Abstract
Wheat plants (Triticum aestivum L. ‘Lyallpur’), limited to a single culm, were grown at day/night temperatures of either 18/13 °C (moderate temperature), or 27/22 °C (chronic high temperature) from the time of anthesis. Plants were either non‐droughted or subjected to two post‐anthesis water stresses by withholding water from plants grown in different volumes of potting mix. In selected plants the demand for assimilates by the ear was reduced by removal of all but the five central spikelets. In non‐droughted plants, it was confirmed that shading following anthesis (source limitation) reduced kernel dry weight at maturity, with a compensating increase in the dry weight of the remaining kernels when the total number of kernels was reduced (small sink). Reducing kernel number did not alter the effect of high temperature following anthesis on the dry weight of the remaining kernels at maturity, but reducing the number of kernels did result in a greater dry weight of the remaining kernels of droughted plants. However, the relationship between the response to drought and kernel number was confounded by a reduction in the extent of water stress associated with kernel removal. Data on the effect of water stress on kernel dry weight at maturity of plants with either the full complement or reduced numbers of kernels, and subjected to low and high temperatures following anthesis, indicate that the effect of drought on kernel dry weight may be reduced, in both absolute and relative terms, rather than enhanced, at high temperature. It is suggested that where high temperature and drought occur concurrently after anthesis there may be a degree of drought escape associated with chronic high temperature due to the reduction in the duration of kernel filling, even though the rate of water use may be enhanced by high temperature.
Key words: Wheat, drought, high temperature, kernel filling
INTRODUCTION
Above‐optimal temperatures and drought are common during kernel filling in wheat growing areas of the world with a Mediterranean climate, including Australia (Hsia et al., 1963; Asana, 1966; Connor, 1975; Nix, 1975; Fischer, 1979; Angus et al., 1980; Aggarwal and Sinha, 1984; Nicolas et al., 1985; Kobata et al., 1992; Palta et al., 1994; Wardlaw and Wrigley, 1994; Shackley and Anderson, 1995). However, it is often difficult to distinguish the main cause of yield reduction when both stresses overlap, as there are many apparent similarities in the response of kernel filling to drought and heat.
Drought following heading has little effect on the rate of kernel filling, but its duration (time from fertilization to maturity) is shortened and kernel dry weight at maturity is reduced (Wardlaw, 1967; Aggarwal and Sinha, 1984; Khanna‐Chopra et al., 1994; Wardlaw and Willenbrink, 2000). The decline in net CO2 exchange of the leaves, stems and ears of wheat following anthesis is accelerated under drought, with the possibility of a shortfall of carbohydrate during kernel filling. Part of the response to drought is an earlier mobilization of non‐structural reserve carbohydrates from the stem and leaf sheaths, which provide a greater proportion of the kernel dry weight at maturity (Gallagher et al., 1976; Bidinger et al., 1977; Blum et al., 1991; Palta et al., 1994; Wardlaw and Willenbrink, 2000).
Chronic high temperatures, up to a mean of approx. 27 °C (day time maximum 30 °C), during kernel filling appear to have a similar effect to drought. There is often only a marginal increase in the rate of kernel filling as mean temperatures increase from 18 to 27 °C and a significant reduction in the duration of kernel filling, resulting in smaller kernels at maturity (Sofield et al., 1977a; Chowdhury and Wardlaw, 1978; Wiegand and Cuellar, 1981; Shpiler and Blum, 1986; Tashiro and Wardlaw, 1989). While drought stress may impose a source limitation on kernel filling associated with the availability of both current and stored photosynthate (Gallagher et al., 1976; Bidinger et al., 1977; Aggarwal and Sinha, 1984), chronic high temperature normally appears to act directly on the developing kernels in an ear (Ford et al., 1976; Wardlaw et al., 1980; Bhullar and Jenner, 1983).
Field studies have often been centred on the role of drought in limiting yield, but there are a number of examples, based largely on time of sowing experiments, when high temperature following heading has been considered part of the equation (Fischer and Kohn, 1966; Marcellos and Single, 1972; Doyle and Marcellos, 1974; Woodruff and Tanks, 1983; Saini and Dadwhal, 1986; Stapper and Fischer, 1990; Shackley and Anderson, 1995). The relative importance of drought and high temperature in limiting yield, when these occur concurrently during kernel filling, is difficult to determine under field conditions due to the variable nature of each stress and variation in the growth of individual plants, particularly in leaf area associated with tillering. However, in at least two field trials in Australia, high temperature following heading has been considered of major importance in limiting yield (McDonald et al., 1983, 1984; Cooper, 1992).
Even under controlled environment conditions, equating the degree of water stress when plants are grown at different temperatures is difficult, as both the rate of kernel development and also the rate of water use by the plant are temperature dependent. Davidson and Birch (1978) found that an increase in day/night temperature from 18/13 °C to 24/19 °C improved post‐anthesis water use efficiency (g water used per g grain produced), although grain yield was reduced. This effect of temperature was also evident under both mild and severe drought conditions, and these authors concluded that there was no water × temperature interaction in relation to grain yield per plant. In experiments on wheat where high temperature (28/20 °C) following anthesis was imposed during water stress, Nicolas et al. (1984) concluded that the effects of drought and high temperature on grain yield were additive. However, high temperature in these experiments increased the degree of water stress.
