Abstract
Major differences in primary cell wall (PCW) components between non‐vascular plant taxa are reported. (1) Xyloglucan: driselase digestion yielded isoprimeverose (the diagnostic repeat unit of xyloglucan) from PCW‐rich material of Anthoceros (a hornwort), mosses and both leafy and thalloid liverworts, as well as numerous vascular plants, showing xyloglucan to be a PCW component in all land plants tested. In contrast, charophycean green algae (Klebsormidium flaccidium, Coleochaete scutata and Chara corallina), thought to be closely related to land plants, did not contain xyloglucan. They did not yield isoprimeverose; additionally, charophyte material was not digestible with xyloglucan‐specific endoglucanase or cellulase to give xyloglucan‐derived oligosaccharides. (2) Uronic acids: acid hydrolysis of PCW‐rich material from the charophytes, the hornwort, thalloid and leafy liverworts and a basal moss yielded higher concentrations of glucuronic acid than that from the remaining land plants including the less basal mosses and all vascular plants tested. Polysaccharides of the hornwort Anthoceros contained an unusual repeat‐unit, glucuronic acid‐α(1→3)‐galactose, not found in appreciable amounts in any other plants tested. Galacturonic acid was consistently the most abundant PCW uronic acid, but was present in higher concentrations in acid hydrolysates of bryophytes and charophytes than in those of any of the vascular plants. Mannuronic acid was not detected in any of the species surveyed. (3) Mannose: acid hydrolysis of charophyte and bryophyte PCW‐rich material also yielded appreciably higher concentrations of mannose than are found in vascular plant PCWs. (4) Mixed‐linkage glucan (MLG) was absent from all algae and bryophytes tested; however, upon digestion with licheninase, PCW‐rich material from the alga Ulva lactuca and the leafy liverwort Lophocolea bidentata yielded penta‐ to decasaccharides, indicating the presence of MLG‐related polysaccharides. Our results show that major evolutionary events are often associated with changes in PCW composition. In particular, the acquisition of xyloglucan may have been a pre‐adaptive advantage that allowed colonization of land.
Key words: Bryophyta, mosses, liverworts, hornworts, Charophyta, cell walls, xyloglucan, galacturonic acid, glucuronic acid, mannans, mixed‐linkage glucans, evolution
INTRODUCTION
The primary cell wall (PCW) has many essential biological roles, including tissue cohesion, defence (e.g. against microbes), ion exchange, the production of oligosaccharins and the regulation of cell expansion (Goldberg et al., 1994; Brett and Waldron, 1996; Cassab, 1998; Dumville and Fry, 1999; Fry, 1999). Demands on the PCW, and therefore its optimal composition, may have changed during periods of rapid plant evolution. In particular, colonization of the land by the first bryophytes is likely to have exposed these plants to novel ecological problems, the solution to which may have driven rapid evolution of the PCW.
Few studies have been undertaken of cell wall composition in non‐vascular land plants (Chodat and Cortesi, 1939; Edelmann et al., 1998). Among bryophytes, liverworts broadly resemble gymnosperms in their PCW sugar residue composition, the major neutral monosaccharide residues being Glc > Gal > Man > Xyl ≈ Ara > Fuc ≈ Rha (Thomas, 1977; Thomas et al., 1984). However, there is no information on the nature of the polysaccharides that contain these sugar residues in bryophytes. Even the sugar residue composition is not fully defined: for example, most studies do not report which acidic sugars are present in bryophytes (Thomas, 1977). Das and Rao (1966b) reported the presence of mannuronic acid (ManA) in cell walls of the liverwort Riccardia. However, this report of ManA in liverwort cell walls is unusual as GalA > GlcA > 4‐O‐methyl‐GlcA are the most commonly occurring acidic sugars in angiosperm cell walls. Galactoglucomannans that have a structure similar to those occurring in angiosperms and gymnosperms have been characterized from an aquatic moss (Geddes and Wilkie, 1971).
Variation exists in cell wall composition between different algal taxa and is widely used in algal classifications (Stace, 1981). On the basis of morphological and biochemical evidence, there is now wide support for the closest extant ancestor of land plants being amongst the charophycean green algae (Manhart and Palmer, 1990; Kranz et al., 1995). Although the charophycean green algae Chara and Nitella have been widely used as models for studies of plant cell growth, their PCW composition has not been well studied. Indeed, both Chara and Nitella differ from land plants in lacking detectable hydroxyproline—a component of the major PCW glycoproteins, extensins (Gotteli and Cleland, 1968; Kieliszewski and Lamport, 1994).
In this paper the evolution of the primary cell wall in land plants is documented. The polysaccharide composition of a variety of charophycean green algae and bryophytes is reported. Comparisons are drawn with results obtained from vascular land plants.
MATERIALS AND METHODS
Source of plant material
Sources of non‐vascular botanical material are listed in Table 1. Details of lycopodiophyte and fern material are listed in Popper et al. (2001). Young leaf tissue of Flagellaria guineensis Schum. was obtained from the Royal Botanic Garden Edinburgh (Accession number 19720171). Seeds of Secale cereale L., Triticum aestivum L., Zea mays L. and Hordeum vulgare L. were obtained commercially, grown in the dark, and alcohol‐insoluble residue (AIR) was prepared from the coleoptiles.
Table 1.
Sources of non‐vascular plant material
| Classification | Species | Source* |
| Algae | ||
| Chlorophyte | Ulva lactuca L. | North Berwick |
| Charophytes | Chara corallina (Klein ex Willd. Em. R.D.W.) | Culture (D. Sanders) |
| Coleochaete scutata Brébisson | CCAP 414/4 | |
| Klebsormidium flaccidium (Kützing) Silva, Mattox et Blackwell | Culture (H. Sluiman) | |
| Bryophytes | ||
| Hornwort | Anthoceros caucasicus Spring. | Faial Island (R. Schumacker) |
| Thalloid liverworts | Lunularia cruciata (L.) Dum. Ex. Lindb. | Balerno (P.M. Smith) |
| Pellia epiphylla (L.) Corda | Roslin Glen | |
| Leafy liverworts | Lepidozia reptans (L.) Dum. | Milngavie (D.S. Rycroft) |
| Nardia scalaris (Schrad.) Gray (Alicularia scalaris (Schrad. Corda) | Cairngorms | |
| Marsupellia emarginata (Lindb.) Dum. | Cairngorms | |
| Plagiochila asplenioides (L.) Dum. (P. asplenioides var. major Nees) | Cairngorms | |
| Lophocolea bidentata (L.) Dum. | Edinburgh | |
| Scapania undulata (L.) Dum. | Cairngorms | |
| Pleurozia purpurea Lindb. | Cairngorms | |
| Porella cordaena (Hüb.) Moore (Madotheca cordaena Hüb.) Dum. | Milngavie (D.S. Rycroft) | |
| Mosses | Sphagnum palustre L. (S. cymbifolium (Ehrh.) Hedw.) | Cairngorms |
| Sphagnum molle Sull. | Cairngorms | |
| Andrea rupestris Hedw. (A. petrophila Ehrh.) | Cairngorms (W.M.M. Eddie) | |
| Polytrichum formosum Raddi. | Roslin Glen | |
| Dicranum scoparium Hedw. | Roslin Glen | |
| Mnium hornum Hedw. | Roslin Glen | |
| Philonotis fontana (Hedw.) Brid. | Cairngorms | |
| Rhizomnium punctatum (Hedw.) Kop. (Mnium punctatum Hedw.) | Cairngorms | |
| Hookeria lucens (Hedw.) Sm. Pterygophyllum lucens (Hedw. Brid.) | Mull | |
| Thuidium tamariscinum (Hedw.) B, S & G | Roslin Glen | |
| Plagiothecium undulatum (Hedw.) B, S & G | Roslin Glen | |
| Hypnum cupressiforme Hedw. | Roslin Glen |
* CCAP, Culture Collection of Algae and Protozoa, Ambleside, Cumbria; material was kindly provided by Dr D. Long and Dr H. Sluiman (Royal Botanic Garden, Edinburgh), Dr D. S. Rycroft (Glasgow University), Professor R. Schumacker (University of Liege), Dr W. M. M. Eddie (University of Texas, Austin) and Professor D. Sanders (University of York). Other material was collected by Z.A.P. Collection sites were: Cairngorm Hills (57°05–10′N, 3°35–45′W), Roslin Glen (55°51′N, 3°10′W) and Faial Island (38°40′N, 28°40′W), UK.
