Abstract
The possible effects of climate change on the advance of the tree line are considered. As temperature, elevated CO2 and nitrogen deposition co‐vary, it is impossible to disentangle their impacts without performing experiments. However, it does seem very unlikely that photosynthesis per se and, by implication, factors that directly influence photosynthesis, such as elevated CO2, will be as important as those factors which influence the capacity of the tree to use the products of photosynthesis, such as temperature. Moreover, temperature limits growth more severely than it limits photosynthesis over the temperature range 5–20 °C. If it is assumed that growth and reproduction are controlled by temperature, a rapid advance of the tree line would be predicted. Indeed, some authors have provided photographic evidence and remotely sensed data that suggest this is, in fact, occurring. In regions inhabited by grazing animals, the advance of the tree line will be curtailed, although growth of trees below the tree line will of course increase substantially.
Key words: Review, tree line, krummholz, alpine, arctic, CO2, N‐deposition, global warming
INTRODUCTION
Trees are excluded from the coldest parts of the world. At high latitudes and high elevations they always give way to dwarf shrubs. The boundary of the forest is known as the tree line, although it is usually not a distinct line. Commonly, one observes both immature trees and old dwarf trees scattered above a very ragged ‘line’. This important boundary is observed in all parts of the world, and it exhibits common features (Tranquillini, 1979; Beneke and Davis, 1980; Alden et al., 1993; Holtmeier, 2000). Earlier, it was considered that the tree line coincided with the 10 °C July isotherm of air temperature (e.g. Köppen, 1931) but closer scrutiny revealed this to be a coarse approximation, and one that holds only for the latitude range 40–70 ° (Tuhkanen, 1993; Körner, 1998). In tropical and subtropical mountains the tree line occurs where the summer isotherms are as low as 3–6 °C (Körner, 1998). It is not surprising that the correlation with air temperature is only a weak one, as the temperature of plant tissues, in the shoots, stems and roots, is unlikely to be the same as air temperature.
Currently, there is much interest in the rate at which the tree line may advance in response to environmental change, especially global warming. There are at least three aspects of environmental change to which plants are generally thought to respond: increasing temperature, rising concentration of carbon dioxide, and increasing deposition of nitrogen. There may be other contributing factors which are poorly understood: for example, the decrease in solar radiation as a result of increases in cloudiness or aerosols may be especially important at the tree line (Stanhill and Cohen, 2001). The reason for the renewed interest in tree lines is clear: an advancing tree line, or a denser forest below the tree line, would have important implications for the global carbon cycle (increasing the terrestrial carbon sink) and for biodiversity of the alpine ecotone (possibly ousting rare species and disrupting alpine and arctic plant communities). It would also change drastically the montane landscapes and the livelihood of their inhabitants.
In this paper evidence for changes in tree lines that have taken place as a result of environmental changes over the last few decades is discussed, and the possible response to environmental change in the future is considered.
WHAT MAKES TREES DIFFERENT FROM SMALLER PLANTS?
As mentioned previously, dwarf shrubs can survive and reproduce well above the tree line. However, it seems that the basic processes of photosynthetic production and utilization of woody shrubs are not very different from those of trees. The explanation of their success in cold environments is found in the microclimates that develop over and within dwarf vegetation. The temperatures of dwarf plants are decoupled from air temperature, being considerably higher by day than those of the air above, as a result of the lower transfer of heat between vegetation and the atmosphere (Wilson et al., 1987; Grace et al., 1989). Direct measurements show that surface or meristem temperatures of dwarf plants in the daytime are often 5–10 °C above air temperature during the day, and depend largely on the flux density of solar radiation (Fig. 1). Conversely, coupling is strong for tall vegetation, and the plant–atmosphere temperature differential is small (< 5 °C) and relatively insensitive to solar radiation.