The current experiments were designed to examine more precisely the effect of chronic high temperature (day temperatures less than 30 °C) and drought following anthesis on kernel filling, by varying the degree (timing) of water stress under moderate and high temperature conditions, in a situation where the source/sink balance was altered by reducing kernel numbers in some of the ears. A high temperature of 27/22 °C was chosen for this study as this temperature commonly occurs in the field following heading and, at higher temperatures (above 30 °C), grain metabolism may be markedly altered with the formation of heat shock proteins (Wardlaw and Wrigley, 1994).
MATERIALS AND METHODS
Plant growth
Wheat plants (Triticum aestivum L.) of the cultivar Lyallpur (Aus 18804) were selected for this study because of their sensitivity to high temperature (Wardlaw et al., 1989). Plants were grown individually in cylindrical pots of variable volume, containing a 1 : 1 mixture of perlite and vermiculite. To ensure as little variation as possible among plants in the rate of water use, plants were restricted to a single culm by the periodic removal of all tillers up to the time of anthesis. Prior to anthesis, plants were watered with standard nutrient solution each morning (Hoagland No. 2 with minor modification of the trace elements) and with tap water each afternoon. After anthesis, selected plants were no longer supplied with water or nutrients (droughted), while the non‐stressed controls received tap water only. Plants were initially maintained in a naturally lit phytotron glasshouse at a day temperature of 18 °C for 8 h and a night temperature of 13 °C for 16 h, with the natural photoperiod extended to 16 h by low intensity quartz‐iodide flood lights (Morse and Evans, 1962).
Drought and temperature treatments
At anthesis, plants were transferred to artificially lit growth chambers at a day/night temperature of either 18/13 °C (8/16 h) or 27/22 °C (8/16 h), and a daytime irradiance of 600 µmol m–2 s–1 [photosynthetically active radiation (PAR)] supplied by Siemens Power Star HQ1T WD 400W metal arc lamps, measured at the height of the flag leaf blade. The photoperiod was extended to 16 h by low intensity incandescent lamps. Post‐anthesis water stress was induced by ceasing the supply of nutrients and water to the plants at anthesis, and two water stresses were obtained by varying the volume of the perlite/vermiculite in each pot, with a 2·12‐l volume providing a ‘mild’ stress and a 1·54‐l volume providing a ‘severe’ stress. Pot diameter was 85 mm, so differences in volume were associated with the depth of the potting mix.
Sink–source manipulations
The number of kernels per ear of selected plants was limited (reduced sink) to the five central spikelets of an ear by removal of the four basal spikelets and all remaining spikelets above the ninth (each ear typically consisted of 16–18 spikelets).
Limitation of assimilate supply (source reduction) was achieved in selected plants by the use of 50 % shade cloth to reduce cabinet irradiance from 600 to 300 µmol m–2 s–1 PAR.
Roots and young tillers are possible alternative sinks to the developing grain following anthesis. Root growth was not checked in these experiments, but in a preliminary experiment measurements were made of the regrowth (dry weight) of young tillers in the period from anthesis to kernel maturity.
Measurements
Dry weights.
At each harvest, plant parts were separated and oven dried at 80 °C for 48 h, then left under laboratory conditions (approx. 20 °C at 40 % relative humidity) until weighed.
Plant–water relations.
The level of drought stress reached by a particular plant was assessed by measuring the water potential of the flag leaf blade. Two fresh leaf discs (0·7 cm diameter) were punched from the centre of each blade, and the water potential of the discs was measured using a thermocouple psychrometer (Wescor, Logan, UT, USA). In two preliminary experiments, water potential (ψw) and relative water content [RWC = (initial fresh weight – dry weight/saturated fresh weight – dry weight) × 100; Weatherley, 1950] of the flag leaf of droughted and well‐watered control plants were compared with measurements on the stem, glumes and kernels from the ear. Measurements were made between 4 and 6 h after the start of the high light period.
Net CO2 exchange (NCE).
Net CO2 exchange measurements were made on a 6·25 cm2 area of fully expanded flag leaf blades using a portable infrared gas analysis (IRGA) system. A Parkinson leaf chamber was linked to an IRGA (LCA‐2 Analytical Development Co., Hoddeson, UK) and data logger (DL‐1). Air was supplied from a cylinder at a partial pressure of 330 µmol CO2 mol–1 and zero humidity. Light at the leaf surface was 500 µmol m–2 s–1 PAR and airflow through the leaf chamber was 0·5 l min–1. Measurements were made between 1000 and 1600 h with a mixed sequence across treatments to reduce any bias due to timing.
Preliminary studies on the response to temperature and a change in source–sink balance during kernel filling
(1) The pattern of kernel filling and stem weight at 18/13 °C and 27/22 °C was determined for non‐droughted plants, with harvests of 22 plants at 12, 24 and 30 d after anthesis and at maturity (all green coloration lost from the glumes and the grain).
(2) In a second set of plants, from the same planting, the number of kernels per ear was either unaltered or limited to those in the five central spikelets (reduced sink) and these plants were either maintained under full light in the 18/13 °C cabinet (600 µmol m–2 s–1 PAR), or light was reduced with 50 % shade cloth (reduced source). All plants were harvested at maturity, with 22 plants per treatment.
Response to drought and variable sink capacity during kernel filling at low temperature
From anthesis onwards, plants were held in an artificially lit cabinet at a day/night temperature of 18/13 °C and a relative humidity of 60 %. In half of the plants, sink size was reduced by removing all but the five central spikelets of an ear. Two drought stresses (‘mild’, large pot volume; ‘severe’, small pot volume) were imposed by ceasing watering at anthesis. Kernels were harvested and water potential measurements made at 10, 15, 20, 25, 30 and 35 d after anthesis, and at maturity. There were five to six replicates for each intermediate harvest and 13 to 14 replicates at maturity.
