Abstract
This study addresses the question of how size‐related changes in leaf morphology and physiology influence light absorption and carbon gain of the epiphytic bromeliad Vriesea sanguinolenta. A geometrically based computer model, Y‐plant, was used for the three‐dimensional reconstruction of entire plants and for calculation of whole plant light interception and carbon gain. Plants of different sizes were reconstructed, and morphological and physiological attributes of young and old leaves, and small and large plants were combined to examine the individual effects of each factor on light absorption and carbon gain of the plant. The influence of phyllotaxis on light absorption was also explored. Departure of measured divergence angles between successive leaves from the ideal 137·5° slightly decreased light absorption. The only morphological parameter that consistently changed with plant size was leaf shape: larger plants produced more slender foliage, which substantially reduced self‐shading. Nevertheless, self‐shading increased with plant size. While the maximum rate of net CO2 uptake of leaves increased linearly with plant size by a factor of two from the smallest to the largest individual, the potential plant carbon gain (based on total foliage area) showed a curvilinear relationship, but with similar numerical variation. We conclude that leaf physiology has a greater impact on plant carbon gain than leaf and plant morphology in this epiphytic bromeliad.
Key words: Crown architecture, ecophysiology, epiphytes, light capture, modelling, photosynthesis, plant size, scaling, Vriesea sanguinolenta
INTRODUCTION
The effective display of leaves in a canopy is crucial for plant functioning because it determines the efficiency of the photosynthetic conversion of available light to assimilate and thus influences the potential growth of plants. Ecophysiological studies on plant responses to the light environment have typically focused on individual organs, primarily leaves (Boardman, 1977; Evans et al., 1988; Lambers et al., 1998), while the functional implications of light‐induced changes in plant morphology for whole plant performance have received much less attention (Givnish, 1988; Pearcy and Yang, 1996; Valladares and Pearcy, 1998). Strong selection pressure is presumed to reduce mutual shading among leaves of the same plant and this presumption has motivated a number of studies on crown architecture and adaptations that may reduce self‐shading (Niklas, 1988, 1992; Yamada et al., 2000; Valladares et al., 2002). To minimize self‐shading during growth, plants may change leaf orientation, petiole length and angle, and leaf deployment and turnover (Pearcy and Yang, 1998; Yamada et al., 2000). However, no architectural solution prevents self‐shading completely, especially when leaf area increases as a result of plant growth.
Architectural changes during growth, which may also be related to other limitations such as mechanical stresses or the translocation of water and nutrients (Niklas and Kerchner, 1984), are difficult to predict and can decrease or, conversely, increase the effects of physiological changes (Valladares, 1999). Hence, any study on the importance of leaf processes in an ecological context remains incomplete without proper analysis of the consequences for the whole canopy. This need for understanding has motivated the present study, which addresses the importance of size‐related changes in physiological parameters of organs of vascular epiphytes (reviewed in Zotz, 2000) for the functioning of entire plants.
Recent studies have shown that the photosynthetic performance of vascular epiphytes changes with plant size. There is both direct evidence from oxygen electrode measurements with cut leaf discs in the laboratory (Zotz, 1997a; Schmidt et al., 2001) and from diel CO2 exchange measured on intact leaves in the field (Schmidt and Zotz, 2001), as well as indirect evidence from determinations of carbon isotope ratios (Zotz and Ziegler, 1999). As the scaling from leaf to whole plant is not straightforward, the importance of these changes for the entire organism remains an open question. The present paper addresses this problem by using a deterministic, geometrically based computer model, Y‐plant (Pearcy and Yang, 1996), that allows the three‐dimensional reconstruction of the entire above‐ground structures of a plant, and the calculation of whole plant light absorption and carbon gain. Moreover, it allows the simulation of plants with hypothetical features (e.g. morphologically ‘old’ plants with ‘young’ leaf‐level physiology and vice versa), essential for our goal of understanding the relative importance of size‐related physiological and architectural changes. Since its development, Y‐plant has been applied successfully to analogous questions, for example, the importance of variation in leaf and petiole morphology for carbon gain in the understorey plant, Adenocaulon bicolor (Pearcy and Yang, 1998), the importance of structural photo‐protection for different plant species from arid environments (Valladares and Pearcy, 1999; Valladares and Pugnaire, 1999), and the functional convergence in light capture of tropical understorey species of contrasting architecture (Valladares et al., 2002).
We chose the bromeliad, Vriesea sanguinolenta Cogn. & Marchal for detailed study. This large epiphyte has already been investigated extensively, and data on leaf photosynthesis (Schmidt and Zotz, 2001; Schmidt et al., 2001), leaf demography (Schmidt and Zotz, 2002), and habitat preferences (Croat, 1978; Zotz, 1997b; Zotz et al., 1999) allowed realistic simulations of changes in photosynthetic capacity with leaf age, or with respect to a range of external conditions.