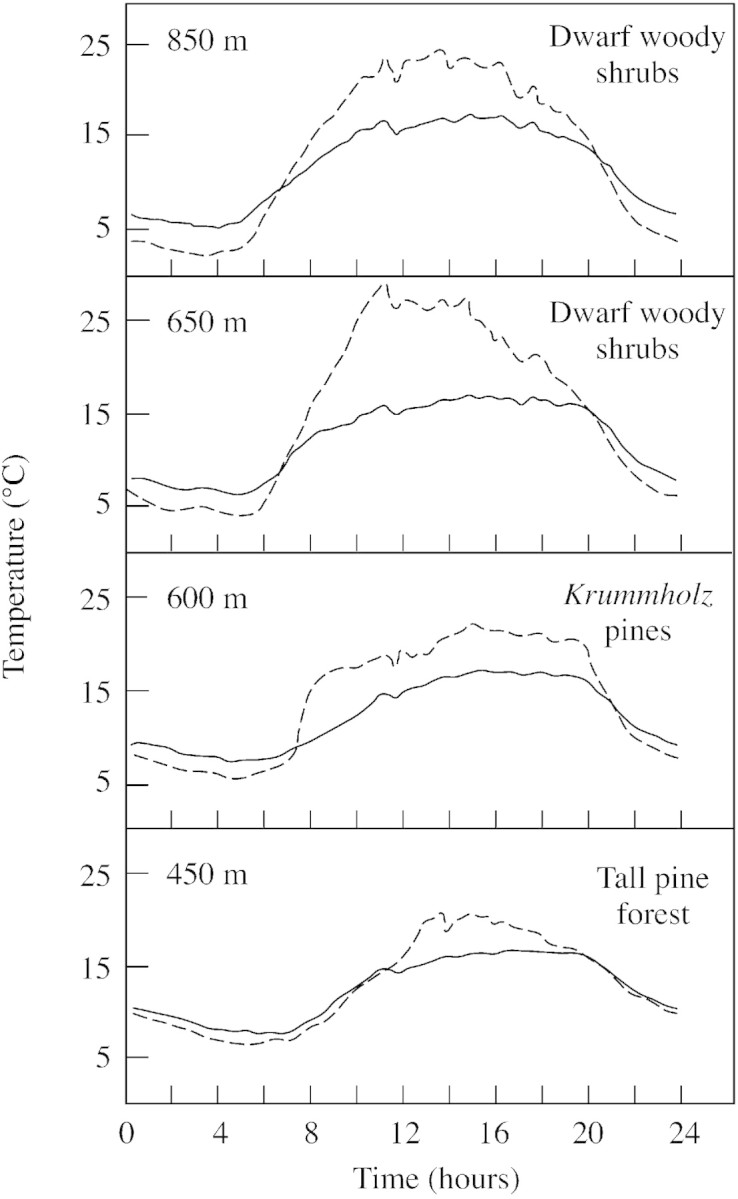
Fig. 1. Mean diurnal trends of temperature in June at four sites near the tree line in the Cairngorm mountains of Scotland. Solid lines are air temperatures, broken lines are the temperatures measured within terminal meristems (Wilson et al., 1987).
There are a few implications of this for the extension of the tree line. Scattered seedlings of trees above the tree line are likely to experience the beneficial microclimates of the dwarf plants. However, as they grow taller, the temperatures of their apical meristems may become closer to air temperature, i.e. cooler, and so their growth rate may be reduced. Sometimes they appear as dwarf and crooked specimens (in the krummholz zone). Otherwise, as their canopy expands, the shallow soils in which they are rooted may be cooler by day as a consequence of shading of the surface. Overall, it seems as if there should be a microclimatological bottle‐neck in the development from seedlings to mature trees. Indeed this is observed: there are often slowly growing populations of seedlings, saplings and krummholz trees above the tree line.
At night, when radiation is negative, the surface temperature of the dwarf vegetation is slightly below that of air (Fig. 1). In alpine environments this difference is more than balanced by the elevated daytime temperatures. In arctic regions, however, when the days are short and solar radiation is weak in the spring, the reverse might often be true.
Another difference between trees and dwarf shrubs is the relationship between productive tissues (leaves and roots) and their support structures: most of the living biomass of trees is in woody tissues that do not photosynthesize but which are used to support the ‘productive’ foliage and fine roots. Stevens and Fox (1991) emphasized the idea that the size and complexity of trees cause, at the tree line, a particularly unfavourable balance between resource acquisition and consumption. Herbaceous plants, on the other hand, have a much more favourable relationship between productive tissues and their support structures, which may explain their predominance above the tree line.