Response to drought and variable sink size at high temperature
Plants were held in an artificially lit cabinet from anthesis at a day/night temperature of 27/22 °C and a relative humidity of 40 %. In half of the plants, sink size was reduced by removing all but the five central spikelets of an ear. Two drought stresses (‘mild’ and ‘severe’) were imposed by ceasing watering at anthesis. Kernels were harvested and water potential measurements made at 7, 14, 17 and 21 d after anthesis, and at maturity. There were five replicates at each intermediate harvest and 13 to 24 replicates at maturity.
Interaction between drought, temperature and sink size in regulating kernel dry weight at maturity
At anthesis, plants were transferred to artificially lit cabinets with a day/night temperature of either 18/13 °C or 27/22 °C and a relative humidity of 65 and 40 %, respectively. Drought was induced by ceasing the supply water and nutrients at anthesis. In an attempt to balance the timing of the stress, pot volume at low temperature was 1·54 l and at high temperature was 2·12 l. In half of the plants, sink size was reduced by retaining only the five central spikelets of an ear.
In a limited study, the effect of treatment on flag leaf water potential and NCE was determined for both droughted and control plants 19–20 d after anthesis in the 18/13 °C treatment, and 13–14 d after anthesis in the 27/22 °C plants, i.e. towards the beginning of the linear phase of kernel filling in each case.
RESULTS
Preliminary studies
Tiller regrowth following anthesis was reduced by 84 % in droughted plants at 18/13 °C and by 91 % in droughted plants at 27/22 °C. Limiting the ear demand, by reducing kernel number, significantly increased tiller regrowth of both control and droughted plants at 18/13 °C, but only in control plants at 27/22 °C. Tiller regrowth was reduced by 87–97 % with the increase in temperature from 18/13 °C to 27/22 °C.
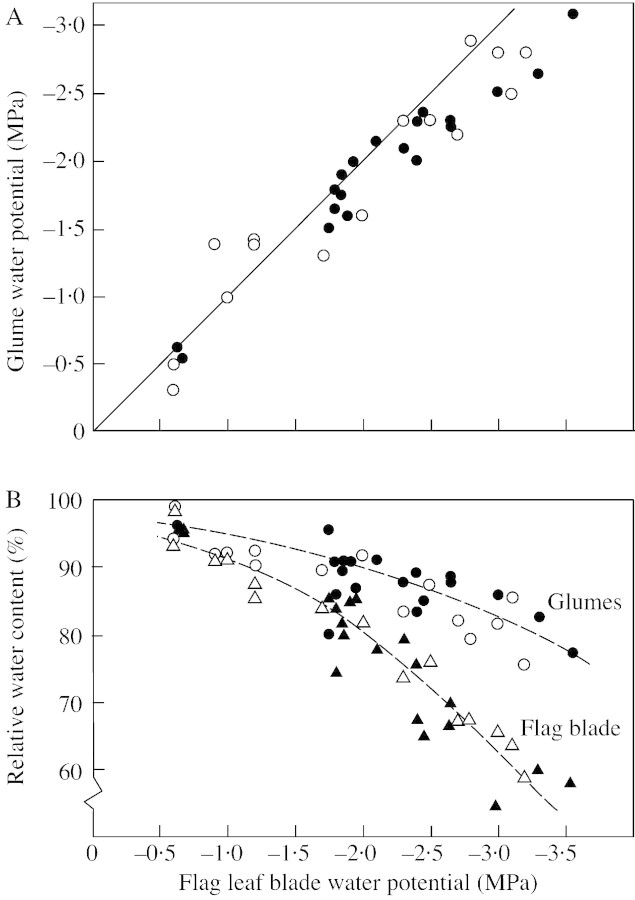
Not all organs responded in a similar way to increasing drought, with the grains showing only a small drop in ψw and RWC (Table 1) and the glumes largely following the pattern of the flag leaf (Fig. 1) until this fell to around –2 MPa. With greater stress, indicated by lower flag leaf water potentials, the glumes showed a somewhat smaller drop in water potential than the flag leaf and maintained higher relative water contents. The water potential of the peduncle of stressed plants was intermediate between that of the flag leaf and grains, with that part of the peduncle enclosed by the flag leaf sheath having a lower water potential than the exposed part of the peduncle (Table 1). Water potential differences between the flag leaf, glumes, stem and grain were maintained as the water stress increased further (data not shown). These findings are broadly in agreement with those of Xu and Ishii (1990).
Table 1.
A comparison of water potential measurements for different organs of wheat plants subjected to drought following anthesis
| Organ | Water potential (MPa)* | Range (MPa) |
| Flag blade | –1·88 ± 0·04 | 1·75–2·10 |
| Glumes | –1·79 ± 0·08 | 1·60–2·00 |
| Enclosed peduncle | –1·45 ± 0·07 | 1·10–1·75 |
| Exposed peduncle | –1·00 ± 0·03 | 0·91–1·21 |
| Kernels | –0·57 ± 0·03 | 0·46–0·73 |
* Mean values ± s.e., from measurements on eight plants.