MATERIALS AND METHODS
Plant material
Vriesea sanguinolenta Cogn. & Marchal (syn. Werauhia sanguinolenta; Grant, 1995) occurs in tropical moist and wet forests from Costa Rica to Colombia. In the Barro Colorado Nature Monument, Panama (BCNM, 9°10′ N, 79°51′ W), this epiphytic bromeliad can be found on a range of different host tree species, but it is particularly abundant on Annona glabra L. (Annonaceae) (Zotz et al., 1999). Eight individuals were collected from populations growing on A. glabra trees, and these were transferred to a glasshouse at the University of Würzburg (Germany). At the time of transfer, lengths of the longest leaf (LLmax) ranged from 7 to 76 cm. We used LLmax as a measure of size throughout the study as it is highly correlated with more rigorous measures of plant size, such as plant dry mass (Schmidt and Zotz, 2001).
Optical properties, crown architecture and simulation of light absorption
All morphological data of the eight individuals of V. sanguinolenta required to reconstruct their crowns with the computer model Y‐plant (Pearcy and Yang, 1996) were collected within 3 weeks of transferring the plants to Würzburg. Y‐plant simulates above‐ground crown architecture and calculates light absorption and carbon gain by the whole crown using optical and physiological properties of leaves. We determined the total length and width of each leaf, the divergence angles to neighbouring leaves, and elevation angles in 5 % steps from the leaf base to the tip. Measurements of leaf azimuths and elevation angles were made using a compass and an angle finder constructed from a level and protractor. Optical properties of leaves [leaf reflectance and transmittance for photosynthetic photon flux (PPF)] were measured as a function of leaf segment type, leaf age class and plant size using an integrating sphere attached to a spectral radiometer (LICOR model LI‐1800; Nebraska, USA). Absorptance was calculated as 1 – reflectance – transmittance.
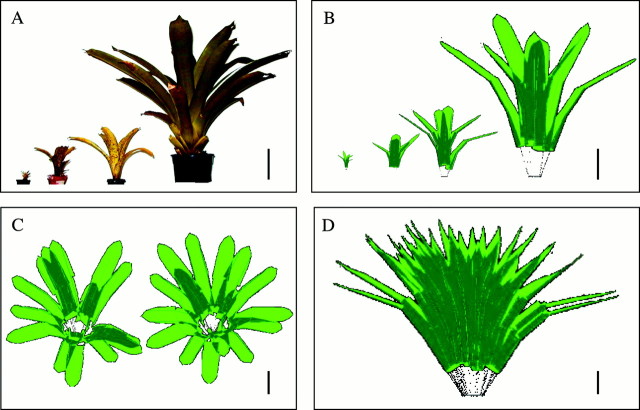
Y‐plant uses this information to produce a three‐dimensional image of the plant crown, reconstructing the plant node by node, creating a leaf as a three‐dimensional vector space with a given azimuth, angle from the horizontal, and distance from base to tip. The vector for petioles (the non‐chlorophyllous leaf bases were treated as ‘petioles’) was determined in a similar way. Leaves were connected to the distal end of the petiole with their position in space determined by the angle and azimuth of the normal to the leaf surface, the azimuth of the longitudinal axis corresponding to the (imaginary) midrib. Y‐plant was designed for plants with simple, flat leaves. To simulate the relatively complex morphology of the arched and curved bromeliad leaves, each leaf was treated as the combination of several units. Dividing the leaf into a total of nine parts rendered a very realistic 3‐D reconstruction of each leaf (see Fig. 1). Firstly, three segments were used to mimic the longitudinal curvature, and their lengths (from base to tip) were set to 25, 25 and 50 % of the total, following morphological data in Schmidt (2000). Secondly, each of these segments was divided into one broad central section and two narrow lateral sections to simulate the U‐shape of the leaves’ cross‐section. Examples of real and reconstructed plants are given in Fig. 1. By rotating this 3‐D image to specific vectors corresponding to a direction of incident PPF, Y‐plant calculates the fraction of PPF that would be intercepted from that direction. Simulations of direct PPF interception were based on the interception of light from the angle and azimuth of the solar disc every 30 min throughout the day, while simulations of diffuse PPF absorption were based on vectors for 160 different sky sectors (eight azimuth and 20 zenith angle classes).
Fig. 1. A, Photograph of four individuals of Vriesea sanguinolenta differing in size. B, The same four plants in A as reconstructed using Y‐plant; differences betweeen the reconstructions and photographs are partly due to different angles of view. C, Apical view of a V. sanguinolenta plant reconstructed using Y‐plant showing, on the left‐hand side, a simulated, normal plant, and on the right a modified plant with a divergence angle of 137·5° between successive leaves. D, The largest individual of V. sanguinolenta studied as reconstructed using Y‐plant. Bars = 25 cm.
