Abstract
The vascularization of the pedicel in Marisol clementine (Citrus clementina Hort. ex Tanaka) has been characterized in relation to fruit growth. Phloem and xylem formation occurred during the first half of the period of fruit growth. Phloem cross‐sectional area reached its maximum value by the end of fruitlet abscission, 78 d after anthesis (DAA), shortly after the rate of accumulation of dry matter in fruitlets reached its maximum value. Secondary xylem formation occurred until day 93, well after the end of fruitlet abscission. At fruit maturity, xylem accounted for 42–46 % of the cross‐section of the pedicel. Vessels differentiated in this late‐formed xylem. Formation of phloem and early xylem was directly related to fruitlet size (and growth rate). Differences in the rate of formation of conductive tissues in the pedicel of the developing fruitlets followed rather than preceded the differences in growth rate. Specific mass transfer (SMT) in the phloem was highest in the fastest growing fruitlets, and peaked during the late stages of fruitlet abscission (72–78 DAA) and during the main period of fruit growth (107–121 DAA). Application of a synthetic auxin to developing fruits, either at the end of flowering (2,4‐D) or by day 64 after flowering (2,4‐DP), increased the growth rate of the fruit and fruit size at maturity (8–13 % increase in fruit diameter at maturity). These auxin applications also enhanced the formation of conductive tissues in the pedicel, with a specific effect on phloem formation. Applying auxin at flowering resulted in a reduction in the phloem SMT by days 72–78, whereas auxin application on day 64 increased this parameter. Despite this difference in behaviour, which resulted from the different time‐course of the growth response of the fruit to auxin applications, these applications increased fruit size to a similar extent. Severing 37 % of the phloem of the pedicel during the main period of fruit growth resulted in an increase in the specific mass transfer in the phloem but had no influence on fruit growth. These observations demonstrate that the transport capacity in the phloem of the pedicel does not limit fruit growth and, within the limits of our experiments, an increase in demand by the fruit appeared to be matched by an increase in SMT. The dependence of late xylem formation (after the period of fruitlet abscission) on fruitlet growth was demonstrated in Salustiana orange [Citrus sinensis (L.) Osbeck] by means of controlling fruit growth through the manipulation of leaf area. Fruit growth at this time was more closely related to leaf area than to carbohydrate levels, suggesting that it may be limited by current photosynthesis.
Key words: Auxin, Citrus clementina, Citrus sinensis, clementine, fruit growth, mandarin, orange, pedicel vascularization, phloem formation, specific mass transfer, transport in the phloem
INTRODUCTION
The transport of dry matter to developing fruits is driven by their sink capacity. The actual rate of accumulation may be smaller than the potential capacity as it may be limited by assimilate supply and by the resistance in, and the capacity of, the path of transport. Assimilate production in leaves is modulated by the demand for photoassimilates (Goldschmidt and Koch, 1996; Bruchou and Genard, 1999; Lakso et al., 1999). Thus, to some extent, the supply is adjusted to demand by the developing fruits. However, the modulation of photosynthesis by crop load is only significant when crop loads are very low. Changes in fruit load from moderately low to very high have little effect on the photosynthetic rate of leaves (Goldschmidt and Koch, 1996; Lakso et al., 1999). The photosynthetic capacity of leaves, rather than demand by the fruits, usually determines the availability of photoassimilates. On the other hand, there is evidence that assimilate supply is a limiting factor in the growth of Citrus fruit. Thus, fruit size is inversely related to fruit number, and this has been considered a demonstration of competition between sinks for carbohydrates (Goldschmidt and Monselise, 1977; Guardiola, 1988). Furthermore, both fruit set and initial growth rate increased in Valencia orange trees when grown in a CO2‐enriched atmosphere, i.e. in conditions that increased photosynthesis and sugar availability (Downton et al., 1987).
The capacity of the transport system is not usually considered a limiting factor for fruit growth (Canny, 1973). In Citrus (Fishler et al., 1983) and in deciduous fruit trees (Bruchou and Genard, 1999), the distance to the source leaves has no effect on fruit growth so long as no competing fruits are in the path of transport. The increased resistance in longer paths has no effect on transport. On the other hand, a correlation between pedicel diameter and fruit size at harvest has been reported for several Citrus cultivars (Stewart et al., 1952; El‐Otmani et al., 1993; Bustan et al., 1995). These observations suggest the possibility of a transport limitation to Citrus fruit growth (Bustan et al., 1995). Furthermore, Erner and Shomer (1996) showed that the diameter of the peduncle was four times higher in leafy than in leafless inflorescences, a characteristic that was related to the higher fruit set in the former.
Although there are several descriptions of the vascular system in the stem and pedicel of Citrus fruit (Schneider, 1968; Bustan et al., 1995; Erner and Shomer, 1996), the influence that the capacity of the transport system may have on growth of the fruit has not been examined in detail. Guardiola et al. (1993) stated that the effect of exogenous growth regulators on fruitlet growth was not mediated by an effect on pedicel vascularization. In contrast, Bustan et al. (1995) stated that a limitation in transport capacity in the pedicel does occur. In the present study, changes in the vascularization of the pedicel during fruit development are examined in detail to determine whether fruit growth is limited by the transport capacity of the phloem in the pedicel. The influence of carbohydrate supply on fruit growth and pedicel vascularization is determined, as are the effects of sink strength of fruitlets on pedicel vascularization, and the effects on fruitlet growth of partial severing of the pedicel.
MATERIALS AND METHODS
Plant material
With one exception stated below, all experiments and fruit measurements were performed on 8‐ to 9‐year‐old ‘Marisol’ clementine trees (Citrus clementina Hort. ex Tanaka) grafted on Cleopatra mandarin (Citrus reshni Hort. ex Tanaka) rootstock. The effect of leaf area on fruit growth was studied in 30‐year‐old ‘Salustiana’ orange trees [Citrus sinensis (L.) Osbeck] grafted on Troyer citrange [Citrus sinensis (L.) Osbeck × Poncirus trifoliata Raf.] rootstock. Trees were drip irrigated, with the amount of water to be applied being determined from actual evaporation from an A class pan multiplied by a crop coefficient calculated for Citrus. Mineral elements were supplied in the irrigation water from mid‐February until September; amounts were decided based on leaf analysis determinations performed the preceding October.
Fruit growth and abscission
At weekly intervals, starting at the end of anthesis (30 March), the diameter of 200 ovaries/fruits from unifloral leafy inflorescences was measured with a precision of ± 0·1 mm. Inflorescences were chosen at random from 50 trees (four flowers from each tree). The average diameter (± s. e.) of the fruit population in the trees was calculated. For histological studies, fruitlets with a diameter that differed by less than 0·2 mm from the average value of the population were sampled. In one experiment, the growth rate and vascularization of the pedicel during the early stages of growth [up to day 75 after anthesis (DAA)], were determined in the largest fruitlets of the tree. The fruitlets chosen for this experiment had a diameter that was greater than that of average‐sized fruits by at least 0·5 times the standard deviation of the fruitlet population (Ruiz and Guardiola, 1994).
Dry and fresh weights of the fruit were determined from their diameter and regression lines between fruit weight (fresh and dry) and diameter determined each week in a random sample of 30 fruitlets. From these values, the daily rate of accumulation of dry matter in the developing fruits at each sampling date was calculated. From the rate of dry matter accumulation in individual fruits, and the number of developing fruits present on the tree, the daily amount of dry matter accumulated in the fruits per tree was also calculated. To determine the number of developing fruits, nets were placed under the canopy of three trees, and the organs that fell were weighed and counted at weekly intervals. From the total number of fruits at harvest and the abscission counts, the number of developing fruits present on the tree at each sampling date was back‐calculated. Abscission is presented in absolute (number of organs fallen per day) and in relative terms (as a percentage of the flowers/fruits present on the tree at the beginning of the week; Zucconi et al., 1978).