MECHANISMS CONTROLLING TREE GROWTH AT THE TREE LINE
The mechanisms whereby growth, reproduction and survival of trees are limited at the tree line are not properly understood. One of the earliest and most enduring hypotheses was that the short, cold growing season at the tree line was insufficient for the full development of the leaf cuticle, and that leaves were thus susceptible to desiccation during winter and spring when the soil was frozen (Tranquillini, 1979). Subsequent research findings did not support this as a global explanation of tree lines, although depressed water potentials do sometimes occur during the winter months, as shown by early studies in Austria (see Tranquillini, 1979). In general, however, cuticular development is not usually impaired, although the leaf surface may become abraded by the action of wind and wind‐borne particles (Grace, 1990). Even then, the store of water retained in the tree is likely to be sufficient to sustain cuticular transpiration over long periods.
However, the cool growing season can be expected to reduce markedly the rate of photosynthesis and the utilization of the products of photosynthesis. Here, we examine the rather different effects of low temperature on each of these.
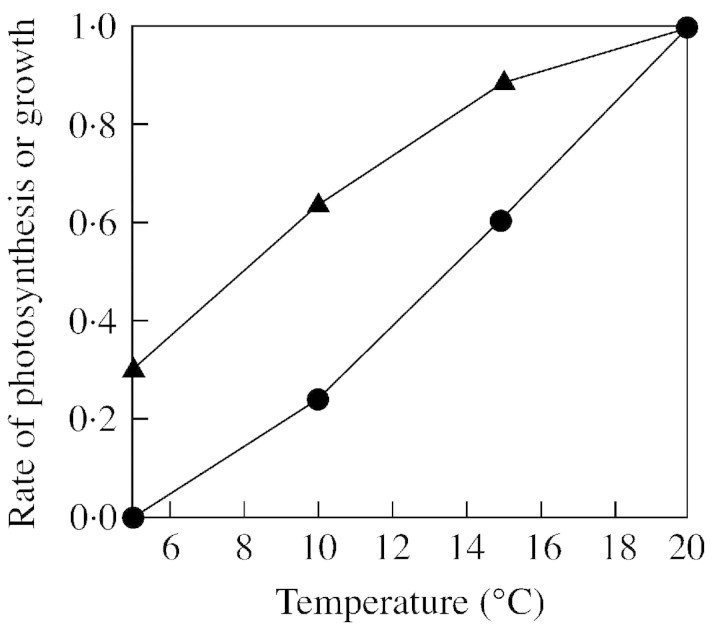
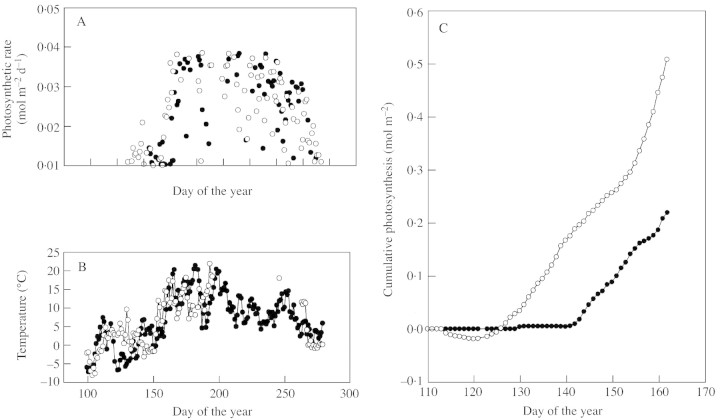
Rates of net photosynthesis at the tree line have been measured many times (e.g. Häsler, 1982; James et al., 1994). Rates reported during the period of growth are not reduced very much by the cooler environment. This is more or less as expected from the well‐known temperature response of C3 photosynthesis: temperate trees generally show a temperature optimum for photosynthesis at around 20 °C, falling only slightly at 10 °C, and still showing positive (albeit low) rates of net photosynthesis at 5 °C (Fig. 2). The situation in the early part of spring and late autumn is somewhat different, as one night’s exposure to temperatures much below 0 °C causes damage to photosystems and subsequent lower rates of photosynthesis that may persist for several days (Öquist and Huner, 1991). For example, Suni et al. (2002) showed that for a southern pine forest the year‐to‐year variation in net ecosystem exchange was dominated by the temperature variation in the spring months. A similar picture emerged from work near the tree line at Värriö, Finland, where photosynthesis was measured for several years (Fig. 3). In 2001 the spring was early, leading to a much‐enhanced production of photosynthate. Because the growing season is very short, such small temperature differences at the start of the year can have a large effect on annual assimilation. Similar results were found by Wieser (2000) who reported a close relationship between stomatal conductance and temperature for Pinus cembra at the tree line. However, this does not imply that the supply of the products of photosynthesis limits growth of the trees, as emphasized by Körner (1998) in his recent ‘growth limitation hypothesis’. On the contrary, it is well known that plants in cold places accumulate carbohydrates in leaves and woody tissues, simply because the rate at which glucose can be used in biosynthetic processes is generally more restricted by low temperatures than is the rate of net photosynthesis. Such accumulation of carbohydrates in artic, alpine or tree line habitats has been demonstrated (e.g. Warren‐Wilson, 1966; Sveinbjörnsson et al., 1992; Skre, 1993). This