The implications for light capture of the crown architecture of plants of different size were studied using several different measures of efficiency. The projected leaf area normal to incident PPF is the actual leaf area reduced by the cosine of the angle of incidence, and the projection efficiency (Ep) is the ratio of potential projected leaf area to actual leaf area. It expresses the angular effects on light interception in the absence of leaf overlap (self‐shading). The displayed area is the projected area as reduced by leaf overlap. The display efficiency (ED) is the ratio of displayed area to actual leaf area.
Estimations of carbon gain by the whole crown
Y‐plant calculates whole‐crown carbon gain by combining the estimated light absorption by plant foliage with the photosynthetic light response of leaves. The physiological data needed from light response curves measured in the field under well‐watered conditions are maximum net photosynthetic rate, dark respiration rate and photosynthetic quantum yield. Much of this information was already available for V. sanguinolenta from previous studies (e.g. Schmidt and Zotz, 2001). To include age‐related changes in physiological characteristics in our simulations we assigned leaves to three age classes. Plants exchange their foliage about once a year (Schmidt and Zotz, 2002), so we estimated leaf age (in months) by dividing leaf number (starting with the youngest leaf = 1) by the total number of leaves and multiplying it by 12. Potential carbon gain of plants was simulated for 21 June, considering that seasonal effects in tropical lowlands should be minimal as long as changes in water availability are ignored. Two different light environments (referred to as high and low light conditions) were simulated by manipulating the inputs of Y‐plant until the outputs given by the program for a horizontal surface matched those obtained in the field with PPF sensors placed at two positions in the canopy of the host trees. These two positions, and the corresponding light environments, represented extremes of the range of light conditions experienced by V. sanguinolenta in the field.
Estimated light absorption and carbon gain of simulated, normal plants were compared with those of equivalent plants that differed in their phyllotaxy (simulated, modified plants), i.e. the divergence angle of the spirally arranged leaves of V. sanguinolenta was modified in the plant files used by Y‐plant to achieve a divergence angle of 137·5°, which minimizes leaf overlap (Niklas, 1997).
Data analysis
Statistical analysis was carried out using STATISTICA software (STATISTICA 5·1, StatSoft Inc., Tulsa, OK, USA). Prior to analysis, variables were tested for normality and homoscedasticity. The slopes of regression lines were compared with an ANCOVA with log‐transformed elevation angles to achieve linearity. Otherwise, percentages and angles were arcsine square root transformed before analysis (Sokal and Rohlf, 1995). All t‐tests are for dependent samples.
RESULTS
Form: size‐related morphological and architectural changes
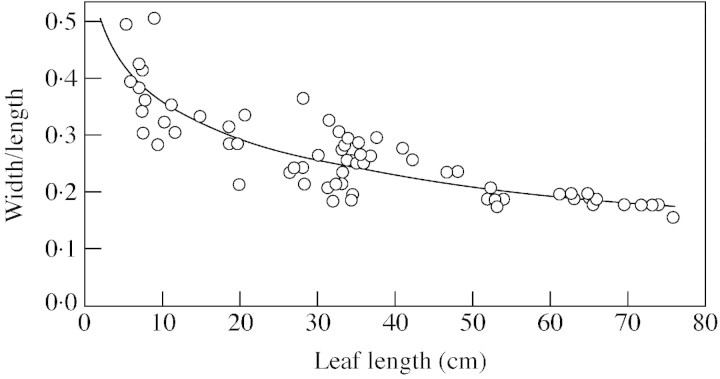
Total leaf areas of the eight plants analysed varied by almost three orders of magnitude. The smallest individual had five leaves (LLmax = 7·4 cm) and its total leaf area was 0·004 m2, whilst the largest plant had 37 leaves (LLmax = 76 cm) and a total leaf area of 1·1 m2. The only morphological parameter investigated that changed over this size range was leaf shape (Fig. 2): longer leaves were significantly narrower than smaller leaves. Average divergence angles of the plants ranged from 159 to 137° with no significant trend associated with plant size (r = –0·39, P = 0·3, n = 8). Neither did the elevation angles of the three longitudinal leaf segments into which each leaf was divided change with plant size (Pearson Product Moment correlation, n = 8, P > 0·05), but angles did change significantly between segments (one‐way ANOVA, F2,21 = 25·8, P < 0·001). Elevation angles of basal, central and apical segments were also influenced by their position within the plants, with angles decreasing continuously with leaf age and slopes of the three regression lines being significantly different (ANCOVA, leaf age as a co‐variate, F1,317 = 176·7, P < 0·001). For example, while the apical part of 1‐month‐old leaves had an elevation angle of approx. 80°, it decreased to approx. 25° in 12‐month‐old leaves. In the basal part, the decrease was only from approx. 90° to approx. 70°.