Auxin applications
Auxin applications were performed as a full coverage tree spray either at the end of flowering (15 mg l–1 of 2,4‐D, as isopropyl ester), or 64 DAA (75 mg l–1 of 2,4‐DP, as butylglycol ester). These auxin applications (concentration used and the time of application) have been reported to be optimal for increasing sink strength of fruitlets without causing overthinning or having an adverse effect on fruit quality (Guardiola and García‐Luis, 2000). A wetting agent (alkyl polyglycol ether, at a final concentration of 0·04 % by volume) was added to both auxin solutions. Each treatment was replicated five times, using units of 50 trees, in a random block design. The effect of 2,4‐D was studied during 2 consecutive years. Due to small differences in the dates of flowering, 2,4‐D was applied 5 d earlier in the first year (1 April; expt 1) than in the second year (6 April; expt 2).
Partial girdling of the pedicel
On 5 August, 127 DAA, 60 developing fruitlets from unifloral leafy inflorescences were tagged. These fruitlets were chosen on the basis that their diameter differed by less than 0·2 mm from the average diameter of the fruitlet population at that date (35·8 mm). Thirty fruitlets were used as controls, while the pedicel of the remaining 30 fruitlets was partially girdled, removing a strip of bark (2 mm wide) from 37 % of the pedicel circumference at a distance of 1·5 mm from the calyx. Anatomical observation of the pedicels showed that the girdle severed 37 ± 4 % of the bark (and phloem) cross‐sectional area, with individual values ranging from 32 to 43 %. Ten fruits per treatment were sampled after 30 d (157 DAA). The remaining 20 fruits were sampled 59 d after girdling (186 DAA).
The influence of leaf area
The effect of leaf area on fruit growth and pedicel vascularization was studied in Salustiana sweet orange. Uniform 1‐year‐old shoots with at least 50 leaves and a single fruit borne in a unifloral leafy inflorescence formed during the current year were girdled on 15 June. Along with the fruit, 10, 20, 30 or 40 leaves remained distal to the girdle and thus contributed to further fruit growth. Each treatment was replicated ten times. The ten replicates of each treatment were on different trees, and different treatments were performed on the same trees. At the time of girdling, differences among treatments in average fruitlet diameter were less than 0·2 mm. Fruit diameter was measured periodically, and two independent leaf samples (two leaves per sample) were taken at weekly intervals. Therefore, the number of leaves on the shoots at the end of the experiment (27 July, 42 d after girdling) was smaller by two units than the initial number. At the end of the experiment shoots were sampled for carbohydrate analysis of leaves, bark and wood, and for histological study of pedicel development.
Anatomical studies
Pedicels were fixed in a formalin : propionic acid : ethanol mixture (1 : 1 : 18 by volume) until processing. After re‐hydrating the samples in an ethanol series, transverse sections 20 µm thick were cut with a freezing microtome at a distance of 1·5 mm from the calyx. These sections were stained with carmine iodine green (Johansen, 1940). Measurements were obtained from 10–15 pedicels per sample. Phloem and xylem cross‐sectional areas (CSA) were calculated from the mean diameters of the vascular cylinder assuming the sections to be circular. During the early stages of pedicel vascularization there were separate vascular bundles, and this assumption was not fulfilled. In these samples, the CSA of phloem and xylem was estimated through digital analysis of the microscope images, by counting the number of pixels contained in the region of interest. This procedure was followed also in the experiment in which the pedicels were partially girdled.
The specific mass transfer (SMT) in phloem was calculated by dividing the rate of dry matter accumulation in the developing fruits (mg d–1) by the phloem CSA of the pedicel (mm2) at the same sampling date. The values thus determined were underestimates, since no correction was made for photosynthesis and respiratory losses in the fruit.
Determination of carbohydrates
Carbohydrate analyses were performed as described previously (Ruiz and Guardiola, 1994). Soluble sugars and starch were extracted in succession with hot 80 % ethanol and with perchloric acid, respectively, and determined using anthrone. Sucrose content was estimated as the difference between total sugars and reducing sugars, an approximation that in Salustiana sweet orange leaves has an error smaller than 10 % (C. Monerri and F. Almeida, unpubl. res.).
Statistical analysis
Comparison of pairs of values was carried out by means of a Student’s t‐test using the variance of the means. In those parameters that are a quotient of two measurements (i.e. the specific mass transfer in the phloem), the estimated variance of the mean was calculated as recommended by the International Organisation for Standardisation (1995). When more than two treatments were compared, analysis was performed by ANOVA (Snedecor and Cochran, 1967). When the F‐value was significant, mean separations were performed with the Duncan multiple range test. A simple regression analysis was performed to determine the relationships among parameters (Snedecor and Cochran, 1967).
RESULTS
Fruitlet growth and pedicel vascularization
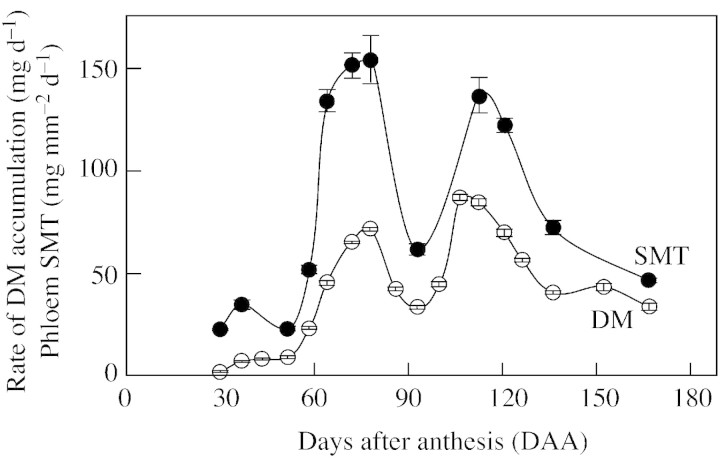
The growth curve of the fruit of Marisol clementine fitted a single sigmoid curve well (Fig. 1A). A linear phase of growth began about 93 DAA and continued beyond day 190, when the palatable but still growing fruit was harvested. The rate of accumulation of dry matter in the developing fruitlets increased steadily from a value less than 0·1 mg d–1 immediately after anthesis to a near maximum value of 77 mg d–1 72 DAA (Figs 1B and 3). Dry matter accumulation then fell to approx. 40 mg d–1 between days 86 and 100, increasing again to a value close to 80 mg d–1 107–113 DAA, prior to a further decrease towards maturity (Figs 1B and 3).