build‐up of carbohydrates demonstrates that it is not the uptake of CO2 that limits growth, but the rate at which glucose can be utilized. This seems to be an important conclusion, implying that trees at the tree line are unlikely to respond appreciably to elevated CO2, simply because they are not short of the products of photosynthesis. They may well, on the other hand, respond to warming as this will increase the rate of cell division and therefore the rate at which the assimilated carbon can be utilized. Warming will also prolong the growing season and, in the already‐short growing season at the tree line, this effect will be proportionately greater than in lowland or more southern conditions.
Fig. 2. Effect of temperature on photosynthesis (triangles) and growth (circles), expressed relative to the value at 20 °C. The photosynthetic data are the mean of eight C3 species plotted in Grace (1977) and the growth data are from Pinus sylvestris (Junttila and Nilsen, 1993).
Fig. 3. Rates of photosynthesis near to the northern limit of trees at Värriö, northern Finland (67°46′N, 29°35′E, 390 m a.s.l.) in contrasting years: 1999 (closed circles) and 2001 (open circles). Graphs show daily totals of photosynthesis, measured with in situ branch chambers (A), annual patterns of temperature (B) and cumulative photosynthesis over the first half of the growing season (C). Data kindly provided by Professor P. Hari.
The strong temperature sensitivity in the range 5–20 °C for the use of photosynthetic products in growth may be illustrated by the effect of temperature on plant respiration, or the effect of temperature on leaf growth, shown schematically in Fig. 2. It is generally found that many aspects of plant growth, such as opening of buds and germination of seeds, require a temperature of about 6 °C, a fact that has long been known to agricultural meteorologists as well as plant physiologists. Bernoulli and Körner (1999) found that in a forest plantation at the tree line, tree biomass did not change along the 140 m elevational gradient, but tree height decreased consistently for all species (Pinus cembra, Pinus uncinata and Larix decidua).
Another possibility is that tree growth at high elevation is limited by nutrients, in particular the supply of nitrogen. Low nitrogen concentrations in tissues are found in the vicinity of the tree line in Alaska (Schulze et al., 1994), and mountain birch (Betula pubescens ssp. tortuosa) has been shown to be nitrogen‐limited at the Swedish tree line (Sveinbjörnsson et al., 1992). In addition, Timoney (1995) found that anomalies of the Canadian arctic tree line ecotone are related to geology, i.e. the tree line tends to be further north on nutrient‐rich soils. Litter decomposition is a strongly temperature‐sensitive process in the upper tree line ecotone. Indeed, the mass loss rate of buried pine needles at the tree line was only half of the rate in a southern boreal location (Berg et al., 1993). Warming of the soil in the tundra of Abisco at a number of sites across the tree line resulted in sustained high increases in N mineralization (Press et al., 1998; Hartley et al., 1999). Current enhanced deposition rates of nitrogen (Pitcairn et al., 1995) might also be stimulating growth at the tree line. Locally, increases in oceanicity may also have an impact, especially on the northern limit of trees, by causing water‐logging of soils and damaging anaerobic conditions (Crawford, 2000).