Fig. 2. Changes in leaf shape with size. Regression line: y = 0·57–0·09 ln (x); r2 = 0·73, n = 70.
Function: size‐related changes in physiological and optical properties
Maximum rates of net CO2 uptake (Amax, in µmol m–2 s–1) were a function of plant size, leaf segment (Table 1), and leaf age (Table 2). As shown in Tables 1 and 2, Amax was greatest in the apical segments of leaves of intermediate age. Dark respiration was around 10 % of the corresponding Amax for any given size. Saturating PPF (allowing 90 % of Amax) was generally low irrespective of size and was approx. 400 µmol m–2 s–1 (Schmidt and Zotz, 2001).
Table 1.
Changes in Amax as a function of plant size (LLmax) and position along the longitudinal axis of a leaf (leaf segment)
| Leaf segment | Regression | r 2 |
| Basal | y = 0·1862x – 0·3174 | 0·45 |
| Central | y = 0·2939x – 0·1088 | 0·85 |
| Apical | y = 0·3649x + 0·0018 | 0·90 |
Given are regression equations with y = log10 (Amax)(in µmol m–2 s–1) and x = log10 (LLmax) (in cm).
Sample size is eight in all cases, P < 0·05.
Data are from Schmidt and Zotz (2001) and previously unpublished (Schmidt and Zotz).
Table 2.
Changes in Amax as a function of leaf age
| Age class | Age (months) | Amax (%) |
| 1 | 0–1 | 37 |
| 2 | 2–8 | 100 |
| 3 | 9–12 | 77 |
The highest rates are observed in leaves of intermediate age and calculated following Table 1.
Relative values of Amax in younger and older leaves are given as a percentage.
Data are from Schmidt (2000).
Optical properties (transmittance, absorptance and reflectance) of V. sanguinolenta leaves were not size‐related for any leaf segment (Pearson Product Moment correlations, P > 0·05) (Table 3). Leaf age did not influence these parameters in central and upper leaf segments, but age and optical properties were significantly correlated in basal segments (P < 0·01, r2 between 0·3 and 0·6); for example, absorptance was less than 0·4 in the basal segments of recently emerged leaves, but exceeded 0·7 in the oldest leaves. To facilitate computation with Y‐plant, we used mean values of transmittance, absorptance and reflectance in the basal segments as well.
Table 3.
Optical properties of V. sanguinolenta leaves
| Segment | Reflectance | Absorptance | Transmittance |
| Basal | 0·26 ± 0·04 | 0·53 ± 0·08 | 0·21 ± 0·07 |
| Central | 0·17 ± 0·02 | 0·72 ± 0·04 | 0·11 ± 0·02 |
| Apical | 0·13 ± 0·02 | 0·82 ± 0·02 | 0·05 ± 0·02 |
Data are means ± s.d. (n = 27) for each of the three leaf segments of plants ranging in size from 10 to 77 cm LLmax.
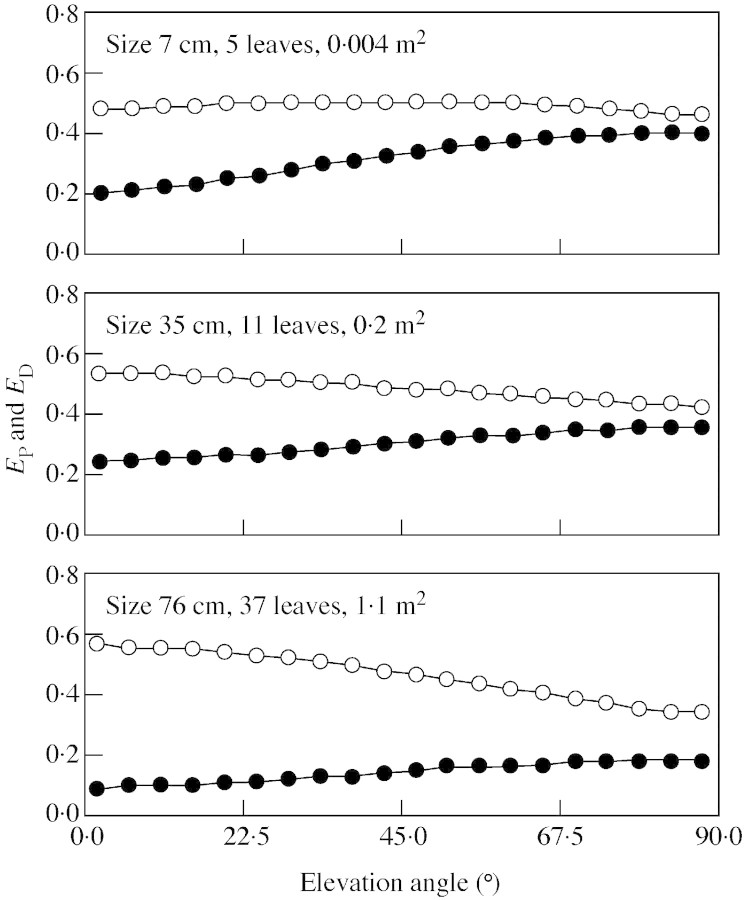
The efficiency of light capture is presented in Fig. 3 for three plants covering the entire size range. Changes in projection efficiency and display efficiency for differ ent elevation angles of incoming radiation were small, indicating similar leaf overlap at any elevation. Self‐shading, which is quantified by the difference between EP and ED, was greatest in the large plant, particularly at low solar elevation. It increased by a factor of two to three from the smallest to largest individual, but the trend was only significant when the sun was low (elevation angle < 30°, P < 0·05).