Fig. 1. The relationship between flag leaf and glume water relations of wheat plants held at 18/13 °C and subjected to drought following anthesis. A, Comparison of flag leaf and glume water potential measurements. Solid line is the one to one relationship between the flag leaf and glume water potentials. B, The relationship between flag leaf water potential and the relative water content of the flag leaf (triangles) and the relative water content of the glumes (circles). Combined data from two experiments identified by open and closed symbols. Dashed lines are hand fitted to illustrate trends. See Table 1 for a comparison with other organs.
At moderate temperatures (18/13 °C), reducing incident radiation by shading following anthesis (reduced source) decreased the dry weight at maturity of kernels taken from the five central spikelets. However, when kernel number was restricted, at the time of anthesis, to these five central spikelets (reduced sink capacity), individual kernel weight was not altered by shading (Table 2).
Table 2.
The effect of shading (reduced photosynthetic assimilate supply) on kernel dry weight at maturity in relation to ear kernel number, for plants held at day/night temperatures of 18/13 °C following anthesis
| Full kernel number | Reduced kernel number | |||
| Measurement | Full light | 50 % shade | Full light | 50 % shade |
| Kernel number | 54·9 ± 1·2 | 56·1 ± 1·2 n.s. | 19·7 ± 0·4 | 20·9 ± 0·5 n.s. |
| Weight per kernel (mg)* | 67·9 ± 0·9 | 59·8 ± 1·5** | 72·4 ± 0·6 | 72·8 ± 0·7 n.s. |
Each value is the mean of 22 replicates ± s.e.
* Kernels from the five central spikelets of an ear.
** Shading effect significant at the 1 % level.
n.s., Shading effect not significant.
Temperature and drought responses
At low temperature (18/13 °C), subjecting plants to drought following anthesis also reduced dry weight of kernels taken from the five central spikelets at maturity. Reducing kernel number resulted in a smaller decrease in the dry weight of the remaining kernels in response to drought; however, this result was confounded by a reduction in drought stress, as indicated by an increased flag leaf water potential in response to degraining (Table 3).
Table 3.
The effect of reduced kernel number on the response of kernel filling in wheat to drought stress following anthesis, under moderate temperature conditions (18/13 °C)
| Treatment | Flag blade Ψw (MPa)* | Dry weight per kernel (mg)† |
| Control | ||
| Full kernel number | –0·64 ± 0·06 | 82·6 ± 1·0a |
| Reduced kernel number‡ | –0·55 ± 0·05 | 85·0 ± 1·0a |
| Mild stress | ||
| Full kernel number | –1·60 ± 0·28 | 49·2 ± 2·2b |
| Reduced kernel number‡ | –1·10 ± 0·15 | 71·3 ± 2·2c |
| Severe stress | ||
| Full kernel number | –7·04 ± 0·84 | 28·6 ± 2·0d |
| Reduced kernel number‡ | –3·20 ± 1·09 | 35·1 ± 2·2e |
* Flag leaf water potential (Ψw) 20 d after anthesis (n = 6).
† Dry weight per kernel at maturity, based on the measurement of the two basal kernels from the five central spikelets of 16 replicates ± s.e. Values with different superscripts differ significantly at the 5 % level.
‡ Kernels removed from all but the five central spikelets of an ear.
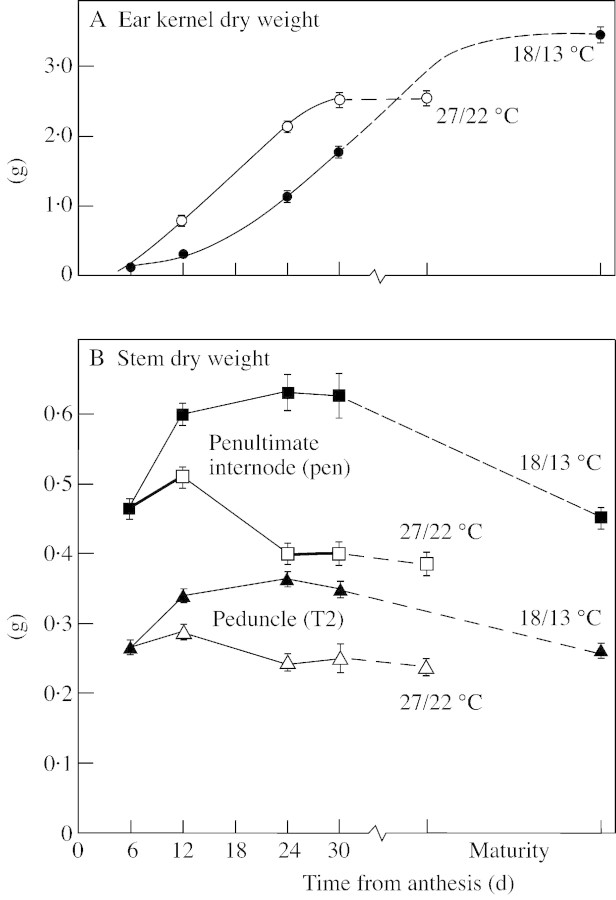
The maximum rate of kernel filling in well‐watered plants increased from 110 to 127 mg per ear d–1 (15 %) with an increase in post‐anthesis temperature from 18/13 °C to 27/22 °C, but the duration of kernel filling was reduced by 32 % with this increase in temperature, resulting in a reduction in kernel weight at maturity (Fig. 2A). The post‐anthesis accumulation of stem dry matter was greatly diminished at the higher temperature, and the subsequent loss of stem dry matter was reduced and occurred earlier (Fig. 2B). Losses in stem dry weight following anthesis are largely accounted for by changes in non‐structural carbohydrate reserves (see Wardlaw and Willenbrink, 2000).