Preparation of alcohol‐insoluble residue
Preparation of alcohol‐insoluble residue was as described by Popper et al. (2001).
Paper chromatography (PC) and thin‐layer chromatography (TLC)
Whatman No. 1 paper was used for analytical PC. One‐dimensional PC was by the descending method, whereas 2‐dimensional PC used the ascending method. Solvent systems used were: (1) butan‐1‐ol : acetic acid : water (12 : 3 : 5 by volume); (2) as for system 1 for 16 h followed, in the same dimension, by ethyl acetate : pyridine : water (8 : 2 : 1 by volume) for 18 h; (3) as for system 1 for 12 h followed, perpendicular to the first dimension, by phenol : water (4 : 1 w/w); and (4) ethyl acetate : pyridine : water (8 : 2 : 1 by volume) for 42 h. Paper chromatograms were stained with aniline hydrogen‐phthalate; faint spots were more readily visible by their fluorescence when viewed under a 366‐nm UV lamp (Fry, 2000). TLC was on Merck silica gel (VWR International, Poole, UK). The solvent system used was either (5) butanol : acetic acid : water (3 : 1 : 1 by volume), or (6) butanol : acetic acid : water (2 : 1 : 1 by volume). TLC plates were stained with thymol–sulfuric acid (Jork et al., 1994).
Paper electrophoresis (PE)
PE was on Whatman No. 1 paper. Samples were loaded 12 cm from the cathode end. The paper was wetted with buffer (acetic acid : pyridine : water; 10 : 1 : 378 by volume), blotted to remove excess and run with pH 3·5 buffer (acetic acid : pyridine : water; 10 : 1 : 189 by volume). Typical running conditions for paper of width 38 cm were 3·0 kV, 100 mA for 1·5 h. Electrophoretograms with radioactive markers were autoradiographed prior to staining with aniline hydrogen‐phthalate.
High‐pressure liquid chromatography (HPLC)
A Dionex HPLC (Camberley, UK) was used with a CarboPac PA1 anion‐exchange column (4 mm internal diameter, 250 mm long) and a pulsed amperometric detector. The flow rate was 1·0 ml min–1 at room temperature, and 20 µl samples in H2O were injected. Before injection, samples were filtered (Millex‐HV4 4 mm syringe filters, acetate membrane, pore size 0·45 µm; Millipore, Bedford, MA, USA). The separation method followed Gibeaut and Carpita (1993).
Ion‐exchange chromatography
Prior to ion exchange, samples were de‐lactonized by adjusting to pH 13 using 0·1 m NaOH and incubating for 8 s. Samples were neutralized with 0·1 m formic acid and made up to 1 ml before loading onto columns (1·5 ml bed volume) of Dowex 1 × 4–200 strongly basic anion exchanger in the chloride form (Sigma, Poole, UK). The resin was pre‐treated by washing (1 h each wash) in (a) 0·5 m NaOH; (b) twice in 0·5 m formic acid; and (c) in 2 m sodium formate. The resin was finally washed in buffer A (10 mm pyridinium formate, pH 5·5). Neutral sugars were eluted with 4 ml buffer A. The acidic fraction was then eluted with 4 ml buffer B (pyridine : formic acid : water; 1 : 1 : 23, pH 5·5). Neutral and acidic fractions were dried, re‐dissolved in 100 µl water and the neutral fraction was de‐salted using cation‐exchange columns of bed‐volume 1·5 ml. The cation‐exchange resin (Dowex 50W 8 100–200, H+ form from Sigma) was pre‐treated by shaking for 1 h in 1 m HCl then rinsing with water until the filtrate was neutral. The neutral sugars were eluted from the columns in 1·5 ml water then dried in vacuo and re‐dissolved in water ready for analysis.
Licheninase digestion
Ten milligrams AIR was heated at 120 °C for 2 h in 1·85 ml 100 mm collidine acetate buffer containing 0·5 % chlorobutanol, pH 7·0, in a tightly sealed tube to solubilize mixed‐linkage glucans. After cooling, 0·5 ml suspension was set aside as a control and 10 units of dialysed licheninase (Megazyme, Dublin, Ireland; E.C. 3.2.1.73) was added to the remaining 1·35 ml. After incubation for 24 h at 20 °C, samples were analysed by TLC (solvent system 5) or HPLC.
Hemicellulose extraction and Driselase digestion
For extraction of hemicelluloses, 20 mg AIR was shaken at 37 °C for 16 h with 10 ml 6 m NaOH containing 1 % NaBH4. The suspension was neutralized before being adjusted to pH 5·0 with acetic acid, dialysed for 24 h and freeze‐dried. Trifluoroacetic acid (TFA) (1 ml 0·1 m) was added to the samples and heated to 85 °C for 1 h; the samples were then dried in vacuo to remove the TFA. Driselase (Sigma; extracted from the basidiomycete fungus Irpex lacteus) solution (1 ml; 1 % w/v in a 2 % pyridinium acetate buffer containing 0·5 % chlorobutanol, pH 4·7) was added, and samples were digested for 2 d. To stop the reaction formic acid was added to a final concentration of 15 % v/v. Samples were dried in vacuo, resuspended in 300 µl water and the solution was then separated into neutral and acidic products. Neutral sugars were analysed by PC (solvent systems 1 and 2), TLC (solvent system 5) and HPLC.
Cellulase digestion
Hemicellulose extracted from 0·1 g AIR was dissolved in 1 ml 50 mm acetate buffer, Na+, pH 4·7. Cellulase (2·5 units; E.C. 3.2.1.4 isolated from Trichoderma longibrachium; Megazyme) was added and the mixture incubated at 20 °C for 2 d before analysis by TLC.
Xyloglucan‐specific endoglucanase (XEG) digestion
One milligram AIR was incubated for 5 min in 0·5 ml 50 mm acetate, Na+, pH 3·0 containing 0·1 % XEG (E.C. 3.2.1.4 isolated from Aspergillus aculeatus; Novo Nordisk, Bagsværd, Denmark; Pauly et al., 1999). The products were analysed by silica gel TLC (solvent systems 5 and 6).