Fig. 3. The dependence of projection efficiency (EP, open symbols) and display efficiency (ED, closed symbols) on the elevation angle of the incoming radiation in three individuals of different size. The difference between EP and ED is due to mutual shading among leaves. Each point is the mean value of all azimuths at that particular elevation angle.
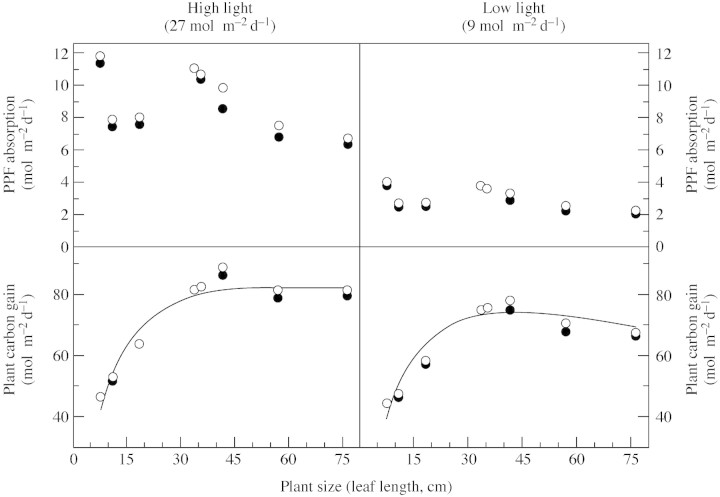
The integrated light absorption of entire plants and the corresponding potential carbon gain for two different light fluences are shown in Fig. 4. Absorbed PPF differed significantly among individuals in both cases, but not with plant size (P > 0·05). The light absorption efficiency of simulated plants with a modified divergence angle of 137·5° was approx. 5 % higher (t‐tests, P < 0·05) than in unmodified plants. In spite of a three‐fold difference in incident light and light absorption, reductions in the integrated plant carbon gain (PCG) due to low PPF averaged only 11 %, with a trend towards greater reductions in PCG in larger individuals. Plants with the ideal divergence angle gained approx. 2 % more carbon (significant at P = 0·05, t‐tests).
Fig. 4. PPF absorption and corresponding carbon gain by V. sanguinolenta plants of different sizes in two light environments as calculated by Y‐plant. Closed symbols indicate simulated, normal plants; open symbols indicate plants modified to exhibit a divergence angle of 137·5° between successive leaves. Regression lines are y = 82 (1–e–0·009x) (r2 = 0·94) and y = 73·9 e(–0·5 (ln (x/428)/1·552) (r2 = 0·92).
The relative importance for plant carbon gain of size‐related changes in leaf physiology vs. varying plant morphology was assessed by simulating plants using either the physiology, the morphology, or both, of the smallest and the largest plants. Both the morphology (narrow leaf shape; Fig. 2) and the physiology (higher Amax; Table 1) of larger plants increased PCG (Fig. 5). However, the effect of leaf physiology was quantitatively more important. While smaller plants showed a very moderate increase in PCG with the leaf morphology of a large plant (Fig. 5B), assuming the physiological properties of a large plant led to an almost two‐fold increase in carbon gain (Fig. 5C). Simulation of large plants with the wide leaves of small plants reduced PCG by approx. 20 % (Fig. 5B), but assuming the physiological characteristics of a small plant gave a much more pronounced reduction (approx. 50 %) (Fig. 5C). Overall, changes in PCG due to physiological modifications (Fig. 5C) were significantly larger than changes due to morphological modifications (Fig. 5B) (t‐tests, P < 0·05).