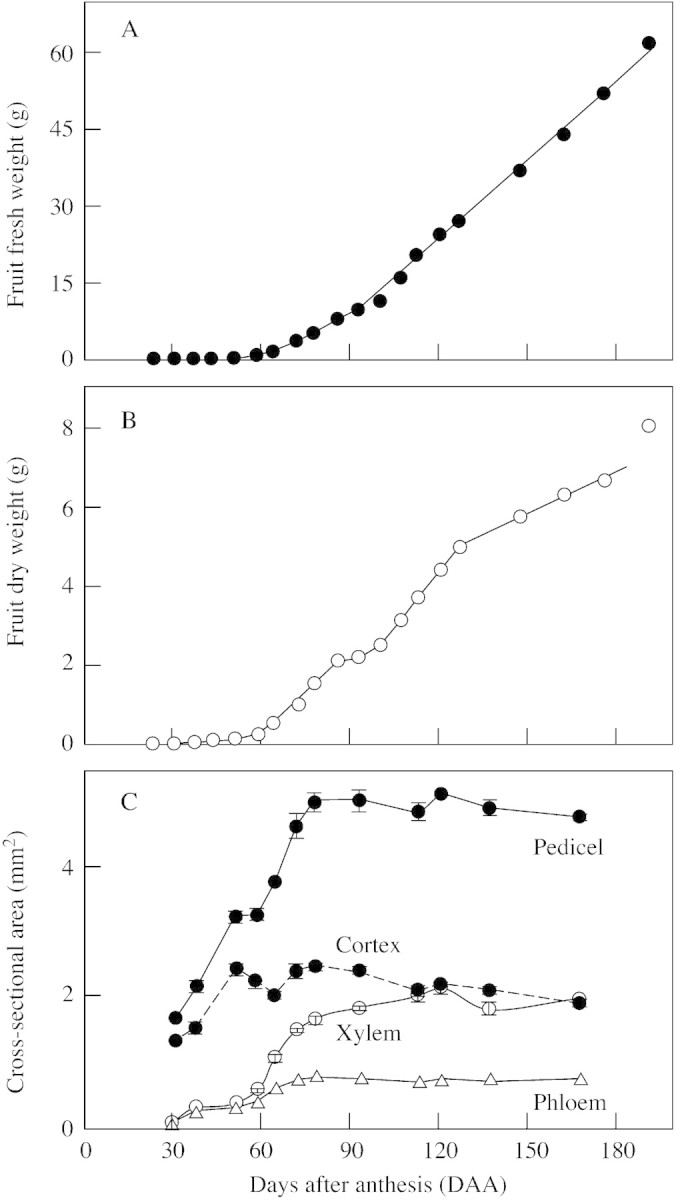
Fig. 1. Fruit fresh weight (A), dry weight (B) and vascularization of the pedicel (C) as related to fruitlet age in Marisol clementine. Cross‐sectional areas of the pedicel, cortex, xylem and phloem are means of 15 fruitlets of average size ± s.e.
Pedicel thickening was completed by day 78, before the beginning of the linear phase of fruit growth (Fig. 1C). The vascular system of the pedicel at anthesis was composed of several discrete bundles (Fig. 2A). As the ovary transformed into a fruit, the activity of the vascular cambium resulted in the formation of a stem‐like structure with a woody central cylinder surrounded by secondary phloem (Fig. 2C). From day 30 to day 78, the CSA of the pedicel increased almost linearly with time (Fig. 1C). Up to day 50, most of this increase in CSA was caused by the growth of the cortex. By day 50, the cross‐sectional areas of the phloem and xylem were similar, and each one of these tissues represented about 10 % of the pedicel CSA. Most of the secondary phloem and xylem formation occurred after day 50. Maximum phloem CSA was achieved by day 78. The increase in xylem formation after day 50 was greater than that observed in the phloem, and continued at a slower rate for a longer period. By day 93, the xylem reached its maximum CSA, accounting for 42–46 % of the pedicel CSA (Fig. 1C). Vessels were present throughout the whole cross‐section of the xylem (Fig. 2C). Any increase in phloem and xylem CSA after day 93 was smaller than the error of our measurements. However, cambium activity was seen under the microscope, and the thickness (and CSA) of the cortex decreased with fruitlet age (Fig. 1C), a consequence of the expansion of internal tissues.
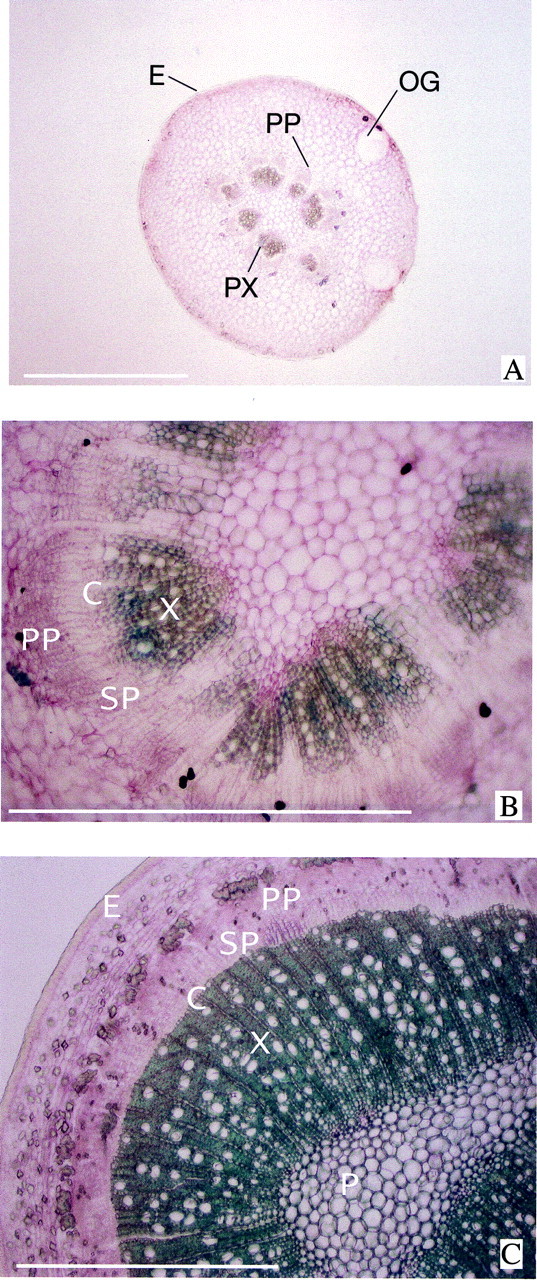
Fig. 2. Cross‐sections of the pedicel of Marisol clementine. A, On 24 April, 24 DAA, showing the presence of discrete vascular bundles. Between some of the vascular bundles initial formation of a vascular cambium has begun. B, On 7 May, 37 DAA. The activity of the cambium has resulted in the formation of a continuous vascular cylinder. C, On 2 October, 180 DAA, showing a stem‐like structure. Vessels differentiate immediately beneath the vascular cambium in the late‐formed xylem. C, vascular cambium; E, cutinized epidermis; OG, oil gland; P, pith; PP, primary phloem; PX, primary xylem; SP, secondary phloem; X, xylem. Bars = 1mm.
Values for phloem SMT of the pedicel during fruit development are presented in Fig. 3. The calculated SMT increased sharply after day 58 to reach a maximum value of 146 mg mm–2 d–1 between days 72–78. After a transient decrease over days 86–100, it rose again to a value close to 125 mg mm–2 d–1 between days 107–121, when the rate of accumulation of fruit dry matter was maximal (Fig. 3).
Fig. 3. Rate of dry matter accumulation (open symbols) and specific mass transfer in the phloem of the pedicel (closed symbols) in fruitlets of Marisol clementine. Data for dry matter accumulation are means of 200 fruits sampled at random. SMT is calculated from the anatomical characteristics of the pedicel of 15 fruits of average size. Bars represent s.e. and are shown when larger than the symbol.