Fig. 2. The effect of high temperature on changes in kernel and stem dry weight with time from anthesis. A, Kernel dry weight per ear. B, Dry weight of the penultimate internode (squares) and that part of the peduncle enclosed by the flag leaf sheath (triangles). Plants held at either 18/13 °C (closed symbols) or 27/22 °C (open symbols) from anthesis. Each value is the mean of 22 replicates. Vertical bars indicate 2 × s.e.
Under chronic high temperature (27/22 °C), mild drought stress decreased the dry weight of kernels from the central spikelets at maturity, with a reduced effect (greater dry weight) on those kernels in ears with fewer kernels. However limiting kernel number also resulted in higher flag leaf water potentials (smaller stress) (Table 4). A similar, but increased response, was observed under greater drought stress.
Table 4.
The effect of reduced kernel number on the response of kernel filling in wheat to drought stress following anthesis, under high temperature conditions (27/22 °C)
| Treatment | Flag blade Ψw (MPa)* | Dry weight per kernel (mg)† |
| Control | ||
| Full kernel number | –0·62 ± 0·06 | 56·8 ± 0·8a (14) |
| Reduced kernel number‡ | –0·62 ± 0·04 | 58·8 ± 1·1a (13) |
| Mild stress | ||
| Full kernel number | –1·10 ± 0·18 | 34·4 ± 1·5b (14) |
| Reduced kernel number‡ | –0·88 ± 0·13 | 49·5 ± 1·0c (16) |
| Severe stress | ||
| Full kernel number | –3·70 ± 0·52 | 23·9 ± 0·8d (23) |
| Reduced kernel number‡ | –2·75 ± 0·52 | 33·7 ± 1·5b (24) |
* Flag leaf water potential measured 12 d after anthesis (n = 5).
† Dry weight per kernel at maturity ± s.e., based on the measurement of the two basal kernels from the five central spikelets of an ear. Values with different superscripts differ significantly at the 5 % level; values in parentheses indicate replication for each treatment.
‡ Kernels removed from all but the five central spikelets of an ear.
In an experiment designed to give more comparable water relations for plants grown from anthesis to kernel maturity at moderate (18/13 °C) and high temperature (27/22 °C), and taking into account differences in the rate of development (see Table 5), similar but not identical trends were obtained to those observed previously. At low temperature, drought stress resulted in lowered water potentials of flag leaf blades and reduced net CO2 exchange rates (NCE) 19–20 d after anthesis. However, there was no significant difference between plants with intact ears or plants with a reduced kernel number (Table 5). The dry weight of floret ‘b’ kernels taken from the central spikelets was reduced by drought stress, but the effect of drought on kernel weight was less in plants with fewer grains (Table 5). At high temperature, drought stress also decreased flag leaf water potentials and net CO2 exchange 13–14 d after anthesis. However, both flag leaf water potential and NCE of drought‐stressed plants were significantly greater where kernel number had been reduced at anthesis (Table 5). This difference in water relations was also evident in the dry weight of the kernels at maturity, with a smaller reduction in kernel weight associated with drought stress in plants having reduced kernel numbers (Table 5).
Table 5.
The effect of reduced kernel number* on the response of kernel filling in wheat to drought and high temperature following anthesis
| Treatment | Mean days after anthesis | Flag blade Ψw (MPa)* | NCE (µmol m–2 s–1)† | Kernel dry weight at maturity(mg)‡ |
| 18/13 °C | ||||
| Control | ||||
| Full kernel number | 20·0 | –0·73 ± 0·08 (7) | 14·9 ± 0·7 (8) | 74·9 ± 0·8 (33) |
| Reduced kernel number | 20·0 | –0·59 ± 0·16 (5) | 15·4 ± 0·7 (6) | 77·6 ± 0·7 (31) |
| Droughted | ||||
| Full kernel number | 19·4 | –1·79 ± 0·24 (9) | 3·0 ± 1·3 (17) | 27·1 ± 1·2 (33) |
| Reduced kernel number | 19·0 | –1·89 ± 0·11 (9) | 3·5 ± 0·9 (17) | 36·2 ± 1·6 (29) |
| 27/22 °C | ||||
| Control | ||||
| Full kernel number | 13·9 | –0·76 ± 0·05 (6) | 14·6 ± 2·0 (6) | 51·0 ± 0·5 (36) |
| Reduced kernel number | 13·6 | –0·75 ± 0·12 (6) | 14·2 ± 1·2 (7) | 51·6 ± 1·7 (35) |
| Droughted | ||||
| Full kernel number | 13·6 | –3·70 ± 0·25 (10) | 1·0 ± 0·3 (10) | 30·5 ± 0·7 (34) |
| Reduced kernel number | 13·1 | –1·79 ± 0·18 (12) | 4·7 ± 0·6 (12) | 42·1 ± 0·7 (34) |
Each value is the mean ± s.e. of the number of replicates shown in parentheses.
* Mean kernel number per ear reduced from 40·1 to 17·2 by removing the kernels from all but the five central spikelets.
† Flag leaf Ψw and NCE measured 19–20 d after anthesis in plants held at 18/13 °C from anthesis and 13–14 d after anthesis in plants held at 27/22 °C from anthesis.
‡ Data relating to floret ‘b’ kernels.