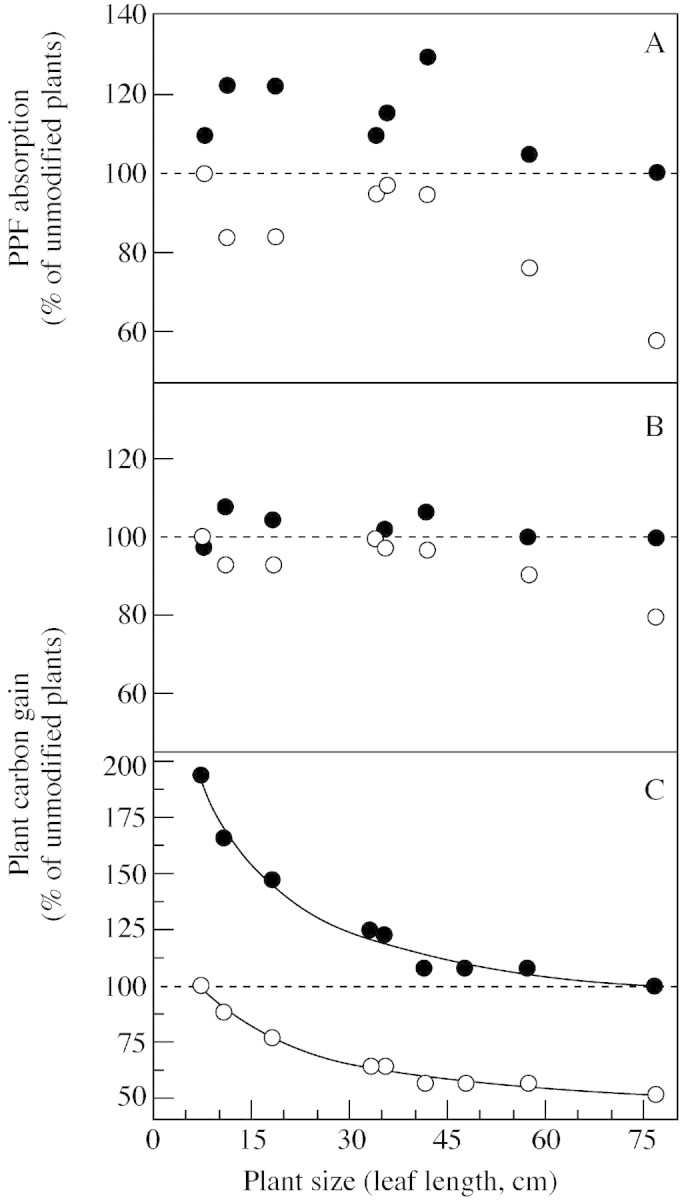
Fig. 5. PPF absorption (A) and carbon gain (B and C) by V. sanguinolenta plants of different sizes. Plants were simulated with Y‐plant using the morphology of the largest (closed symbols) or smallest plants (open symbols) (A and B), or using the physiology of the largest (closed symbols) or smallest plants (open symbols) (C). Dotted lines indicate values for plants simulated without such modifications in morphology or physiology. Regression lines in C are hyperbolic decay functions: PCG = 80 + (17220/(82·1 + LLmax)) for upper line, PCG = 40 + (9720/(90+ LLmax)) for lower line; r2 > 0·98. Calculations were carried out using the smallest daily photon flux that plants experience in nature (9 mol m–2 d–1; Schmidt and Zotz, 2001).
DISCUSSION
Maximum rates of instantaneous net CO2 uptake increase linearly with plant size in V. sanguinolenta and other epiphytes (Schmidt and Zotz, 2001). Similarly, integrated diurnal leaf carbon gain (AL) and plant size show a close, linear correlation (Schmidt and Zotz, 2001; see also Zotz and Winter, 1993). However, potential plant carbon gain and plant size were non‐linearly related: PCG reached saturation in plants of intermediate size in high light, or even decreased slightly in the largest plants in low light (Fig. 4). Increased self‐shading with plant size (Fig. 3) is probably the main reason for this curvilinear relationship. Changes in plant architecture during development (‘ontogenetic drift’; Evans, 1972) frequently lead to reduction in light capture efficiency due to the general increase of self‐shading with plant size (Chazdon, 1985; Ackerly and Bazzaz, 1995). Interspecific, but also intraspecific, comparisons among plants with canopies of different sizes thus always face a problem of scale.
Remarkably, the variations in Amax and PCG associated with changes in plant size were quantitatively quite similar: Schmidt and Zotz (2001) reported that Amax varied by a factor of 2·3 from the smallest to the largest individual used in the present study, while the carbon gain of entire plants based on total leaf area varied by a factor of 1·9 (Fig. 4). This observation and the results of our simulations (Fig. 5) clearly indicate that both physiological and architectural changes influence PCG in V. sanguinolenta. Over the entire range, the former is more important (Fig. 5), but the size‐related variation in Amax of leaves (Schmidt and Zotz, 2001) was strongly modulated by changes in plant morphology.