Fruitlet abscission
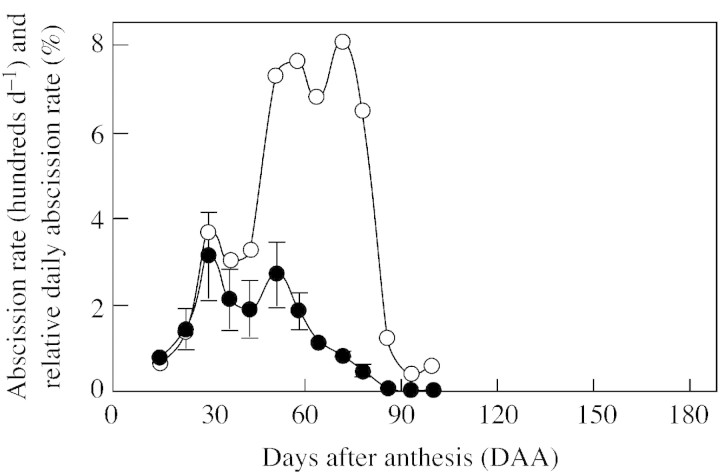
The trees used in the experiments formed over 10 000 flowers. Most (93 %) of the ovaries and developing fruitlets were shed within 86 d of anthesis, coinciding with the continuous increase in fruitlet growth rate. In absolute terms (number of flowers/fruitlets shed per day), two main peaks of abscission occurred around days 30 and 51 (Fig. 4). Up to day 30, abscission occurred mostly at the pedicel, and the shed organs showed little growth. From day 51, abscission occurred mostly at the calyx, and the shed fruitlets had grown markedly. In relative terms (when presented as a percentage of the fruitlets remaining on the tree at that moment), the main peak of abscission occurred from day 51 to day 78 (Fig. 4). Over this period, the number of fruitlets shed per day represented 6–8 % of the number of fruitlets remaining on the tree at the beginning of that week. The number of fruitlets remaining on the tree was a small proportion of the initial number of flowers (20 % by day 51 and 6 % by day 78).
Fig. 4. Daily absolute (fruitlets d–1; closed symbols) and relative (% of surviving fruitlets; open symbols) abscission rates in Marisol clementine. Absolute abscission rate is the mean of three trees ± s.e.
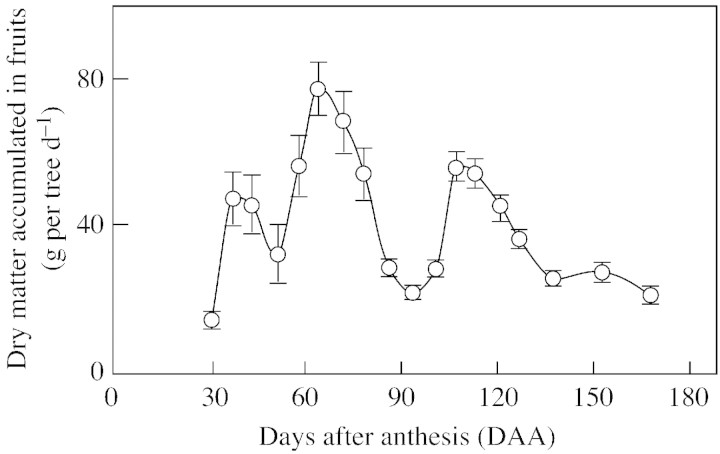
The rate of dry matter accumulation in all the developing fruits per tree increased from flowering until day 64, to reach a maximum value of 77 g per tree d–1 (Fig. 5). It then fell to about one‐third of this value during days 86–100. During the period of the maximum rate of dry matter accumulation in the fruitlets (107–120 DAA), the amount of dry matter incorporated in the fruits ranged between 44–55 g per tree d–1. These values were significantly smaller than the peak value measured at day 64 (Fig. 5).
Fig. 5. Dry matter (g d–1) accumulated in the developing fruit in a tree of Marisol clementine. Values are calculated from the rate of accumulation of dry matter in the fruit (Fig. 3) and the number of developing fruitlets. Values are mean of three trees ± s. e.
Fruit growth rate and pedicel vascularization
The rate of accumulation of dry matter in the most vigorous (biggest) fruitlets post‐anthesis was, on average, twice the rate measured in average‐sized fruitlets. It peaked at a maximum value of 135 mg per fruit d–1 by day 68, compared with a value of 64 mg per fruit d–1 for average‐size fruits at day 72 (Fig. 6A). Differences in the rate of accumulation of dry matter between different‐sized fruitlets were statistically significant (P ≤ 0·01) 23 DAA. Faster growth was accompanied by an increase in pedicel vascularization (Fig. 6C and D). By day 44, the cross‐sectional area of the pedicel and its anatomical components (cortex, phloem and xylem) were tightly correlated (R2 ≥ 0·72; P ≤ 0·01) with fruitlet diameter (Fig. 7). The differences in vascularization of the pedicel among fruitlets differing in growth rate became significant later in development than the initial differences in growth rate. By day 37 there were no significant differences in vascularization of the pedicel between the biggest fruits and those of average size (Fig. 6C and D).
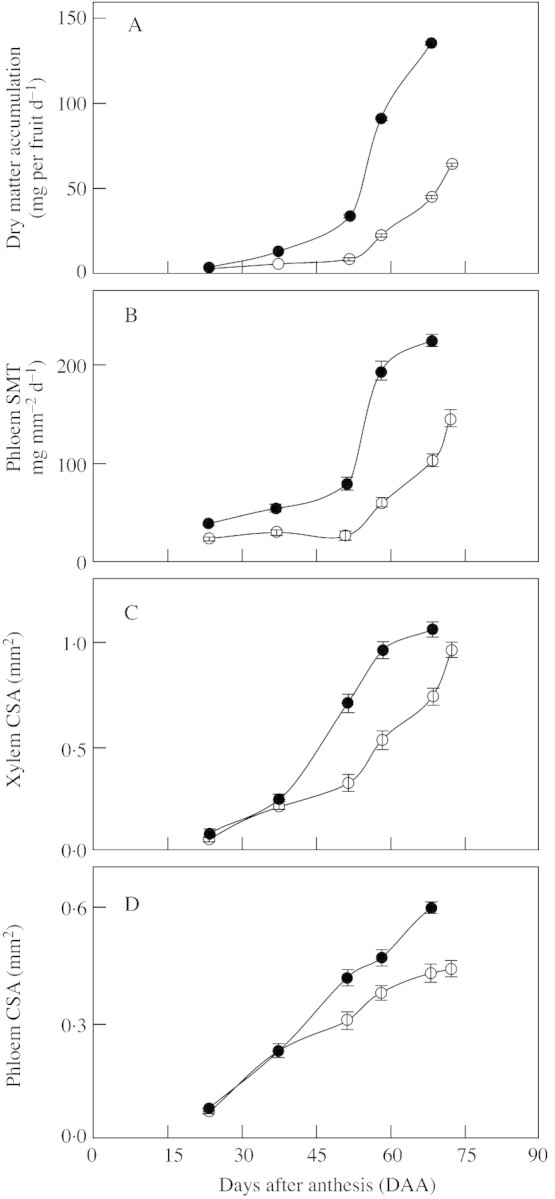
Fig. 6. Rate of dry matter accumulation in the fruitlets (A), specific mass transfer in the phloem of the pedicel (B), and cross‐sectional area of the xylem (C) and phloem (D) during early growth of fruitlets of Marisol clementine. Values from average‐sized fruitlets (open symbols) and from the largest fruitlets (closed symbols) on the tree are means of 100 fruits and 15 pedicels ± s.e.