On a global basis, it is not clear which are the main processes that limit growth at the tree line. The growth limitation hypothesis by Körner (1998) is consistent with the good correlation of tree growth with July tempera tures, frequently observed at the tree line, and can be evoked to explain why the altitude of tree lines is usually well correlated with July temperatures. However, Sveinbjörnsson (2000) claimed that high carbohydrate concentrations of tree line trees could be interpreted as an acclimation to the hazards of an extreme environment with high risks of tissue losses. Also, resource‐based models have yielded a good correlation with growth at the tree line (Scuderi et al., 1993; Rathgeber et al., 2000; Berninger et al., 2002). However, Berninger et al. (2002) found evidence suggesting that trees may, indeed, become increasingly sink‐limited at the tree line. These authors argued that the potential of trees to react to higher photosynthetic production is strictly limited unless other limitations are lifted.
IS GROWTH ALREADY INCREASING AND IS THE TREE LINE ADVANCING?
Over the Holocene, climate change has influenced the level of the tree line profoundly. Many studies have demonstrated that elevational tree lines were significantly higher during the climatic optimum (Pears, 1968; Kullman, 1981, 1988; Dubois and Ferguson, 1988). For example, in the Scottish Cairngorms, the presence of pine stumps in the peat above the present tree line shows that the tree line was 200 m higher during the Boreal period (5000–9000 years ago) than it is at present (Pears, 1968). Assuming temperature lapse rates to be 7 °C km–1, this would imply that the temperature was 1·4 °C higher in that period. In the last century the temperature has increased by about 0·6 °C and is set to increase by 1–5 °C in the next century. This suggests the possibility of a vertical advance of the tree line by 140–700 m over the century. A similar argument may be put for the advance of the latitudinal tree line, where the historical picture is much clearer, being derived from palynological evidence (Birks, 1989).
To what extent can we see changes in growth at the tree line already, caused by the rather modest warming over the last century? Such changes may not be easy to detect because tree growth declines with increasing age (or increasing tree size) and may be influenced by management. Environmentally caused growth trends superimposed upon these patterns may be relatively small. Hari and Arovaara (1988) suggested that an observed growth increase of subartic Scots pine could be attributed either to CO2 or N‐deposition. However, in a north–south transect study from the tree line in Finland, Mäkinen et al. (2000) did not find any growth trend for Norway spruce. Also, for a data set with Scots pine further south, no growth trend was detected by Mielikäinen and Timonen (1996). Graybill and Idso (1986) detected growth increases close to the tree line of the Rocky Mountains, but were unable to decide whether CO2 or changing precipitation was responsible for these changes.
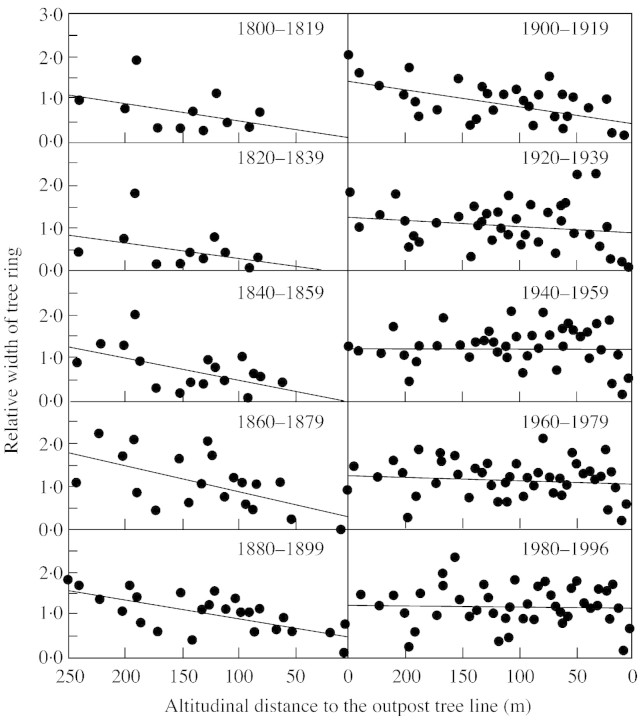
In contrast, recent studies in the Swiss Alps show a dramatic increase in the growth of Picea and Pinus at the tree line (Paulsen et al., 2000). From 1820 to 2000, the temperature of the region increased by 1·02 °C per century, considerably faster than the global average. The authors measured the ring increments in the zone 0–250 m below the current tree line. Before 1940, the annual ring increment declined as expected with altitude as the tree line was approached; after 1940, there was no such decline at all (Fig. 4).