Self‐shading in small plants is relatively modest, and our simulations using the narrow leaves of large plants yielded only minor improvements in carbon gain of a few per cent (Fig. 5B). In contrast, the broad leaves of small plants reduced light absorption in large plants more strongly, reducing light capture by more than 40 % (Fig. 5A) and PCG by more than 20 % (Fig. 5B). Most of the self‐shading in large plants affects the basal portions of leaves which are not very productive anyway (Table 1). By the same token, ignoring size‐related changes in optical properties of basal leaf portions in our simulations should have little influence on the outcome of the model calculations. The relatively modest reduction in PCG compared with that in light capture is also a consequence of the low light‐saturation point of photosynthesis (Schmidt and Zotz, 2001), which is comparable with that of other epiphytes growing at exposed sites (Stuntz and Zotz, 2001).
Although our simulations show that variation in leaf processes scales up to the whole plant, there are a number of simplifications that must be taken into account in the interpretation of our results. For example, all calculations of carbon gain (Figs 5 and 6) assume good water supply. Given that even in the wet season this assumption is frequently not met in Central Panama (Windsor, 1990), our estimates would strongly overestimate long‐term carbon gain in a natural setting. Even more important in the context of this paper, water availability in tank bromeliads is size‐dependent. Tanks, which are formed by overlapping leaf bases, bridge rainless periods much more efficiently in larger plants compared with smaller conspecifics (Zotz and Thomas, 1999; Schmidt and Zotz, 2001). Thus, the expected trend in PCG from smaller to larger plants in situ is probably even steeper than suggested by our results (Fig. 4). It should also be pointed out that our study only scales up to green foliage. To quantify whole plant carbon budgets, all respiring plant parts, e.g. the roots and the achlorophyllous leaf bases, have to be considered. Finally, the present analysis is restricted to carbon relations. Although many studies on crown architecture assume selection for greater carbon gain (e.g. Mooney and Chiariello, 1984; Niklas, 1988), this may not be the most important ‘currency’ in our case. In most tank bromeliads, where roots are primarily holdfasts, leaves serve the triple function of carbon uptake, water storage and water procurement (via absorbing scales) (Zotz and Thomas, 1999; Benzing, 2000; Schmidt and Zotz, 2001). Clearly, future studies should evaluate the trade‐off between these different functions.
ACKNOWLEDGEMENTS
We thank R. W. Pearcy (California, USA) and M. T. Tyree (Vermont, USA), for fruitful discussions, G. Schmidt (Würzburg), for permission to use unpublished data, and the Republic of Panama for making its natural resources available for study. Financial support came from the Deutsche Forschungsgemeinschaft (SFB 251), which covered travel expenses for F.V.
Supplementary Material
Received: 15 March 2002; Returned for revision: 3 June 2002; Accepted: 18 June 2002 Published electronically: 4 September 2002
References
- AckerlyDD, Bazzaz FA.1995. Seedling crown orientation and interception of diffuse radiation in tropical forest gaps. Ecology 76: 1134–1146. [Google Scholar]
- BenzingDH.2000. Bromeliaceae – Profile of an adaptive radiation. Cambridge: Cambridge University Press. [Google Scholar]
- BoardmanNK.1977. Comparative photosynthesis of sun and shade plants. Annual Review of Plant Physiology 28: 355–377. [Google Scholar]
- ChazdonRL.1985. Leaf display, canopy structure and light interception of two understory palm species. American Journal of Botany 72: 1493–1502. [Google Scholar]
- CroatTB.1978. Flora of Barro Colorado Island. Stanford: Stanford University Press. [Google Scholar]
- EvansGC.1972. The quantitative analysis of growth. Berkeley: University of California Press. [Google Scholar]
- EvansJR, Von Caemmerer S, Adams III WW.1988. Ecology of photosynthesis in sun and shade. Melbourne: CSIRO. [Google Scholar]
- GivnishTJ.1988. Adaption to sun and shade: a whole‐plant perspective. Australian Journal of Plant Physiology 15: 63–92. [Google Scholar]
- GrantJR.1995. Bromelienstudien. Tropisch Subtropische Pflanzenwelt91: 1–57. [Google Scholar]
- LambersH, Chapin III FS, Pons TL.1998. Plant physiological ecology. New York: Springer. [Google Scholar]
- MooneyHA, Chiariello N.1984. The study of plant function – the plant as a balanced system. In: Dirzo R, Surakhan J, eds. Perspectives in plant population ecology Sunderland, MA: Sinauer Associates, 305–323. [Google Scholar]