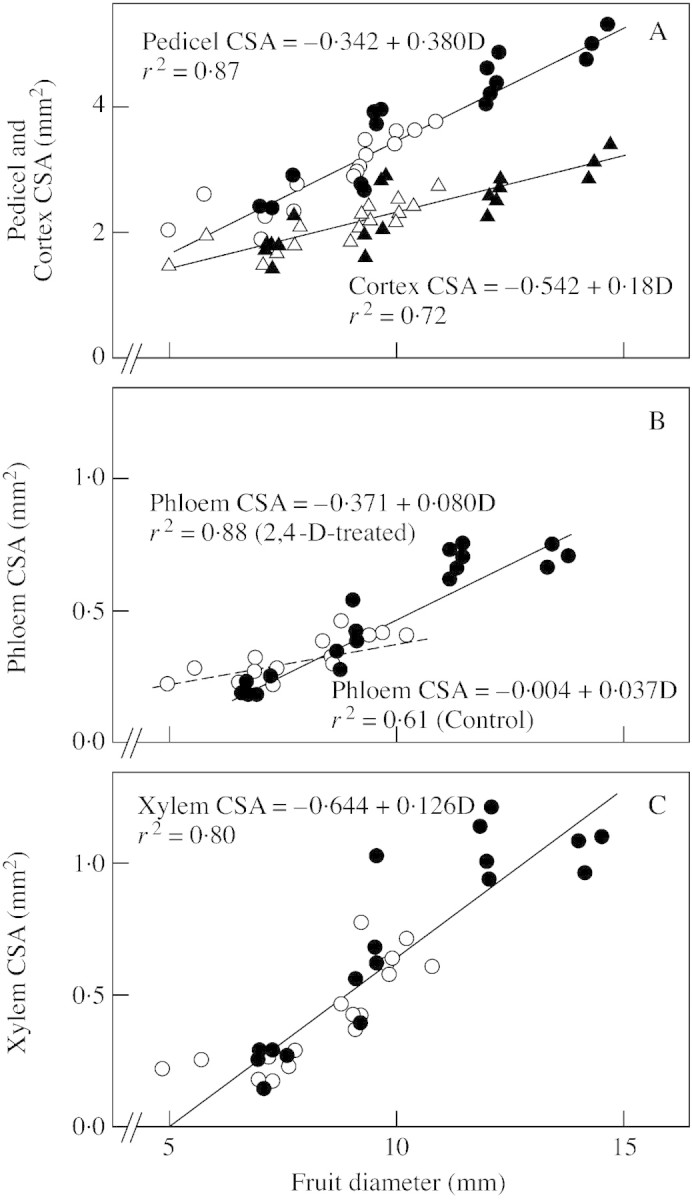
Fig. 7. Pedicel CSA and vascularization as related to fruitlet diameter (D) in Marisol clementine. Data from fruitlets from untreated controls (open symbols) and 2,4‐D‐treated (closed symbols) trees sampled on 14 May, 44 DAA. The regression lines between phloem CSA and fruitlet diameter are significantly different for control (dashed line) and 2,4‐D‐treated trees (full line). Circles represent pedicel CSA values and triangles represent cortex CSA values.
Despite this additional formation of phloem tissue, the SMT of pedicel phloem was always higher in the larger fruitlets (Fig. 6B). By day 68 it had reached a value of 225 mg mm–2 d–1, 50 % more than the peak value of 146 mg mm–2 d–1 recorded in average‐sized fruitlets on day 72 (Fig. 6B).
The effect of partial girdling of the pedicel
The partial girdling of the pedicel resulted in callus formation at the cut edges (Fig. 8). These calli originated from the cambium at the edge of the cut, and partially covered the exposed xylem. However, the calli did not fill the gap caused by the cut in the bark of the pedicel. The surface of the calli and the cut edge of the bark were suberized. The innermost part of these calli was lignified. No vascularization occurred at any region of these calli. In the lignified portion, no vessels were apparent. In the non‐lignified portion, even the highly sensitive aniline blue staining failed to detect the presence of callose, which would be indicative of sieve tube formation. Therefore, the vascular continuity affected by girdling was not restored and the severed phloem was not substituted.
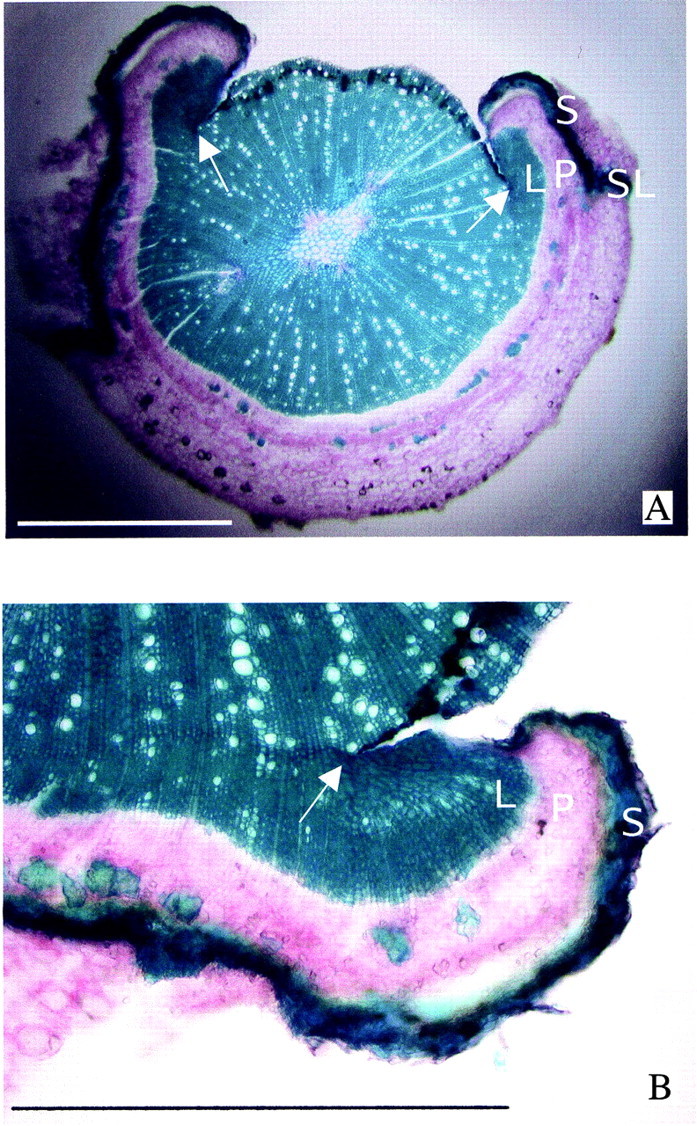
Fig. 8. Transverse section of pedicels taken on 2 October, 153 DAA and 59 d after partial girdling. A, Ringed pedicel showing the proliferation of calli from the innermost part of the cut edge of the bark. Calli consist on an inner lignified region (L), covered by parenchyma (P) protected by suberized cells (S). The calli are not fused with the secondary xylem (arrows). The absence of vessels in the lignified regions of the calli contrasts with their presence in the outermost layers of secondary xylem, which formed at about the same time as the calli. The cut edges of the bark are protected by a suberized layer (SL). B, Enlarged view of a lignified callus showing linear rows of cells converging in the innermost part of the cut edge (arrow), were the callus originated, and the absence of vessels. In the late‐formed secondary xylem, vessels are present. The rows of cells are continuous with those from the early‐formed xylem.
Fruit growth was not affected by partial girdling, either during the first 30 d after girdling (evidence not presented), or in the following 29 d (Table 1). The reduction in cross‐sectional area of the phloem (mean 37 %) was compensated by a similar increase in the SMT in the severed portion of the pedicel (Table 1).
Table 1.
The effect of partial girdling of the pedicel on fruitlet growth and specific mass transfer (SMT) in the pedicel of Marisol clementine
| Parameter | Control | Girdled |
| Accumulation of dry matter (mg d–1) | 42 ± 3 | 39 ± 2 n.s. |
| Phloem cross‐sectional area (mm2) | 0·62 ± 0·04 | 0·39 ± 0·02** |
| Phloem SMT (mg mm–2 d–1) | 69 ± 6 | 99 ± 9* |
Pedicels were girdled on 5 August (127 DAA). Measurements performed 59 d after girdling (186 DAA). Growth values are means of 20 fruits ± s. e. Anatomical measurements are means of ten fruits ± s.e.