Fig. 4. Changes in ring width on approaching the tree line in the Swiss and Austrian Alps (Paulsen et al., 2000).
There is evidence of recent changes in the control of growth at the tree line from dendrochronologists. Briffa et al. (1998) showed that the density of tree rings from the boreal region has decreased since 1960. Their data set was composed of mountain and boreal chronologies, with a high number of chronologies close to the tree line. Whilst this work was mainly based on maximum latewood density, the authors claimed that similar trends are observable for ring width (no decrease in ring width) and wood density. In addition, the slope of the regression between latewood density and summer temperatures decreased.
Hustich (1958) summarized much evidence for an advance in the pine tree line in northern Europe, remarking that a period of warm summers encouraged seeding and regeneration north of the tree line in the 1920s and 1930s. Later, Kullman (1993) reviewed the Scandinavian literature on the location of the tree line in the 20th century, using mainly photographic evidence. Before 1930, summer temperatures were rather low and the tree line was in retreat. Summers between 1933 and 1939 were warmer than average, and Betula pubescens ssp. tortuosa regenerated at higher elevations. Kullman (2001, 2002) recently reported a new active phase of tree limit advance with the mild winters of the 1990s. In the Swedish Scandes mountains, several species have advanced very rapidly since the 1950s: Betula pubescens, Picea abies, Pinus sylvestris, Sorbus aucuparia and Salix spp. Moreover, the non‐native Acer platanoides has become established just below the tree line, suggesting that floristic composition, as well as position, will change. However, in the Alps the tree line is said to behave in a ‘conservative’ way. Petersson (1998) reported changes in elevation of less than 100 m over palaeoperiods differing by 2–3 °C. High resolution palynological analysis at the tree line in the Cairngorms of Scotland showed a similar sluggishness over the last 1000 years (McConnell, 1996), though photographic evidence over the last 20 years suggested that trees are carrying more foliage than previously (Fig. 5). The unresponsiveness of the tree line to environmental change in the Alps and in Scotland, compared with Sweden, may reflect an increasing intensity of human activities: grazing of livestock, fire and, more recently, the increase in deer populations due to the elimination of most of their natural predators. A further consideration which may slow down the responsiveness of the tree line to climate change is the need for relatively high summer temperatures for successful reproduction. This aspect has been less studied than growth or gas exchange, but it has often been observed that the production of viable seeds at high elevation fails, except in exceptionally warm years (Tranquillini, 1979; Millar and Cummins, 1982; Barclay and Crawford, 1984).
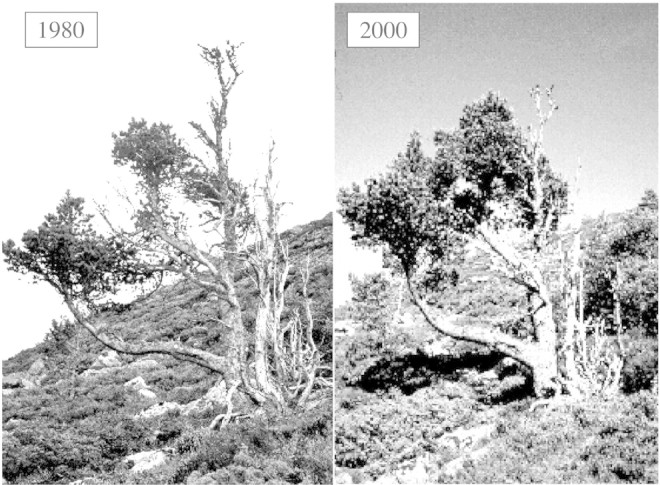
Fig. 5. Photographs of Pinus sylvestris at the tree line in the Cairngorms Scotland, taken from the same point in 1980 and 2000. Note the height of the crown relative to the dead main stem.