DISCUSSION
An analysis of the response of kernel filling to terminal drought and high temperature following anthesis, in relation to the demand for and availability of stored and current photosynthate, is needed to assess the mechanism by which these two stress conditions interact in limiting grain yield.
Where water and nutrients are not limiting, a change in the source–sink balance by reducing kernel number does not greatly influence the reduction in kernel weight associated with exposure of the whole plant to chronic high temperature (day temperatures up to 30 °C) during kernel filling (see Tables 3–5 and Wardlaw et al., 1980), suggesting that the response to high temperature is not governed by the overall availability of photosynthate. Although shoot and root temperatures were varied together in these experiments, there is good evidence from other experiments in which ear temperature has been controlled separately from that of the rest of the plant (Ford et al., 1976; Bhullar and Jenner, 1983; I. A. Dawson, unpubl. res.), or experiments that have been carried out on isolated ears (Bhullar and Jenner, 1986), that chronic high temperatures (up to 27 °C) have a direct effect on developing kernels and not an indirect effect through the remaining shoot or roots. There are considerable differences between the experiments presented here and those described, for example, by Kuroyanagi and Paulsen (1988), which suggested a role for root temperature in relation to kernel filling. In the latter case, root temperatures were in the heat shock range (35 °C) and also were of a significantly longer daily duration (16 h).
In contrast to the temperature response, it is generally assumed that the smaller kernels associated with terminal drought following anthesis are the result, at least in part, of a limited overall supply of photosynthate and that this effect may be minimized by maximizing the storage of non‐structural carbohydrate accumulating in the stem prior to the onset of the stress (Aggarwal and Sinha, 1984; Blum et al., 1991; Kiniry, 1993; Regan et al., 1993), although this is not universally accepted (Brooks et al., 1982).
In preliminary experiments reported here (Table 2), it is shown that the reduced growth of individual kernels in an ear of wheat, when the overall supply of photosynthate is limited by shading, could be compensated for by a reduction in the number of competing kernels. Reducing kernel number (sink size), under both high and low temperature conditions, did result in a greater dry weight of the remaining kernels at maturity of plants subjected to drought during kernel filling, which suggests that droughted kernels were receptive to the availability of photosynthate. However, this response was confounded by a change in plant water relations and a slower onset of stress (higher flag leaf water potential and greater NCE) in plants with a reduced kernel number. This finding supports the earlier observations made by Blum et al. (1988) that degraining causes a decrease in leaf stomatal conductance and transpiration, slower water use and an increase in flag leaf water potential. In the current experiments, the reduction in ear surface (photosynthetic) area by the removal of spikelets, as well as the change in sink size may also have influenced the overall rate of water use.
An analysis of the response of kernel filling to drought stress under variable temperature conditions is complex. While flag leaf water potential measured at one point in time can be used as an indicator of the degree of stress, it must be remembered that where a crop relies entirely on stored soil moisture, the water status of stressed plants declines progressively throughout kernel filling. Also, the rate (duration) of kernel development is temperature dependent. In this instance, allowance was made for the variation in the rate of kernel development due to high temperature by recording flag leaf water potential 19–20 d after anthesis in the plants at 18/13 °C and 12–14 d after anthesis in the plants at 27/22 °C.
In the first experimental series under mild stress, which significantly reduced kernel dry weight at maturity, the reduction in kernel number did not appear to favour kernel filling when changes in plant–water relations, based on flag leaf water potential, were taken into account. However, under more severe drought stress and at high temperature, there was evidence of a positive response to a reduction in kernel number that could not be explained by differences in water relations. In the subsequent experiment, where there was an attempt (although not entirely successful) to compensate for differences in water use between the treatments, reducing kernel number favoured the growth of the remaining kernels under low temperature and relatively mild drought conditions, but apparently not under high temperature conditions.
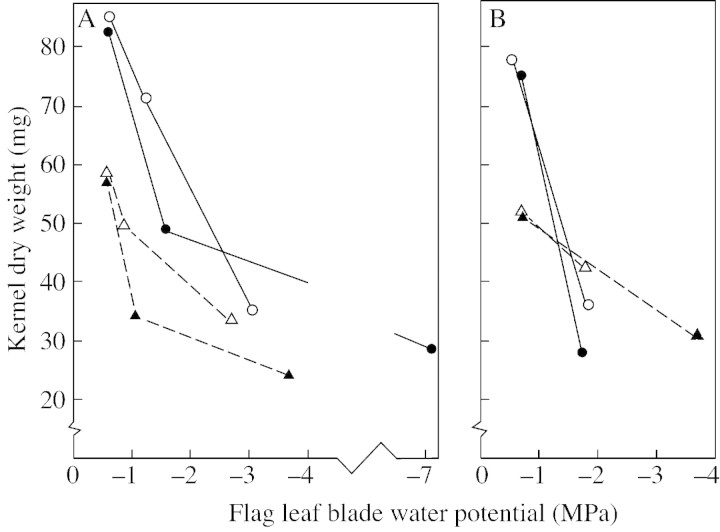
By comparing kernel dry weight against flag leaf water potential across the range of experiments (see Fig. 3), it can be seen that the effect of drought stress and high temperature on kernel weight at maturity are certainly not additive, as suggested by Nicolas et al. (1984), and the drought effect was relatively smaller at the higher temperature than at the moderate temperature. A possible explanation for this response relates to the shorter duration of kernel filling and smaller kernels formed at the higher temperature and, despite a greater rate of water use (implied from the measurement of flag leaf water potentials, but not measured directly in these experiments), a degree of drought escape, with kernel filling largely being completed before the lack of water could reduce the availability of photosynthate to a critical level.