** P ≤ 0·01; * P ≤ 0·05; n.s., non significant.
Effect of auxin on fruit growth and pedicel vascularization
The application of either 2,4‐D or 2,4‐DP resulted in a similar increase (7–10 %) in the final weight of the fruit (Table 2). The auxin applications also increased the phloem and the xylem CSA in the pedicel. At the end of fruit growth, both pedicel diameter and xylem CSA were greater in 2,4‐DP‐treated fruits than in 2,4‐D‐treated fruits. No differences in phloem CSA were found between the two auxin treatments (Table 2). A specific effect on phloem formation unrelated to fruit size (and growth rate) was demonstrated for the 2,4‐D applications. Forty‐four days after anthesis, the slope of the regression line relating phloem CSA to fruitlet diameter (Fig. 7B) was greater (P ≤ 0·01) for the 2,4‐D‐treated fruits (0·080 mm2 mm–1) than for the untreated controls (0·037 mm2 mm–1). For xylem CSA, the difference in the slope of the regression lines was not significant (P ≤ 0·32). At the time of the 2,4‐DP application (64 DAA), the CSA of the phloem in the pedicel was 34 % greater in 2,4‐D‐treated fruits (0·60 mm2) than in the untreated controls (0·44 mm2).
Table 2.
Effect of application of a synthetic auxin on fruit final fresh weight and pedicel vascularization
| Experiment and auxin treatment | Fruit weight (g) | Pedicel diameter (mm) | Phloem CSA (mm2) | Xylem CSA (mm2) |
| Expt 1 | ||||
| Control (none) | 77·8a | 2·41a | 0·68 | 1·90a |
| 2,4‐D | 83·0b | 2·67b | 0·75 | 2·56b |
| s.e. | 0·6 | 0·07 | 0·06 | 0·04 |
| Expt 2 | ||||
| Control (none) | 57·4a | 2·18a | 0·64a | 1·38a |
| 2,4‐D | 63·0b | 2·30b | 0·76b | 1·54a |
| 2,4‐DP | 64·6b | 2·43c | 0·83b | 1·97b |
| s.e. | 0·4 | 0·04 | 0·04 | 0·05 |
2,4‐D (16 mg l–1) was sprayed on 1 April, at the end of anthesis (day 0). 2,4‐DP (75 mg l–1) was sprayed on 3 June (64 DAA). Fruit fresh weight and pedicel characteristics were determined at commercial maturity (7 October; 190 DAA). Data from two experiments performed in different years. Pedicel data are means of 30 determinations.
Within one experiment, values in the same column followed by different superscripts differ at P ≤ 0·05 according to Student’s t‐test (expt 1), or Duncan’s multiple range test.
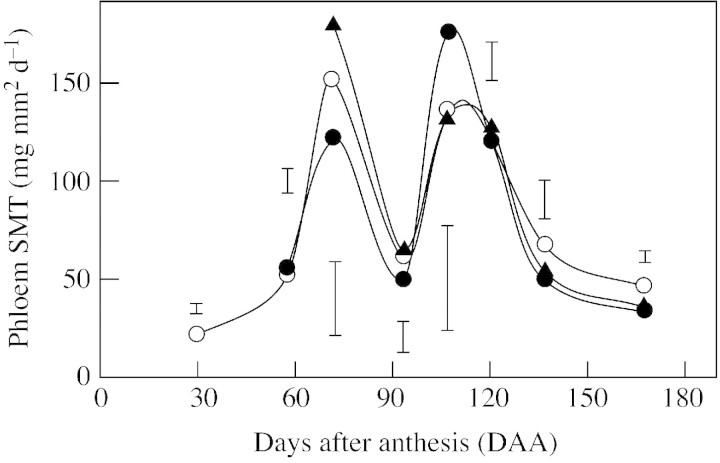
SMT in the phloem of the pedicel 72 DAA was greater in 2,4‐DP‐treated fruits than in 2,4‐D‐treated fruits (Fig. 9). No significant difference in STM between these two treatments or the untreated controls was found at any other date.
Fig. 9. Specific mass transfer in the pedicel of fruits from untreated control (open circles), 2,4‐D‐treated (closed circles) and 2,4‐DP‐treated (triangles) trees. Values calculated from the rate of accumulation of dry matter in a random sample of 100 fruits from 20 trees, and the anatomical characteristics of 15 pedicels from average‐sized fruits. Vertical bars show the lowest significant difference (P ≤ 0·05) on each date.
Effects of leaf area on fruit growth and pedicel vascularization
The effect of leaf area on the increase in dry weight of fruits and the carbohydrate contents in leaves of Salustiana orange is presented in Table 3. The accumulation of dry matter in fruits was closely related to the total leaf area of the shoots (R2 = 0·952; P ≤ 0·01). This accumulation of dry matter in the fruits represented more than 80 % of the total increase in dry weight of the shoots over the duration of the experiment (42 d). Although significant, the relationships between the increase in dry weight of the fruits and leaf sucrose and starch contents were much weaker (R2 = 0·662, P ≤ 0·05; and R2 = 0·748, P ≤ 0·05, respectively). Levels of starch in the leaves fell significantly (P ≤ 0·05) during the experiment, from an initial value of 7·3 ± 0·8 % to a value below 4·9 % (Table 3). The lowest starch content was found in shoots with ten leaves. There was also a significant decrease (P ≤ 0·05) in the carbohydrate reserves (starch + sugars) from the bark and the wood of the shoots. These reserves fell by 2·5 % (of the dry weight) in the 40‐leaf treatment, and by 4·2 % in the ten‐leaf treatment. At the end of the experiment there was a close relationship (P ≤ 0·01) between carbohydrate reserves in the leaves and those of the bark (R2 = 0·899) and the wood (R2 = 0·813). Overall, reserves mobilized from these organs represented less than 2 % of the dry matter accumulated in the fruit during the experiment. Sucrose content in the leaves did not change during the experiment in shoots with 30 or 40 leaves. Lower sucrose contents were found in the leaves of shoots with ten and 20 leaves (Table 3). This marked reduction in sucrose concentration occurred 7 d after girdling (data not shown).
Table 3.
The influence of leaf area on carbohydrate availability and the rate of accumulation of dry matter in fruitlets of Salustiana sweet orange
| Carbohydrates in leaves | |||||
| Date of measurement and treatment | Leaf area (dm2) | Dry weight of fruits (g) | DM accumulation in fruits (mg d–1) | Starch (%) | Sucrose (%) |
| Initial value (15 June) | – | 3·6 ± 0·2 | – | 7·3 ± 0·8 | 4·0 ± 0·1 |
| Final values (27 July) | |||||
| 40 leaves | 5·0 ± 0·4 | 10·0a | 152a | 4·7a | 4·1bc |
| 30 leaves | 3·4 ± 0·6 | 9·0b | 129b | 4·9a | 4·6ab |
| 20 leaves | 2·2 ± 0·3 | 6·4c | 90c | 4·6a | 3·8cd |
| 10 leaves | 0·9 ± 0·02 | 5·3d | 40d | 3·4b | 3·4d |
| s.e. | 0·3 | 7 | 0·16 | 0·2 | |
Shoots with a single fruit were girdled on 15 June above the number of leaves indicated. Measurements were performed on 27 July. Values are means of ten fruitlets or two independent leaf samples (± s.e.)
Values within a column followed by different superscripts differ at P ≤ 0·05 (Duncan’s multiple range test).
During the experiment, the pedicel of fruitlets increased in diameter from an initial value of 2·50 ± 0·08 mm to up to 3·47 ± 0·04 mm in shoots with 40 leaves (Table 4). Pedicel diameter was related to the final size (diameter) of the fruits (R2 = 0·64; P ≤ 0·01). Most of this increase in pedicel diameter was caused by xylem formation. At the end of the experiment, xylem CSA was closely correlated with fruit diameter (R2 = 0·78; P ≤ 0·01). The cross‐sectional area of the phloem (Table 4) and cortex (data not shown) did not increase significantly during the experimental period.
Table 4.