Acid hydrolysis
Each AIR sample (20 mg) was subjected to hydrolysis in 1 ml 2 m TFA at 120 °C for 1 h. The hydrolysate was then dried in vacuo and re‐dissolved in 50 µl water. The hydrolysate was either subjected to 2‐dimensional PC or separated into neutral sugars and sugar acids by ion exchange. Neutral sugars were analysed by PC (solvent systems 1, 2 and 3), and the sugar acids by PE.
RESULTS
Mixed‐linkage glucan
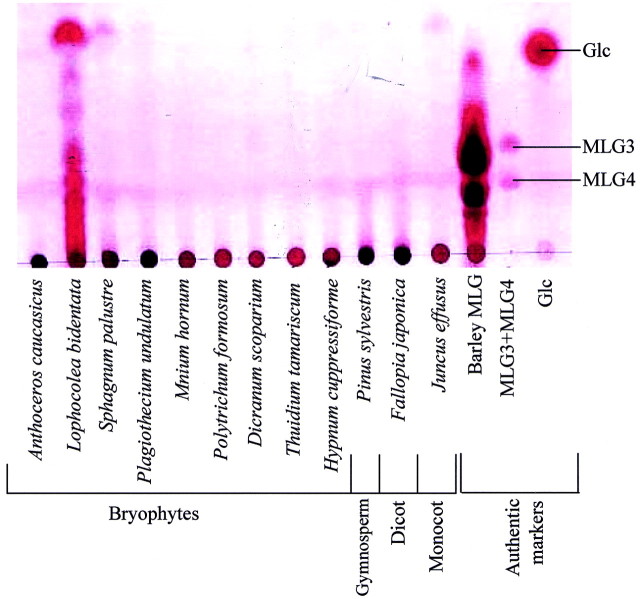
In an investigation of the taxonomic distribution of MLG, licheninase digests of PCWs of a variety of plants were analysed by TLC and HPLC. MLG was clearly detected in rye, maize, barley, wheat and Flagellaria guineensis, confirming the suitability of the methods used. MLG was undetectable in PCW‐rich material derived from the charophytes Chara corallina and Coleochaete scutata. In all but one of the bryophytes tested, MLG was undetectable (Fig. 1; Table 2). The exception was the leafy liverwort Lophocolea bidentata, in which licheninase digestion yielded a range of oligosaccharides. The major oligosaccharides [degree of polymerization (DP) approx. 2–6] were purified by gel‐permeation chromatography on Bio‐Gel P‐2 (Bio‐Rad, Hemel Hempstead, UK); acid hydrolysis of these yielded Glc and Ara, showing that these oligosaccharides differed from those of barley MLG, which contained only Glc (Woodward et al., 1983). However, the licheninase‐digestible polysaccharide in L. bidentata was not always detectable, being clearly visible in only five out of seven replicates of L. bidentata collected in May, and undetectable in four replicates collected in July.
Fig. 1. Licheninase digestion of bryophyte AIRs (alcohol‐insoluble residues). Digestion products were loaded on silica gel TLC, developed in butanol : acetic acid : water (3 : 1 : 1) and stained with thymol–H2SO4. MLG3, MLG4, Tri‐ and tetrasaccharide repeat‐units of barley mixed‐linkage glucan.
Table 2.
Polysaccharide and monosaccharide content of plant taxa
| Uronic acids | |||||||||
| Classification | Species | MLG | Xyloglucan | GalA | GlcA | αGlcA‐(1→3)‐Gal | Mannose | 3‐O‐Methylrhamnose | |
| Algae | Chlorophyte | Ulva lactuca | * | – | ++ | ++ | ± | ||
| Charophytes | Chara corallina | – | – | ++ | + | – | ± | + | |
| Coleochaete scuata | – | – | ++ | + | ? | + | |||
| Klebsormidium flaccidium | – | – | + | ||||||
| Bryophytes | Hornwort | Anthoceros caucasicus | – | + | +++ | ++ | +++ | + | + |
| Thalloid liverworts | Lunularia cruciata | – | + | ++ | + | ++ | |||
| Pellia epiphylla | – | – | + | + | |||||
| Leafy liverworts | Trichocolea tomentella | – | + | ? | +++ | + | |||
| Lepidozia reptans | – | + | +++ | ||||||
| Nardia scalaris | – | + | + | ||||||
| Marsupella emarginata | – | + | – | ++ | + | ||||
| Plagiochila asplenioides | – | + | +++ | ||||||
| Lophocolea bidentata | (*) | + | ++ | ||||||
| Scapania undulata | – | ++ | ++ | ++ | |||||
| Pleurozia purpurea | – | + | +++ | ||||||
| Porella cordaeana | – | + | – | +++ | + | ||||
| Mosses | Sphagnum palustre | – | + | ++ | ++ | ++ | |||
| Sphagnum molle | – | + | – | ++ | + | ||||
| Andrea rupestris | – | + | – | + | + | ||||
| Polytrichum formosum | – | +++ | |||||||
| Dicranum scoparium | – | + | ++ | ± | ++ | ||||
| Mnium hornum | – | + | ++ | ± | ++ | ||||
| Philonotis fontana | – | + | ++ | ||||||
| Rhizomnium punctatum | – | + | +++ | ||||||
| Hookeria lucens | – | +++ | |||||||
| Thuidium tamariscinum | – | + | ++ | ± | ++ | ||||
| Plagiothecium undulatum | – | + | ++ | ± | ++ | ||||
| Hypnum cupressiforme | – | + | ++ | ± | – | ++ | |||
| Vascular | Lycopodiophytes | – | + | + | ± | – | +++ | † | |
| plants | Eusporangiate ferns | – | + | + | ± | – | ± | – | |
| Leptosporangiate ferns | – | + | + | ± | – | ± | – | ||
| Gymnosperms | – | + | + | ± | – | ± | – | ||
| Angiosperms, dicotyledons | – | + | + | ± | – | ± | – | ||
| Angiosperms, gramineous monocotyledons and Flagellaria | + | ± | + | ± | – | ± | – | ||
–, Not detectable; ±, trace; +, present at low concentration; ++, present at moderate concentration; +++, present at high concentration; blank cell, not tested.
* Licheninase‐digestible polysaccharide present (in the case of Lophocolea not always detectable; see text), but not simple MLG.
† 3‐O‐methylrhamnose was present in homosporous lycopodiophytes but absent from homosporous lycopodiophytes.
Licheninase was also able to digest polysaccharides from the chlorophyte Ulva lactuca to products that differed from those typically produced on digestion of MLG from gramineous monocotyledons in having a higher DP and containing xylose as well as glucose (data not shown). The reducing terminus of these oligosaccharides was glucose, as shown by production of [3H]glucitol upon reaction with NaB3H4 (data not shown).
