Photosynthetic inorganic carbon use by freshwater plants. Journal of Ecology 71: 705–724. [Google Scholar]
- MaberlySC, Spence DHN.1989. Photosynthesis and photorespiration in freshwater organisms: Amphibious plants. Aquatic Botany 34: 267–286. [Google Scholar]
- MakerethFJH, Heron J, Talling JF.1978. Water analysis. Ambleside, Cumbria: Freshwater Biological Association. [Google Scholar]
- OleksynJ, Zytkowiak R, Karolewski P, Reich PB, Tjoelker MG.2000. Genetic and environmental control of seasonal carbohydrate dynamics in trees of diverse Pinus sylvestris populations. Tree Physiology 20: 837–847. [DOI] [PubMed] [Google Scholar]
- RidgeI.1987. Ethylene and growth control in amphibious plants. In: Crawford RMM, ed. Plant life in aquatic and amphibious habitats Oxford: Blackwell Scientific Publications, 53–78. [Google Scholar]
- SarukhánJ, Harper JL.1973. Studies on plant demography: Ranunculus repens L., R. bulbosus L. and R. acris L. I. Population flux and survivorship. Journal of Ecology 61: 675–716. [Google Scholar]
- SculthorpeCD.1967. The biology of aquatic vascular plants. Ireland: Edward Arnold. [Google Scholar]
- SetterTL, Waters I, Atwell BJ, Kapanchanakul T, Greenway H.1987. Carbohydrate status of terrestrial plants during flooding. In: Crawford RMM, ed. Plant life in aquatic and amphibious habitats Oxford: Blackwell Scientific Publications, 411–434. [Google Scholar]
- SetterTL, Ellis M, Laureles EV, Ella ES, Senadhira D, Mishra SB, Sarkarung S, Datta S.1997. Physiology and genetics of submergence tolerance in rice. Annals of Botany 79: 67–77. [Google Scholar]
- SmirnoffN, Crawford RMM.1983. Variation in the structure and response to flooding of root aerenchyma in some wetland plants. Annals of Botany 51: 237–249. [Google Scholar]
- SpenceDHN.1981. Light quality and plant response underwater. In: Smith H, ed. Plants and the daylight spectrum London: Academic Press, 245–267. [Google Scholar]
- StummW, Morgan JJ.1981. Aquatic chemistry. New York: Wiley‐Interscience. [Google Scholar]
- UsherwoodJR, Ennos AR, Ball DJ.1997. Mechanical and anatomical adaptations in terrestrial and aquatic buttercups to their respective environments. Journal of Experimental Botany 48: 1469–1475. [Google Scholar]
- Van der SmanAJM, Blom CWPM, Barendse GWM.1993. Flooding resistance and shoot elongation in relation to developmental stage and environmental conditions in Rumex maritimus L. and Rumex palustris Sm. New Phytologist 125: 73–84. [DOI] [PubMed] [Google Scholar]
- WalkerNA, Smith FA.1977. Circulating electric currents between acid and alkaline zones associated with HCO3– assimilation in Chara Journal of Experimental Botany 28: 1190–1206. [Google Scholar]
- WellsR.2002. Stem and root carbohydrate dynamics of two cotton cultivars bred fifty years apart. Agronomy Journal 94: 876–882. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