The influence of leaf area on pedicel vascularization in fruitlets of Salustiana sweet orange
| Date of measurement and treatment | Pedicel diameter (mm) | Xylem CSA (mm2) | Phloem CSA (mm2) | SMT in phloem (mg mm–2 d–1) |
| Initial value (15 June) | 2·5 ± 0·08 | 1·1 ± 0·1 | 0·52 ± 0·08 | – |
| Final values (27 July) | ||||
| 40 leaves | 3·47a | 3·0a | 0·55 | 277a |
| 30 leaves | 3·27b | 2·5bc | 0·60 | 214b |
| 20 leaves | 2·98c | 1·9cd | 0·52 | 159c |
| 10 leaves | 2·81d | 1·5d | 0·51 | 79d |
| s.e. | 0·04 | 0·25 | 0·08 | 15 |
Shoots were girdled on 15 June. Measurements performed on 27 July. Values are means of ten fruitlets. For further details see Table 3.
Values within a column followed by different superscripts differ at P ≤ 0·05 (Duncan’s multiple range test).
The rate of transport of dry matter to fruits paralleled the value of the SMT in the phloem of the pedicel. This value was 3·5 times higher in shoots with 40 leaves (277 mg mm–2 d–1) than in shoots with ten leaves (79 mg mm–2 d–1; Table 4).
DISCUSSION
A correlation between fruit size and pedicel CSA has been reported in Citrus at fruit maturity (Stewart et al., 1952; Bustan et al., 1995; El‐Otmani et al., 1993). We confirmed these observations (Table 2) and further demonstrated that fruit size was tightly correlated to both phloem CSA and xylem CSA (Fig. 7; Table 2). We also found that pedicel vascularization was complete before the linear phase of fruit growth (Fig. 1). Phloem formation was completed by the time the rate of dry matter accumulation in the fruit became maximal (78 DAA). Xylem formation lasted longer, and was only complete by the time the growth rate of the fruit was maximal (93 DAA). Both phloem and early xylem formation were correlated with early fruit growth (Fig. 7). In a fruit population within a tree there is a close relationship between the growth rate of individual fruits during early and late development (Praloran et al., 1981; Guardiola and Lázaro, 1987; Guardiola, 1988). Thus, pedicel size and vascularization become adjusted during early fruit development to the forthcoming transport and mechanical requirements of the fruit. The greater phloem CSA will potentially enable faster transport of dry matter to the more vigorously growing fruitlets. The mechanical strength of the woody xylem will allow the pedicels to hold the increasingly heavier fruitlets. At variance with reports on apple, which demonstrated that the xylem formed in the pedicel after flowering is non‐functional (Lang and Ryan, 1994), vessels differentiated continuously in the pedicel of Citrus (Figs 2 and 8). This xylem may not contribute directly to the transport of water and mineral elements to the fruit, since in vines (Lang and Thorpe, 1989), deciduous fruit trees (Lang, 1990; Lang and Ryan, 1994) and Citrus (Elfving and Kaufman, 1972; Goldschmidt and Koch, 1996) there is a net water backflow from the fruit into the xylem. This water backflow may help to dissipate excess water incorporated along with the sugars in the phloem, thus helping to maintain the necessary pressure gradient for further transport (Huang et al., 1992).
At any time during fruit development, the transport capacity of the phloem equals the CSA of this tissue multiplied by its (unknown) potential SMT. The possibility that demand by the fruit becomes higher than this transport capacity, thus making transport the limiting factor for fruit growth, is most likely at times when the actual (measured) SMT in the pedicel is highest. In agreement with previous reports (Guardiola et al., 1993; Bustan et al., 1995), the maximum SMT value was reached in our experiments during late fruitlet abscission (72–78 DAA; Figs 3 and 4), at the time that the transport of carbohydrates to the population of developing fruits was maximal (Fig. 5). This late abscission mainly affects the slow‐growing fruitlets (Zucconi et al., 1978; Ruiz and Guardiola, 1994) and occurs at a time when tree carbohydrate reserves have been utilized and transport to the fruit depends on current photosynthesis (Sanz et al., 1987; Ruiz et al., 2001). The decrease in late fruitlet abscission when carbohydrate availability was increased through enhanced photosynthesis (Downton et al., 1987) or by trunk girdling (Ruiz et al., 2001) supports the hypothesis that late abscission is related to competition by fruitlets for carbohydrates (Goldschmidt, 1999). In contrast, we found no evidence that the transport capacity in the pedicel was determining the rate of incorporation of dry matter into fruitlets at that time. The markedly lower phloem SMT in the pedicel in fruits with a smaller growth rate (Fig. 6) demonstrates that the lower sink strength of these fruitlets, rather than the differences in phloem CSA, was a determining factor for the reduced transport to them.
Phloem formation in the pedicel was in excess of the transport requirements of the fruits during the main period of growth, as demonstrated by the lower phloem SMT values during this period (Fig. 3). In agreement with Bustan et al. (1995), we found that the removal of a significant proportion (up to 43 %) of the pedicel phloem was matched by a compensatory increase in the SMT, but that it had no effect on transport to the fruit (Table 1). Bustan et al. (1995) also reported that the reduction in fruit growth rate that occurred when most (90 %) of the phloem of the pedicel was removed, was accompanied by a 3·8‐fold increase in the SMT in this tissue. These observations prove that transport in the phloem occurred at a rate markedly lower than its transport capacity. Hence, the role that the 19–30 % increase in phloem CSA resulting from auxin applications may have played in the 10–12 % increase in fruit weight (expt 2; Table 2) is not clear. In contrast, the immediate (measured after 8 d) growth response to 2,4‐DP application (72 DAA) occurred before there was any significant change in phloem formation, and resulted from a 1·4‐fold increase in the phloem SMT above that of 2,4‐D‐treated fruits (Fig. 8).
On the other hand, the close relationship between fruit growth and leaf area (Table 3) demonstrates the dependence of late fruit growth on current photosynthesis. Leaf photosynthesis was modulated by demand of the fruit. The apparent rate of photosynthesis, calculated from changes in dry weight of the fruits (reported in Table 3) and shoot leaf area, ranged from 5·9 µmol CO2 m–2 s–1 (40‐leaf shoots) to 8·6 µmol CO2 m–2 s–1 (ten‐leaf shoots). These values, which are underestimates since no correction was made for respiratory losses in the fruit, are within the range of photosynthetic rates reported for Citrus (4–8 µmol CO2 m–2 s–1; Syvertsen and Lloyd, 1994). Under our experimental conditions, the modulation of photosynthesis by the fruit did not fully compensate for the reduction in leaf area. The values of the SMT in the phloem of the pedicel (up to 277 mg mm–2 d–1) were within the range of the values reported for fruit trees (apple, pear and peach; Canny, 1973; Bruchou and Genard, 1999).
As mentioned above, the fate of developing fruitlets is determined early in their development, and the initial growth rate (= sink strength) of the fruitlets is related to their probability of abscission and to their late growth rate. From previously published evidence, it is likely that differences in growth rate were already determined at flowering. At anthesis, ovaries are bigger in leafy inflorescences (Guardiola et al., 1984). These inflorescences set a higher proportion of fruit and form fruits with a higher initial growth rate than leafless inflorescences (Guardiola and Lázaro, 1988). Bangerth (1989, 1997) examined in detail the establishment of dominance among young fruitlets and concluded that the mechanism did not involve carbohydrate shortage. The results of the present work agree with this conclusion. The differences in growth rate of the fruitlets occurred at a time when there was no limitation on carbohydrate supply in the tree. Furthermore, differences in the vascularization of the pedicel related to differences in fruit size (= growth rate) were only significant by day 44 (Fig. 7), markedly later in development than the initial differences in growth rate and in phloem SMT (Fig. 6), and therefore may not be the causal factor.