Xyloglucan
Driselase digestion yields the disaccharide isoprimeverose from xyloglucan but from no other known polymer. PC and HPLC of the Driselase digests of PCW‐rich material from a variety of land plants showed isoprimeverose to be an appreciable component in all the land plants tested, including bryophytes (Table 2). However, isoprimeverose was not detectable in Driselase digests of PCW‐rich material derived from the charophyte Chara (Figs 2 and 3). If exogenous xyloglucan was added to Chara AIR prior to Driselase digestion, isoprimeverose was detectable among the products. The limits of detection were 0·01 % xyloglucan (w/w, xyloglucan : AIR) by PC (Fig. 2) and 0·02 % (w/w, xyloglucan : AIR) by HPLC (Fig. 3). Some monosaccharides are released from Chara by pre‐treatment with 0·1 m TFA; they can be clearly seen in the non‐digested Chara sample.
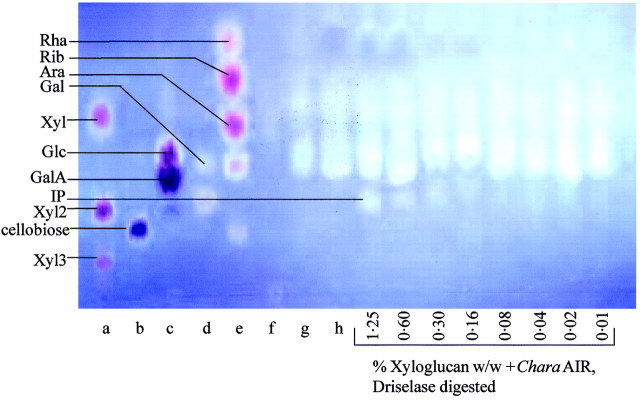
Fig. 2. Paper chromatogram (PC) of Driselase digest of Chara corallina AIR. Samples were pre‐treated with 0·1 m TFA prior to Driselase digestion. Samples (with the exception of non‐digested and Driselase‐digested Chara AIR) had an internal marker of xyloglucan (0·01–1·25 %, w/w, with respect to the weight of AIR) added prior to Driselase digestion. The PC was developed in butanol : acetic acid : water (12 : 3 : 5), stained with aniline hydrogen‐phthalate, and photographed under UV. IP, Isoprimeverose; Xyl2, xylobiose; Xyl3, xylotriose. Markers were (a) xylose, xylobiose and xylotriose; (b) cellobiose; (c) Glc + GalA; (d) IP + Gal; and (e) Rha, Rib, Ara, Gal, lactose. Samples were (f) Driselase; (g) mild‐acid treated Chara AIR; and (h) Driselase digest of mild‐acid pre‐treated Chara AIR in the absence of exogenous xyloglucan.
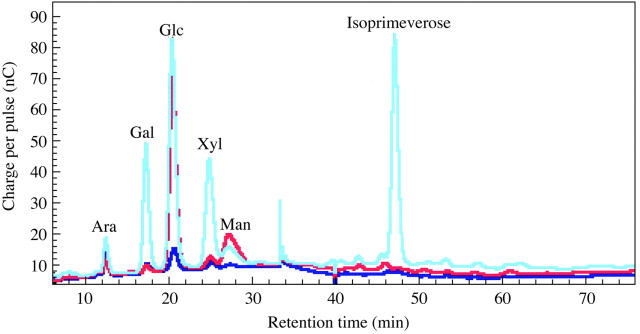
Fig. 3. HPLC of Driselase digestion‐products from Chara AIR. Samples are a Driselase digest of mild‐acid pre‐treated Chara AIR (red line), a Driselase digest of mild‐acid pre‐treated tamarind xyloglucan (cyan line) and mild‐acid pre‐treated Chara AIR (blue line).
Sequential pectic and hemicellulosic extracts of Chara PCW‐rich material also yielded no isoprimeverose on Driselase digestion (results not shown). Driselase digestion of Coleochaete AIR also did not result in the production of isoprimeverose in a quantity detectable by our methods (limit of detection approx. 0·01 % w/w; results not shown).
Although cellulase digested Chara, none of the digestion products corresponded to the xyloglucan oligosaccharides (e.g. XXXG, XXFG, XLLG, XFFG; Fry et al., 1993) typically produced by cellulase digestion of xyloglucans from dicotyledons. The cellulase digestion products of Chara AIR appeared to correspond to oligosaccharides of xylan, which is also partially digested by cellulase (TLC results not shown). XEG, a specific xyloglucanase, did not digest any of the polysaccharide fractions sequentially extracted from Chara (Fig. 4A). Additionally, XEG did not digest AIR from Klebsormidium (Fig. 4B) or Nitella (M. Pauly, Max Planck Institut für Pflanzenphysiologie, Golm, Germany, pers. comm.). XEG digested bryophyte AIR to yield low concentrations of the xyloglucan‐derived oligosaccharide XXXG (TLC results not shown).
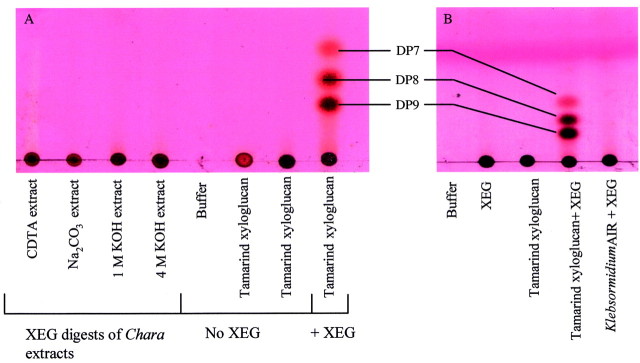
Fig. 4. XEG digestion products of charophyte AIR. A, Products from Chara polysaccharide extracts prepared by the method of Morrison et al. (1993). B, Products from Klebsormidium flaccidium AIR. Products were developed on silica gel TLC in either butanol : acetic acid : water 3 : 1 : 1 (A) or butanol : acetic acid : water 2 : 1 : 1 (B) and stained with thymol–H2SO4. The streaking seen in the CDTA extract is due to the presence of CDTA still remaining in the preparation after dialysis for 72 h. DP 7, 8 and 9 indicate the xyloglucan‐derived oligosaccharides XXXG, XXLG and XLLG (XLLG and XXFG are not separated by these methods).
TFA hydrolysis of Coleochaete and Klebsormidium AIRs showed that they each had approximately equimolar xylose and glucose residues. Since xyloglucan was undetectable, this Xyl probably arose from β‐xylans. Very little xylose, a major component of xyloglucan, was released on TFA hydrolysis of Coleochaete AIR; again, this is compatible with the absence of xyloglucan. According to all the above criteria, xyloglucan appears to be absent from charophycean green algae.