- NiklasKJ.1988. The role of phyllotactic pattern as a “developmental constraint” on the interception of light by leaf surfaces. Evolution 42: 1–16. [DOI] [PubMed] [Google Scholar]
- NiklasKJ.1992. Petiole mechanics, light interception by lamina and “economy of design”. Oecologia 90: 518–526. [DOI] [PubMed] [Google Scholar]
- NiklasKJ.1997. The evolutionary biology of plants. Chicago: Chicago University Press. [Google Scholar]
- NiklasKJ, Kerchner V.1984. Mechanical and photosynthetic constraints on the evolution of plant shape. Palaebiology10: 79–101. [Google Scholar]
- PearcyRW, Yang W.1996. A three‐dimensional shoot architecture model for assessment of light capture and carbon gain by understory plants. Oecologia 108: 1–12. [DOI] [PubMed] [Google Scholar]
- PearcyRW, Yang W.1998. The functional morphology of light capture and carbon gain in the Redwood forest understorey plant, Adenocaulon bicolor Hook. Functional Ecology 12: 543–552. [Google Scholar]
- SchmidtG.2000. Plant size and intraspecific variability in vascular epiphytes. PhD Thesis, Bayerische Julius‐Maximilians‐Universität. [Google Scholar]
- SchmidtG, Zotz G.2001. Ecophysiological consequences of differences in plant size – in situ carbon gain and water relations of the epiphytic bromeliad, Vriesea sanguinolenta Plant, Cell and Environment 24: 101–112. [Google Scholar]
- SchmidtG, Zotz G.2002. Growth in two Carribbean epiphyte species – a demographic approach. Journal of Vegetation Science 13 (in press). [Google Scholar]
- SchmidtG, Stuntz S, Zotz G.2001. Plant size – an ignored parameter in epiphyte ecophysiology. Plant Ecology 153: 65–72. [Google Scholar]
- SokalRR, Rohlf FJ.1995. Biometry 3rdedn. New York: Freeman and Co. [Google Scholar]
- StuntzS, Zotz G.2001. Photosynthesis in vascular epiphytes – a survey of 27 species of diverse taxonomic origin. Flora 196: 132–141. [Google Scholar]
- ValladaresF.1999. Architecture, ecology, and evolution of plant crowns. In: Pugnaire FI, Valladares F, eds. Handbook of functional plant ecology New York: Marcel Dekker, 121–194. [Google Scholar]
- ValladaresF, Pearcy RW.1998. The functional ecology of shoot architecture in sun and shade plants of Heteromeles arbutifolia M. Roem., a Californian chaparral shrub. Oecologia 114: 1–10. [DOI] [PubMed] [Google Scholar]
- ValladaresF, Pearcy RW.1999. The geometry of light interception by shoots of Heteromeles arbutifolia: morphological and physiological consequences for individual leaves. Oecologia 121: 171–182. [DOI] [PubMed] [Google Scholar]
- ValladaresF, Pugnaire FI.1999. Tradeoffs between irradiance capture and avoidance in semi‐arid environments assessed with a crown architecture model. Annals of Botany 83: 459–469. [Google Scholar]
- ValladaresF, Skillman JB, Pearcy RD.2002. Convergence in light capture efficiencies among tropical forest understory plants with contrasting crown architectures: a case of morphological compensation. American Journal of Botany 89: 1275–1284. [DOI] [PubMed] [Google Scholar]
- WindsorDM.1990. Climate and moisture variability in a tropical forest: long‐term records from Barro Colorado Island, Panamá. Washington: Smithsonian Institution. [Google Scholar]
- YamadaT, Okuda T, Abdullah M, Awang M, Furukawa A.2000. The leaf development process and its significance for reducing self‐shading of a tropical pioneer tree species. Oecologia 125: 476–482. [DOI] [PubMed] [Google Scholar]
- ZotzG.1997a Photosynthetic capacity increases with plant size. Botanica Acta 110: 306–308. [Google Scholar]
- ZotzG.1997b Substrate use of three epiphytic bromeliads. Ecography 20: 264–270. [Google Scholar]
- ZotzG.2000. Size‐related intraspecific variability in physiological traits of vascular epiphytes and its importance for plant physiological ecology. Perspectives in Plant Ecology, Evolution and Systematics 3: 19–28. [Google Scholar]
- ZotzG, Thomas V.1999. How much water is in the tank? Model calculations for two epiphytic bromeliads. Annals of Botany 83: 183–192. [Google Scholar]
- ZotzG, Winter K.1993. Short‐term photosynthesis measurements predict leaf carbon balance in tropical rainforest canopy plants. Planta 191: 409–412. [Google Scholar]
- ZotzG, Ziegler H.1999. Size‐related differences in carbon isotope discrimination in the epiphytic orchid, Dimerandra emarginata Naturwissenschaften 86: 39–40. [Google Scholar]
- ZotzG, Bermejo P, Dietz H.1999. The epiphyte vegetation of Annona glabra on Barro Colorado Island, Panama. Journal of Biogeography 26: 761–776. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.