Fig. 3. The effect of temperature and sink size on the relation between flag leaf water potential and dry weight per kernel of the five central spikelets at maturity. A, Data from the two experiments presented in Tables 2 and 3. B, Data from the experiment presented in Table 4. Plants held at either 18/13 °C (circles) or at 27/22 °C (triangles) during kernel filling. Solid symbols, Plants with intact ears; open symbols, plants with a reduced number of kernels.
In conclusion, the data indicate that where water use by a crop following anthesis is increased in parallel with, and related to, an increase in temperature, the reduction in kernel weight that occurs in response to this stress may result as much from the effect of temperature on the duration of kernel filling as from a direct effect of drought. These results lend support to some of the conclusions reached in earlier field experiments (McDonald et al., 1983, 1984; Cooper, 1992) and highlight the need for plant breeders to consider the response of kernel filling to high temperature as well as drought in a Mediterranean climate where both these factors occur in the period from heading to maturity.
ACKNOWLEDGEMENTS
I thank Mrs L. Moncur for her valuable technical assistance throughout these experiments and the staff of the Canberra phytotron (CERES) for maintaining the experimental material.
Supplementary Material
Received: 27 February 2002; Returned for revision: 24 April 2002; Accepted: 2 July 2002
References
- AggarwalPK, Sinha SK.1984. Effect of water stress on grain growth and assimiliate partitioning in two cultivars of wheat contrasting in their yield stability in a drought‐environment. Annals of Botany 53: 329–340. [Google Scholar]
- AngusJF, Nix HA, Russell JS, Kruizinga JE.1980. Water use, growth and yield of wheat in a subtropical environment. Australian Journal of Agricultural Research 31: 873–886. [Google Scholar]
- AsanaRD.1966. Physiological analysis of yield in wheat in relation to water‐stress and temperature. Journal of the Post Graduate School (Delhi) 4: 17–31. [Google Scholar]
- BhullarSS, Jenner CF.1983. Responses to brief periods of elevated temperature in ears and grains of wheat. Australian Journal of Plant Physiology 10: 549–560. [Google Scholar]
- BhullarSS, Jenner CF.1986. Effects of temperature on the conversion of sucrose to starch in the developing wheat endosperm. Australian Journal of Plant Physiology 13: 605–615. [Google Scholar]
- BidingerF, Musgrave RB, Fischer RA.1977. Contribution of stored pre‐anthesis assimilate to grain yield in wheat and barley. Nature 270: 431–433. [Google Scholar]
- BlumA, Mayer J, Golan G.1988. The effect of grain number per ear (sink size) on source activity and its water relations in wheat. Journal of Experimental Botany 39: 106–114. [Google Scholar]
- BlumA, Shpiler G, Golan G, Mayer J, Sinmena B.1991. Mass selection of wheat for grain filling without transient photosynthesis. Euphytica 54: 111–116. [Google Scholar]
- BrooksA, Jenner CF, Aspinall D.1982. Effects of water deficit on endosperm starch granules and on grain physiology of wheat and barley. Australian Journal of Plant Physiology 9: 423–436. [Google Scholar]
- ChowdhurySI, Wardlaw IF.1978. The effect of temperature on kernel development in cereals. Australian Journal of Agricultural Research 29: 205–223. [Google Scholar]
- ConnorDJ.1975. Growth, water relations and yield of wheat. Australian Journal of Plant Physiology 2: 353–366. [Google Scholar]
- CooperJL.1992. Effect of time of sowing and cultivar on the development and grain yield of irrigated wheat in the Macquarie valley, New South Wales. Australian Journal of Experimental Agriculture 32: 345–353. [Google Scholar]
- DavidsonJL, Birch JW.1978. Responses of a standard Australian and Mexican wheat to temperature and water stress. Australian Journal of Agricultural Research 29: 1091–1106. [Google Scholar]
- DoyleAD, Marcellos H.1974. Time of sowing and wheat yield in northern New South Wales. Australian Journal of Experimental Agriculture and Animal Husbandry 14: 93–102. [Google Scholar]
- FischerRA.1979. Growth and water limitations to dryland wheat yield in Australia: a physiological framework. The Journal of the Australian Institute of Agricultural Science 45: 83–94. [Google Scholar]
- FischerRA, Kohn GD.1966. The relationship of grain yield to vegetative growth and post‐flowering leaf area in the wheat crop under conditions of limited soil moisture. Australian Journal of Agricultural Research 17: 281–295. [Google Scholar]
- FordMA, Pearman I, Thorne GN.1976. Effects of variation in ear temperature on growth and yield of spring wheat. Annals of Applied Biology 82: 317–333. [Google Scholar]
- GallagherJN, Biscoe PV, Hunter B.1976. Effects of drought on grain growth. Nature 264: 541–542. [Google Scholar]
- HsiaCA, Waon HS, Wang FT.1963. The effect of temperature on the physiological changes of wheat during grain development. Acta Botanica Sinica 11: 338–349. [Google Scholar]
- Khanna‐ChopraR, Rao PSS, Maheswari M, Xiaobing L, Shivshankar KS.1994. Effect of water deficit on accumulation of dry matter, carbon and nitrogen in the kernel of wheat genotypes differing in yield stability. Annals of Botany 74: 503–511. [Google Scholar]