Uronic acids
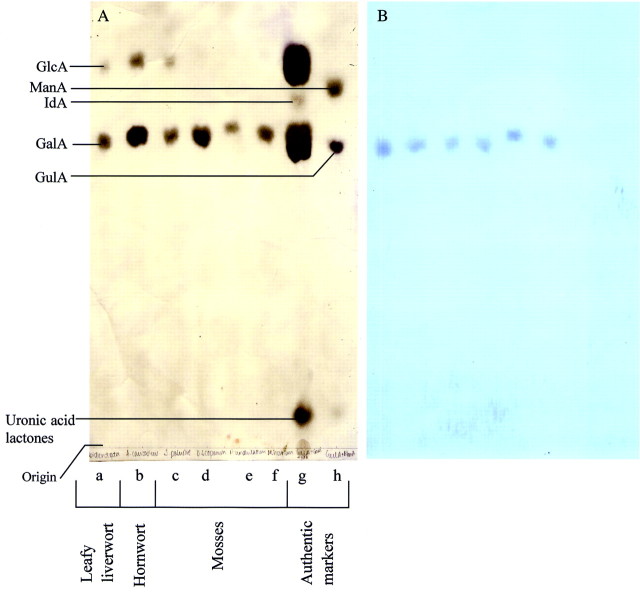
In the PCW, uronic acid residues are found as constituents of xylans, pectins, glucuronomannans and arabinogalactan‐proteins. The acidic fraction of TFA hydrolysates from a variety of land plants was analysed by PE. GalA was found to be the major uronic acid in all the bryophytes and charophycean green algae tested (Table 2; Fig. 5). The concentration of GalA was higher in the charophycean green algae and bryophytes than in any of the vascular plants (Table 3). Additionally, GlcA was found in high concentration in the two charophytes tested (Chara and Coleochaete), leafy and thalloid liverworts, and Sphagnum (one of the most basal moss genera) (Fig. 5 and similar results, not shown). GlcA was not present in high concentration in any of the more recently evolved moss lineages or in vascular plants.
Fig. 5. Paper electrophoresis (PE) of the acidic fraction of acid hydrolysates of bryophyte AIRs. Samples were run by PE at pH 3·5 and 3·0 kV for 1·5 h. An internal marker of [14C]GalA (trace; not detectable by staining) was added to each hydrolysate (a–f) before loading onto the PE. A, PE stained with silver nitrate; B, autoradiogram of PE. Hydrolysates were from (a) Lophocolea bidentata, (b) Anthoceros caucasicus, (c) Sphagnum palustre, (d) Dicranum scoparium, (e) Plagiothecium undulatum and (f) Mnium hornum. Markers were (g) GalA + iduronic acid (IdA) + GlcA and (h) ManA + guluronic acid (GulA).
Table 3.
Summary of the major trends in polysaccharide composition of plant taxa
| Group | Isoprimeverose (xyloglucan) | GalA | GlcA | αGlcA‐ (1→3)‐Gal | Man | 3‐O‐MeGal | 3‐O‐MeRha |
| Charophytes | – | ++ | + | – | ± | – | + |
| Hornwort | + | +++ | ++ | +++ | + | – | + |
| Liverworts & Sphagnum | + | ++ | ++ | – | +++ | – | + |
| Advanced mosses | + | ++ | ± | – | ++ | – | + |
| Homosporous lycopodiophytes | + | + | ± | – | +++ | + | + |
| Heterosporous lycopodiophytes | + | + | ± | – | +++ | + | – |
| Euphyllophytes | + | + | ± | – | + | – | – |
–, not detectable; ±, trace; +, present at low concentration; ++, present at moderate concentration; +++, present at high concentration.
Mannuronic acid has been reported as the major uronic acid residue in the PCWs of the leafy liverwort Riccardia (Das and Rao, 1963, 1966a, b). However, our methods did not detect ManA in any land plants, including liverworts. Both GlcA and GalA residues were clearly detected in the cell walls of all liverworts tested, both thalloid and leafy, as well as in the hornwort Anthoceros. Das and Rao (1963, 1966) did not report the presence of either GlcA or GalA in the cell walls of Riccardia, but reported that the RF values (mobility of compund relative to that of the solvent front) of GlcA and ManA on PC in ethyl acetate : pyridine : acetic acid : water (5 : 5 : 1 : 3) only differed slightly. Therefore, it is possible that ManA is not present in liverwort cell walls and that Das and Rao (1966a, b) mistook either GlcA or GalA for ManA.
By our method of separation (PE), 4‐O‐methylglucuronic acid (4‐O‐Me‐GlcA) is only just distinguishable from GlcA. We found that 4‐O‐Me‐GlcA was either undetectable or indistinguishable from GlcA in any of the land plants analysed. 4‐O‐Me‐GlcA is known from xylem (secondary cell walls) and may be present at much lower concentrations in primary cell walls. Darvill et al. (1980) reported that in suspension‐cultured sycamore cells, 4‐O‐Me‐GlcA constitutes approx. 0·25 % of the monosaccharide residues present in the wall (on a dry weight basis). This correlates with the results of Harris et al. (1997), who reported that the unlignified cell walls of dicotyledons and non‐gramineous monocotyledons contain 0·3–0·8 % 4‐O‐Me‐GlcA w/w of the cell wall.
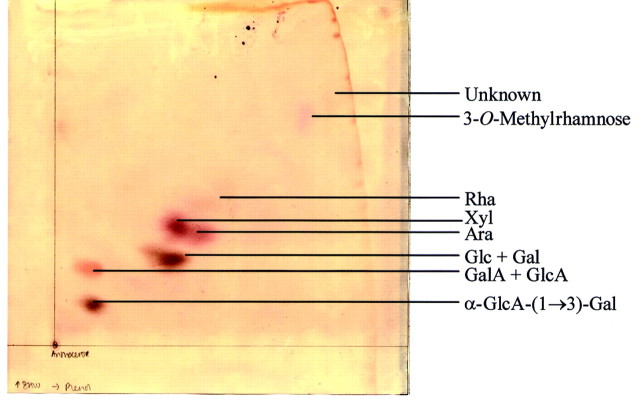
TFA hydrolysis of AIR from Anthoceros gave large amounts of an acidic disaccharide (Fig. 6). This product was purified by chromatographic methods and has recently been identified as α‐GlcA‐(1→3)‐Gal by N.M.R. (I. H. Sadler, pers. comm.). It appears to be confined to Anthoceros.
Fig. 6. Acid hydrolysis products of Anthoceros caucasicus (hornwort) AIR. Products were separated by 2‐dimensional PC in butanol : acetic acid : water (12 : 3 : 5) (vertical on image) followed by 80 % w/w phenol (horizontal) and stained with aniline hydrogen‐phthalate.
3‐O‐Methylrhamnose
3‐O‐Methylrhamnose (Table 2; Fig. 6) has not previously been reported from charophytes, bryophytes or homosporous lycopodiophytes. We have purified this unusual sugar from acid hydrolysates of the AIR of the hornwort Anthoceros by PC and characterized it by NMR (data not shown; collaboration with I. H. Sadler, Department of Chemistry, The University of Edinburgh, UK). We found it to occur in the cell walls of charophytes, bryophytes and homosporous lycopodiophytes (Table 2).