In summary, we have demonstrated that both early and late xylem formation are related to the actual rate of fruit growth. At late stages of fruit development, xylem makes the major contribution to the cross‐sectional area of the pedicel, and is the major component of the reported relationship between pedicel diameter (and CSA) and fruit size at maturity. Phloem formation was completed by the end of fruitlet abscission. The rate of phloem formation was related to the initial growth rate of the fruit, and occurs in excess of the transport requirements of the developing fruit. We found no evidence that, at any stage of fruit development, the transport capacity of the phloem in the pedicel was limiting transport to the fruit.
ACKNOWLEDGEMENTS
This research was funded by grants from Fundación Séneca, Consejería de Medio Ambiente, Agricultura y Agua de la Región de Murcia (Grant 00502/CU/98), Comisión Interministerial de Ciencia y Tecnología (Grant PB 95‐0736) and Consejería de Agricultura, Pesca y Alimentación, Generalitat Valenciana (Grant GV‐CAPA00‐11). D.L.S. was on leave from Universidade Federal de Viçosa, MG, Brazil, with the support of CAPES (Ministerio da Educaçao e do Desporto, Brasil). We thank Ing. Agron. M. Bernard and J. M. Torres (Anecoop, Valencia, Spain) for providing orchard facilities, and Ing. Ind. Carlos Guardiola for help with statistical analysis.
Supplementary Material
Received: 19 July 2002; Returned for revision: 1 August 2002; Accepted: 12 September 2002 Published electronically: 24 October 2002
References
- BangerthFK.1989. Dominance among fruits/sinks and the search for a correlative signal. Physiologia Plantarum 76: 608–614. [Google Scholar]
- BangerthFK.1997. Can regulatory mechanisms in fruit growth and development be elucidated through the study of endogenous hormone concentration? Acta Horticulturae 463: 77–87. [Google Scholar]
- BustanA, Erner Y, Goldschmidt EE.1995. Interactions between developing Citrus fruits and their supportive vascular system. Annals of Botany 76: 657–666. [Google Scholar]
- BruchouC, Génard M.1999. A space‐time model of carbon translocation along a shoot bearing fruits. Annals of Botany 84: 565–576. [Google Scholar]
- CannyMJ.1973. Phloem translocation. London: Cambridge University Press. [Google Scholar]
- DowntonWJS, Grant WJR, Loveys BR.1987. Carbon dioxide enrichment increases yield of Valencia orange. Australian Journal of Plant Physiology 14: 493–501. [Google Scholar]
- ElfvingDC, Kaufmann MR.1972. Diurnal and seasonal effects of environment on plant water relations and fruit diameter of Citrus.Journal of the American Society for Horticultural Science 97: 556–570. [Google Scholar]
- El‐OtmaniM, Agusti M, Aznar M, Almela V.1993. Improving the size of Fortune mandarin fruits by auxin 2,4‐DP. Scientia Horticulturae 55: 283–290. [Google Scholar]
- ErnerY, Shomer I.1996. Morphology and anatomy of stems and pedicels of spring flush shoots associated with Citrus fruit set. Annals of Botany 77: 537–545. [Google Scholar]
- FishlerM, Goldschmidt EE, Monselise SP.1983. Leaf area and fruit size on girdled grapefruit branches. Journal of the American Society for Horticultural Science 108: 218–221. [Google Scholar]
- GoldschmidtEE.1999. Carbohydrate supply as a critical factor for Citrus fruit development and productivity. HortScience 34: 1020–1024. [Google Scholar]
- GoldschmidtEE, Koch KE.1996. Citrus. In: Zamski E, Schaffer AA, eds. Photoassimilate distribution in plants and crops New York: Marcel Dekker Inc., 797–823. [Google Scholar]
- GoldschmidtEE, Monselise SP.1977. Physiological assumptions toward the development of a Citrus fruiting model. Proceedings of the International Society of Citriculture 2: 668–672. [Google Scholar]
- GuardiolaJL.1988. Factors determining productivity in Citrus A physiological approach. Proceedings of the International Society of Citriculture 2: 381–394. [Google Scholar]
- GuardiolaJL, García‐Luis A.2000. Increasing fruit size in Citrus Thinning and stimulation of fruit growth. Plant Growth Regulation 31: 121–132. [Google Scholar]
- GuardiolaJL, Lázaro E.1987. The effect of synthetic auxins on fruit growth and anatomical development in Satsuma mandarin. Scientia Horticulturae 31: 119–130. [Google Scholar]
- GuardiolaJL, García‐Marí F, Agustí M.1984. Competition and fruit set in the Washington navel orange. Physiologia Plantarum 62: 297–302. [Google Scholar]
- GuardiolaJL, Barrés MT, Albert C, García‐Luis A.1993. Effects of exogenous growth regulators on fruit development in Citrus unshiu Annals of Botany 71: 169–176. [Google Scholar]
- HuangTB, Darnell RL, Koch KE.1992. Water and carbon budgets for developing Citrus fruit. Journal of the American Society for Horticultural Science 117: 287–293. [Google Scholar]
- International Organisation for Standardisation.1995. Guide to the expression of uncertainty in measurement. Corrected and reprinted. Genève: ISO. [Google Scholar]
- JohansenDA.1940. Plant microtechnique. New York: McGraw–Hill. [Google Scholar]
- LaksoAN, Wünsche JN, Palmer JW, Corelli Grappadelli L.1999. Measurement and modeling of carbon balance of the apple tree. HortScience 34: 1040–1047. [Google Scholar]
- LangA.1990. Xylem, phloem and transpiration flows in developing apple fruits. Journal of Experimental Botany 41: 645–651. [Google Scholar]
- LangA, Ryan KG.1994. Vascular development and sap flow in apple pedicels. Journal of Experimental Botany 74: 381–388. [Google Scholar]
- LangA, Thorpe MR.1989. Xylem, phloem and transpiration flows in a grape: application of a technique for measuring the volume of attached fruits to high resolution using Archimedes’ principle. Journal of Experimental Botany 40: 1069–1078. [Google Scholar]
- PraloranJC, Vullin G, Jacquemond C, Despierre D.1981. Observations sur la croissance des clementines in Corse. Fruits 36: 755–767. [Google Scholar]
- RuizR, Guardiola JL.1994. Carbohydrate and mineral nutrition of orange fruitlets in relation to growth and abscission. Physiologia Plantarum 90: 27–36. [Google Scholar]
- RuizR, García‐Luis A, Monerri C, Guardiola JL.2001. Carbohydrate availability in relation to fruitlet abscission in Citrus.Annals of Botany 87: 805–812. [Google Scholar]
- SanzA, Monerri C, González‐Ferrer J, Guardiola JL.1987. Changes in carbohydrates and mineral elements in Citrus leaves during flowering and fruit set. Physiologia Plantarum 69: 93–98. [Google Scholar]
- SchneiderH.1968. The anatomy of Citrus. In: Reuther W, Batchelor LD, Webber HJ, eds. The Citrus industry. Vol. 2 Riverside: University of California, 1–87. [Google Scholar]
- SnedecorGW, Cochran WG.1967. Statistical methods Ames: The Iowa University Press, 65–99.. [Google Scholar]
- StewartWS, Hield HZ, Brannaman BL.1952. Effects of 2,4‐D and related substances on fruit drop, yield, size and quality of Valencia oranges. Hilgardia 21: 301–309. [Google Scholar]
- SyvertsenJP, Lloyd J.1994. Citrus. In: Schaffer B, Anderson PC, eds. Handbook of environmental physiology of fruit crops. Vol. 2 Boca Raton: CRC Press, 65–99. [Google Scholar]
- ZucconiG, Monselise SP, Goren R.1978. Growth‐abscission relationships in developing orange fruit. Scientia Horticulturae 9: 137–146. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.