- KiniryJR.1993. Nonstructural carbohydrate utilization by wheat shaded during grain growth. Agronomy Journal 85: 844–849. [Google Scholar]
- KobataT, Palta JA, Turner NC.1992. Rate of development of postanthesis water deficits and grain filling of spring wheat. Crop Science 32: 1238–1242. [Google Scholar]
- KuroyanagiT, Paulsen GM 1988. Mediation of high‐temperature injury by roots and shoots during reproductive growth of wheat. Plant, Cell and Environment 11: 517–523. [Google Scholar]
- McDonaldGK, Sutton BG, Ellison FW.1983. The effect of time of sowing on the grain yield of irrigated wheat in the Namoi valley, New South Wales. Australian Journal of Agricultural Research 34: 229–240. [Google Scholar]
- McDonaldGK, Sutton BG, Ellison FW.1984. The effect of sowing date, irrigation and cultivar on the growth and yield of wheat in the Namoi river valley, New South Wales. Irrigation Science 5: 123–135. [Google Scholar]
- MarcellosH, Single WV.1972. The influence of cultivar, temperature and photoperiod on post‐flowering development of wheat. Australian Journal of Agricultural Research 23: 533–540. [Google Scholar]
- MorseRN, Evans LT.1962. Design and development of CERES – an Australian phytotron. Journal of Agricultural Engineering Research 7: 128–140. [Google Scholar]
- NicolasME, Gleadow RM, Dalling MJ.1984. Effects of drought and high temperature on grain growth in wheat. Australian Journal of Plant Physiology 11: 553–566. [Google Scholar]
- NicolasME, Lambers H, Simpson RJ, Dalling MJ.1985. Effect of drought on metabolism and partitioning of carbon in two wheat varieties differing in drought‐tolerance. Annals of Botany 55: 727–742. [Google Scholar]
- NixHA.1975. The Australian climate and its effects on grain yield and quality. In: Lazenby A, Matheson EM, eds. Australian field crops 1. Wheat and other temperate cereals North Ryde, NSW: Angus & Robertson, 183–226. [Google Scholar]
- PaltaJA, Kobata T, Turner NC, Fillery IR.1994. Remobilization of carbon and nitrogen in wheat as influenced by postanthesis water deficits. Crop Science 34: 118–124. [Google Scholar]
- ReganKL, Whan BR, Turner NC.1993. Evaluation of chemical desiccation as a selection technique for drought resistance in a dryland wheat breeding program. Australian Journal of Agricultural Research 44: 1683–1691. [Google Scholar]
- SainiAD, Dadhwal VK.1986. Influence of sowing date on grain‐growth duration and kernel size in wheat. Indian Journal of Agricultural Science s56: 439–447. [Google Scholar]
- ShackleyBJ, Anderson WK.1995. Responses of wheat cultivars to time of sowing in the southern wheatbelt of Western Australia. Australian Journal of Experimental Agriculture 35: 579–587. [Google Scholar]
- ShpilerL, Blum A.1986. Differential reaction of wheat cultivars to hot environments. Euphytica 35: 483–492. [Google Scholar]
- SofieldI, Evans LT, Cook MG, Wardlaw IF.1977. Factors influencing the rate and duration of grain filling in wheat. Australian Journal of Plant Physiology 4: 785–797. [Google Scholar]
- StapperM, Fischer RA.1990. Genotype, sowing date and plant spacing influence on high‐yielding irrigated wheat in southern New South Wales. III Potential yields and optimum flowering dates. Australian Journal of Agricultural Research 41: 1043–1056. [Google Scholar]
- TashiroT, Wardlaw IF.1989. A comparison of the effect of high temperature on grain development in wheat and rice. Annals of Botany 64: 59–65. [Google Scholar]
- WardlawIF.1967. The effect of water stress on translocation in relation to photosynthesis and growth. I. Effect during grain development in wheat. Australian Journal of Biological Sciences 20: 25–39. [PubMed] [Google Scholar]
- WardlawIF, Willenbrink J.2000. Mobilization of fructan reserves and changes in enzyme activities in wheat stems correlate with water stress during kernel filling. New Phytologist 148: 413–422. [DOI] [PubMed] [Google Scholar]
- WardlawIF, Wrigley CW.1994. Heat tolerance in temperate cereals: an overview. Australian Journal of Plant Physiology 21: 695–703. [Google Scholar]
- WardlawIF, Dawson IA, Munibi P.1989. The tolerance of wheat to high temperatures during reproductive growth. II Grain development. Australian Journal of Agricultural Research 40: 15–24. [Google Scholar]
- WardlawIF, Sofield I, Cartwright PM.1980. Factors limiting the rate of dry matter accumulation in the grain of wheat grown at high temperature. Australian Journal of Plant Physiology 7: 387–400. [Google Scholar]
- WeatherleyPE.1950. Studies in the water relations of the cotton plant. I. The field measurements of water deficits in leaves. New Phytologist 49: 81–97. [Google Scholar]
- WiegandCL, Cuellar JA.1981. Duration of grain filling and kernel weight of wheat as affected by temperature. Crop Science 21: 95–101. [Google Scholar]
- WoodruffDR, Tonks J.1983. Relationship between time of anthesis and grain yield of wheat genotypes with differing developmental patterns. Australian Journal of Agricultural Research 34: 1–11. [Google Scholar]
- XuH‐L, Ishii R.1990. Effects of water deficit on photosynthesis in wheat plants. Japanese Journal ofCrop Science 59: 384–389. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.