Mannans
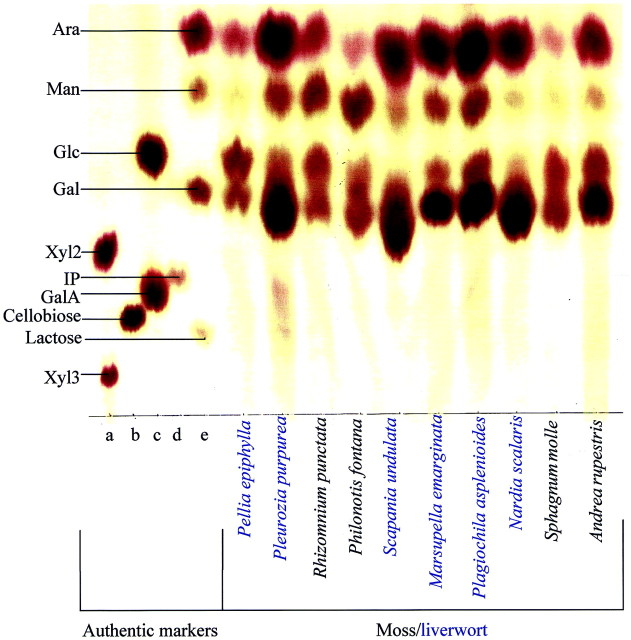
In the PCW, mannose residues are present in mannans, glucomannans, galactoglucomannans and glucuronomannans, with lower concentrations of mannose residues present in many wall enzymes such as peroxidases. In an investigation of the distribution and relative quantity of mannose residues, the neutral fraction of TFA hydrolysates from a variety of land plants was analysed by PC. Mannose residues were found in higher concentration in most of the bryophytes (Fig. 7; Table 2), lycopodiophytes, psilotophytes, equisetophytes and eusporangiate ferns (data not shown) than in Ulva lactuca and the charophytes, leptosporangiate ferns, gymnosperms and angiosperms (Table 2).
Fig. 7. PC of acid hydrolysis products of various bryophyte AIRs. The solvent was butan‐1‐ol : acetic acid : water (12 : 3 : 5) for 16 h followed by ethyl acetate : pyridine : water (8 : 2 : 1) for 18 h, and staining was with aniline hydrogen‐phthalate. Authentic markers were (a) Xyl2 and Xyl3; (b) cellobiose; (c) Glc + GalA; (d) IP; and (e) Ara, Man, Gal and lactose. IP, Isoprimeverose; Xyl2, xylobiose; Xyl3, xylotriose.
DISCUSSION
This is the first detailed study of the cell wall compositions of bryophytes in comparison with those of their charophycean ancestors and vascular successors. Cell walls of charophycean green algae, a hornwort, thalloid and leafy liverworts, and primitive mosses were found to contain high concentrations of GlcA and GalA residues. In contrast, walls of the more advanced (and more drought‐tolerant) mosses have a much lower concentration of GlcA. Additionally, a novel disaccharide is described from hornwort cell walls. The PCW polysaccharide xyloglucan was found to be present in all land plants but is lacking from the PCWs of charophycean green algae. Mannose was found to be present in high concentration in the PCWs of liverworts, mosses and lycopodiophytes, and present at low concentration in the PCWs of charophytes and euphyllophytes (= all vascular plants other than lycopodiophytes; Kenrick and Crane, 1997).
The results indicate that major changes in PCW composition accompanied major evolutionary steps (Table 3). In particular, there are quantitative and qualitative differences between the charophytes and the bryophytes, and between those bryophytes thought to be primitive and those thought to be more advanced (Bopp and Capesius, 1996; Hedderson et al., 1996). This supports Stebbins’ hypothesis (1992) that alterations in cell wall composition may have been associated with bryophyte diversification.
The first land plants are thought to have resembled extant bryophytes (Mishler et al., 1994). Hornworts and liverworts cling to the moist substrata on which they grow, and some primitive mosses grow submerged in water. In the absence of specialized conducting tissues, height is physiologically limited by the time it takes water to diffuse between cells and reach aerial portions of the plant. The time for water to diffuse through a string of cells end on end is relatively long (Nobel, 1983). Therefore, some kind of conducting tissue is a physiological necessity for the continued vertical growth of land plants. Increased vertical growth increases competitiveness and survival by allowing greater access to light. The water‐conducting tissue, hydrom, of advanced mosses, and the apparently homologous xylem of vascular plants (Scheirer, 1980; Mishler and Churchill, 1984, 1985; Mishler et al., 1994) solve this problem in these plants; and the problem does not arise with aquatic charophytes. However, primitive bryophytes may require unique sponge‐like properties of their PCW to permit the efficient distribution of water between all the parenchymatous cells of their aerial tissues. Thus, the characteristic features of lower bryophytes (high GalA, GlcA and Man) may be connected with this requirement. One of the major differences observed between primitive bryophytes (hornworts, liverworts and the moss genus Sphagnum) and more advanced bryophytes (all other moss genera investigated) was the high concentration of GlcA in primitive bryophytes; GlcA was present at very low concentration in more advanced bryophytes and all other land plants investigated.
The shared presence of high GlcA concentration in charophytes and primitive bryophytes is notable as GlcA was present at very low concentration in all other land plants investigated. GalA was also present at higher concentrations in charophytes and bryophytes than in vascular plants. Both these observations support molecular and morphological evidence that suggests that bryophytes (and ultimately all land plants) evolved from a common ancestor within the charophytes (Pickett‐Heaps, 1976; Mattox and Stewart, 1984; Graham, 1993).
However, we found xyloglucan to be absent from the charophytes Chara, Coleochaete and Klebsormidium, although present in all land plants investigated. This conclusion conflicts with previous reports suggesting that xyloglucan is present in the charophycean cell wall. Anderson and King (1961) reported that Chara has a cell wall composition similar to that of land plants. This comparison was based on the relative concentrations of the main classes of cell wall polymers (hemicellulose, pectic polysaccharides and cellulose) present in Chara and a variety of land plants. However, the hemicellulose fraction was not characterized and the presence of hemicellulose does not prove the existence of xyloglucan. Morrison et al. (1993) reported that the 1 m KOH extract of Nitella contained xyloglucan. The extracted polysaccharide was reported to be composed of 10 % Fuc, 21·7 % Xyl, 10·4 % Gal and 45·8 % Glc residues w/w (Morrison et al., 1993). Xyloglucan from dicotyledons has a similar monosaccharide composition. However, methylation analysis revealed that all the Xyl in the Nitella polysaccharide was 2‐ and/or 4‐linked Xyl. If xyloglucan with a similar structure to that in angiosperm cell walls had been present, terminal Xyl would have also been present. Therefore, the results of Morrison et al. (1993) do not conclusively show the presence of xyloglucan in Nitella cell walls; many of the sugar linkages reported could result from the presence of other hemicelluloses such as xylans (4‐linked Xyl) and possible contamination with starch (4‐ and 4,6‐linked Glc). Acid hydrolysis of Coleochaete AIR yielded a very low concentration of xylose residues, confirming that xyloglucan could not have been a major component.
All land plants ultimately descend from a common algal ancestor from which the modern Charophyceae are also descended (Pickett‐Heaps, 1976; Mattox and Stewart, 1984; Graham, 1993). It is possible that charophycean green algae have indigestible xyloglucan with no unsubstituted Glc. Such xyloglucan would fail to yield isoprimeverose upon Driselase digestion or oligosaccharides upon cellulase or XEG digestion. However, Morrison et al. (1993) reported the presence of 4‐linked Glc in Nitella hemicellulose, and xyloglucan with 4‐linked Glc residues would be expected to be digestible by any of the three enzymes used in this study.
Driselase digestion yields only a low concentration of isoprimeverose from bryophyte AIR. Therefore, xyloglucan is likely to be present in bryophyte PCW at a much lower concentration than is typically found in the angiosperm PCW. However, it is possible that xyloglucan in the bryophyte PCW may have reduced digestibility, either because of the structure of the polymer itself, or because of the structure and composition of the PCW. Additionally, XEG digestion of bryophyte AIR yielded only a low concentration of the xyloglucan‐derived oligosaccharide XXXG. Bryophyte xyloglucan therefore appears to differ from typical angiosperm xyloglucan both in concentration and structure. It is possible that the bryophytes do not have all the enzymes required to make typical angiosperm xyloglucan.
Glucomannans have been found as a major hemicellulose component in the secondary cell walls of the gymnosperms (10 % total cell wall w/w) and a minor hemicellulose component in the secondary cell walls of dicotyledons (3–5 % total cell wall w/w) (Matheson, 1990). They have been reported to constitute approx. 15 % of the total secondary cell walls of a fern, Pteridium aquilinum (Bremner and Wilkie, 1971). Our results show that the PCWs of leptosporangiate ferns, gymnosperms and angiosperms contained far less mannose than those of the bryophytes, lycopodiophytes, equisetophytes and psilotophytes. It is possible that the PCW composition of the bryophytes, lycopodiophytes, equisetophytes and psilotophytes is similar to that of the secondary cell wall of leptosporangiate ferns, gymnosperms and angiosperms.
The unusual disaccharide repeat unit α‐GlcA‐(1→3)‐Gal from Anthoceros (hornwort) AIR has been characterized. Hornworts are thought to be the most primitive land plants (Renzaglia et al., 2000). The occurrence of the unusual disaccharide repeat unit α‐GlcA‐(1→3)‐Gal in Anthoceros, and its absence from all other land plants investigated, may suggest that the polysaccharide containing the disaccharide repeat unit was not essential to land plant survival. The disaccharide repeat unit may be associated with mucilage rather than PCW, and potentially may be a way in which algae growing in areas that dry out seasonally, and primitive land plants, prevent desiccation. The high concentration of uronic acid in the mucilage or PCW could potentially increase water retention.
3‐O‐Methylrhamnose (trivial name acofriose) was detected in the PCWs of charophytes, bryophytes and homosporous lycopodiophytes. Best known from bacteria (Williams and Wander, 1980), this sugar has been reported from polymers of the fern Osmunda (Akiyama et al., 1988), as a component of an acidic polysaccharide in the green alga Chlorella (Ogawa et al., 1997), and in resin exudates from gymnosperms (Anderson and Munro, 1969). 3‐O‐Methylrhamnose is present in the PCWs of charophytes, their closest immediate descendants, the bryophytes, and homosporous lycopodiophytes. It seems likely that many different plant taxa, excluding the angiosperms, have the ability to synthesize 3‐O‐methylrhamnose. However, most taxa synthesize low concentrations of 3‐O‐methylrhamnose or have lost the pathway for methylation of rhamnose as it does not appear to be necessary for land plant survival. The existence of 3‐O‐methylrhamnose in a wide range of diverse plants could be explained by the rapid divergence of lineages that occurred during the emergence of the euphyllophytes (Pryer et al., 2001).
In conclusion, primary cell wall composition has been found to differ between monophyletic groups of plants (supported by morphological and molecular data). It is likely that during the emergence of these groups, plants were subjected to particularly rigorous selection pressures. The emergence of these groups is also linked with fundamental changes in plant habitat e.g. the aquatic‐to‐land transition, and with the acquisition of vascular tissue and upright habit. Variation in cell wall composition may be related to ecological pressures experienced during evolution. In particular, the presence of xyloglucan in all land plants and its absence from all charophytes investigated suggests that the acquisition of xyloglucan may have been a pre‐adaptive advantage for the colonization of land.
ACKNOWLEDGEMENTS
We thank Dr D. Long (Royal Botanic Garden, Edinburgh) and Dr D. S. Rycroft (Glasgow University), who kindly assisted in the provision and identification of several bryophytes. Z.A.P. thanks the BBSRC for a research studentship.
Supplementary Material
Received: 26 June 2002; Returned for revision: 24 September 2002; Accepted: 15 October 2002
References
- AkiyamaT, Tanaka K, Yamamoto S, Iseki S.1988. Blood‐group active proteoglycan containing 3‐O‐methylrhamnose (acofriose) from young plants of Osmunda japonica Carbohydrate Research 178: 320–326. [DOI] [PubMed] [Google Scholar]
- AndersonDMW, King NJ.1961. Polysaccharides of the Characeae. III. The carbohydrate content of Chara australis Biochimica et Biophysica Acta 52: 449–454. [DOI] [PubMed] [Google Scholar]
- AndersonDMW, Munro AC.1969. The presence of 3‐O‐methyl rhamnose in Araucaria resinous exudates. Phytochemistry 8: 633–634. [Google Scholar]
- BoppM, Capesius I.1996. New aspects of bryophyte taxonomy provided by a molecular approach. Botanica Acta 109: 1–5. [Google Scholar]
- BremnerI, Wilkie KCB.1971. The hemicelluloses of bracken. II. A galactoglucomannan. Carbohydrate Research 20: 193–203. [DOI] [PubMed] [Google Scholar]
- BrettCT, Waldron KW.1996. Physiology and biochemistry of plant cell walls. London: Chapman and Hall. [Google Scholar]
- CassabGI.1998. Plant cell wall proteins. Annual Review of Plant Physiology and Plant Molecular Biology 49: 281–309. [DOI] [PubMed] [Google Scholar]
- ChodatF, Cortesi R.1939. Sur la coloration des membranes de mousses. Comptes Rendus des Archives des Sciences Physiques et Naturelles de Genève Series 5C 21: 58–61. [Google Scholar]
- DarvillJE, McNeil M, Darvill AG, Albersheim P.1980. Structure of plant cell walls. XI. Glucuronoarabinoxylan, a second hemicellulose in the primary cell walls of suspension‐cultured sycamore cells. Plant Physiology 66: 1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasVSR, Rao MP.1963. Non‐volatile organic acids in some liverworts. Nature 198: 970.13935362 [Google Scholar]
- DasVSR, Rao MP.1966a Cell wall sugars of the liverwort Riccardia Indian Journal of Experimental Biology 5: 193–194. [Google Scholar]
- DasVSR, Rao MP.1966b Mannuronic acid in the cell‐wall of Riccardia, a jungermannialian liverwort. Current Science 1: 20–21. [Google Scholar]
- DumvilleJC, Fry SC.1999. Uronic acid‐containing oligosaccharins: their biosynthesis, degradation and signalling roles in non‐diseased plant tissues. Plant Physiology and Biochemistry 38: 125–140. [Google Scholar]
- EdelmannH, Neinhuis C, Jarvis MC, Evans B, Fischer E, Barthlott W.1998. Ultrastructure and chemistry of the cell wall of the moss Rhacocarpus purpurascens (Rhacocarpaceae): a puzzling architec ture among plants. Planta 206: 315–321. [Google Scholar]
- FrySC.1999. Plant cell walls. In: Encyclopedia of life sciences London: Nature Publishing Group. http://www.els.net [Google Scholar]
- FrySC.2000. The growing plant cell wall: chemical and metabolic analysis. Caldwell, New Jersey, USA: The Blackburn Press. [Google Scholar]
- FrySCet al.1993. An unambiguous nomenclature for xyloglucan‐derived oligosaccharides. Physiologia Plantarum 89: 1–3. [Google Scholar]
- GeddesDS, Wilkie KCB.1971. Hemicelluloses from the stem tissues of the aquatic moss Fontinalis antipyretica Carbohydrate Research 18: 333–335. [Google Scholar]
- GibeautDM, Carpita NC.1993. Synthesis of (1→3),(1→4)‐β‐d‐glucan in the Golgi apparatus of maize coleoptiles. Proceedings of the National Academy of Sciences of the USA 90: 3850–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GoldbergR, Prat R, Morvan C.1994. Structural features of water‐soluble pectins from mung bean hypocotyls. Carbohydrate Polymers 23: 203–210. [Google Scholar]
- GotteliLB, Cleland R.1968. Differences in the occurrence and distribution of hydroxyproline‐proteins among the algae. American Journal Botany 55: 907–914. [PubMed] [Google Scholar]
- GrahamLE.1993. Origin of land plants. New York: Wiley. [Google Scholar]
- HarrisPJ, Kelderman MR, Kendon MF, Mckenzie RJ.1997. Monosaccharide compositions of unlignified cell walls of the monocotyledons in relation to the occurrence of wall‐bound ferulic acid. Biochemical Systematics and Ecology 25: 167–179. [Google Scholar]
- HeddersonTA, Chapman RL, Rootes WL.1996. Phylogenetic relationships of bryophytes inferred from nuclear encoded rRNA gene sequences. Plant Systematics and Evolution 200: 213–224. [Google Scholar]
- JorkH, Funk W, Fischer W, Wimmer H.1994. Thin layer chromatography: reagents and detection methods. Volume 1b, Physical and chemical detection methods; activation reactions, reagent sequences, reagents II. Weinheim: VCH. [Google Scholar]
- KenrickP, Crane PR.1997. The origin and early evolution of plants on land. Nature 389: 33–39. [Google Scholar]
- KieliszewskiMJ, Lamport DTA.1994. Extensin: repetitive motifs, functional sites, post‐translational codes and phylogeny. Plant Journal 5: 157–172. [DOI] [PubMed] [Google Scholar]
- KranzHD, Mikš D, Siegler M‐L, Capesius L, Sensen CW, Huss VAR.1995. The origin of land plants: phylogenetic relationships among charophytes, bryophytes and vascular plants inferred from complete small‐subunit ribosomal RNA sequences. Journal of Molecular Evolution 41: 74–84. [DOI] [PubMed] [Google Scholar]
- ManhartJR, Palmer JD.1990. The gain of two chloroplast tRNA introns marks the green algal ancestors of land plants. Nature 345: 268–270. [DOI] [PubMed] [Google Scholar]
- MattoxKR, Stewart KD.1984. Classification of the green algae: a concept based on comparative cytology. In: Irvine DEG, John DM, eds. Systematics of green algae London, Ontario: Academic Press, 29–72. [Google Scholar]
- MathesonNK.1990. Mannose‐based polysaccharides. In: Dey PM, Harborne JB, eds. Methods in plant biochemistry Volume2: Carbohydrates. London: Academic Press, 371–413. [Google Scholar]
- MishlerBD, Churchill SP.1984. A cladistic approach to the phylogeny of the ‘bryophytes’. Brittonia 36: 406–424. [Google Scholar]
- MishlerBD, Churchill SP.1985. Transition to a land flora: phylogenetic relationships of the green algae and bryophytes. Cladistics 1: 305–328. [DOI] [PubMed] [Google Scholar]
- MishlerBD, Lewis LA, Buchheim MA, Renzaglia KS, Garbary DJ, Delwiche CF, Zechman FW, Kantz, Chapman RL.1994. Phylogenetic relationships of the ‘green algae’ and ‘bryophytes’. Annals of the Missouri Botanic Garden 81: 451–483. [Google Scholar]
- MorrisonJC, Greve LC, Richmond PA.1993. Cell wall synthesis during growth and maturation of Nitella internodal cells. Planta 189: 321–328. [DOI] [PubMed] [Google Scholar]
- NobelPS.1983. Biophysical plant physiology and ecology. New York: Freeman. [Google Scholar]
- OgawaK, Yamaura M, Maruyama I.1997. Isolation and identification of 2‐O‐methyl‐l‐rhamnose and 3‐O‐methyl‐l‐rhamnose as constituents of an acidic polysaccharide of Chlorella vulgaris.Bioscience Biotechnology and Biochemistry 61: 539–540. [DOI] [PubMed] [Google Scholar]
- PaulyM, Anderson LN, Kaupinnen S, Kofod LV, York WS, Albersheim P, Darvill AG.1999. A xyloglucan‐specific endo‐β‐1,4‐glucanase from Aspergillus aculeatus: expression in yeast, purification and characterisation of the recombinant enzyme. Glycobiology 9: 93–100. [DOI] [PubMed] [Google Scholar]
- Pickett‐HeapsJD.1976. Cell division in eucaryotic algae. Bioscience 26: 445–450. [Google Scholar]
- PopperZA, Sadler IH, Fry SC.2001. 3‐O‐Methyl‐d‐galactose residues in lycophyte primary cell walls. Phytochemistry. 57: 711–719. [DOI] [PubMed] [Google Scholar]
- PryerKM, Schneider H, Smith HR, Cranfill R, Wolf PG, Hunt JS, Sipes SD.2001. Horsetails and ferns are a monophyletic group and are the closest living relatives to seed plants. Nature 409: 618–622. [DOI] [PubMed] [Google Scholar]
- RenzagliaKS, Duff RJ, Nickrent DL, Garbary DJ.2000. Vegetative and reproductive innovations of early land plants: implications for a unified phylogeny. Philosophical Transactions of the Royal Society of London Series B—Biological Sciences 355: 769–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ScheirerDC.1980. Differentiation of bryophyte conducting tissues: structure and histochemistry. Bulletin of the Torrey Botanical Club 107: 298–307. [Google Scholar]
- StaceCA.1981. Plant taxonomy and biosystematics. 2nd edn. Cambridge: Cambridge University Press. [Google Scholar]
- StebbinsGL.1992. Comparative aspects of plant morphogenesis: a cellular, molecular and evolutionary approach. American Journal of Botany 79: 589–598. [Google Scholar]
- ThomasJJ, McNeil M, Darvill AG, Albersheim P.1984. Structure of plant cell walls. 29. Isolation and characterization of wall polysaccharides from suspension‐cultured Douglas fir cells. Plant Physiology 83: 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ThomasRJ.1977. Wall analyses of Lophocolea seta cells (Bryophyta) before and after elongation. Plant Physiology 59: 337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WilliamsNR, Wander JD,1980. Deoxy and branched‐chain sugars. In: Pigman W, Horton D, eds. The carbohydrates: chemistry and biochemistry vol. IB 2nd edn New York: Academic Press, 761–798. [Google Scholar]
- WoodwardJR, Fincher GB, Stone BA.1983. Water soluble (1→3)(1→4)‐β‐d‐glucans from barley (Hordeum vulgare) endo sperm. II. Fine structure. Carbohydrate Polymers 3: 207–225. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.