Attention has been given to actual and potential migration rates. Although the seeds of many trees can be dispersed over rather large distances, the bulk of the seeds are usually carried only tens of metres, except in the case of small‐seeded species such as Betula (Crawford, 1989). Much is known from palynological data about the rate at which a tree species may migrate northwards under the influence of climatic warming. Huntley (1991) pointed out that most tree species have been seen to spread at a rate of 150–500 m year–1, and exceptional rates as high as 2000 m year–1 have been reported (in Alnus). Gear and Huntley (1991) commented that the rapid rates of northerly migration of Pinus sylvestris in Britain 4000 years ago were still one order of magnitude slower than the predicted migration of isotherms. Where tree lines have migrated at such very fast rates it is necessary to postulate special dispersal mechanisms (e.g. seeds carried over the surface of the snow and ice by wind), or to hypothesize that a population of founder plants has already been established but has remained suppressed by low temperatures.
Some information is also available on migration rates of herbaceous plants in mountain areas. Grabherr et al. (1994) examined the distribution of mountain plants by re‐surveying old plots. They found evidence of a vertical migration of 11–40 m per century, much slower than the rate at which isotherms have migrated over the same period (in the Alps, the warming over the last century has been about 1 °C, implying a vertical migration of isotherms of 143 m).
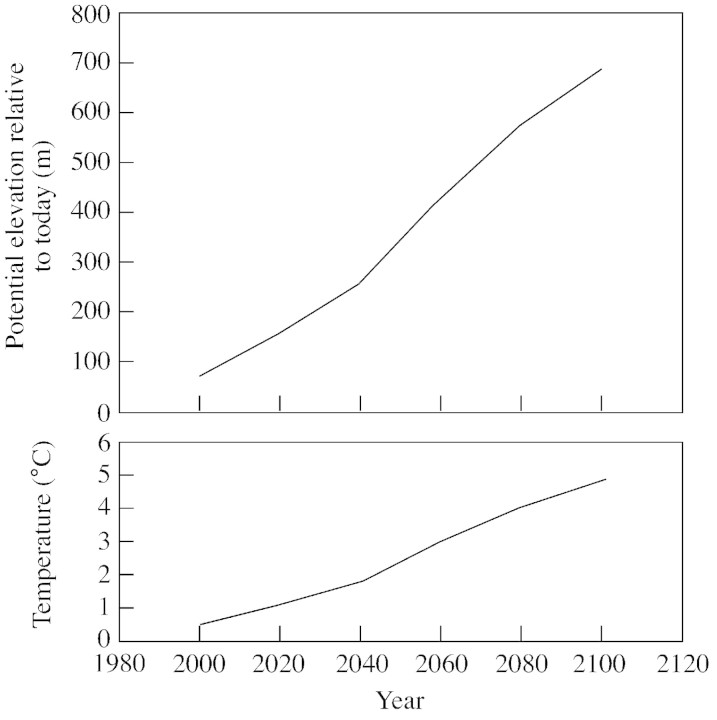
The rapid rates of migration of trees, evident in the pollen isopleths, remain unexplained. Estimates of the likely migration rate under climatic warming may be made, based upon some simple rules. Here we make an ‘order of magnitude calculation’ using data on Scots pine from Carlisle and Brown (1986), from whence a simple rule might be ‘trees at the tree line produce seeds when they are 20 years old and disperse them 20 m vertically’. On this assumption, given a typical scenario of climate change, we would expect tree lines to advance vertically by 100 m in the next 100 years. However, isotherms are expected to move much faster. Using warming rates of 4·5 °C per century (IPCC, 2001), it is estimated that they would move vertically by 640 m (Fig. 6).
Fig. 6. Potential increase in the elevation of the tree line, assuming a 4·5 °C rise in temperature over 100 years.
There is recent photographic evidence that the woody vegetation is migrating northwards. For the northern tree line, additional dispersal mechanisms have been suggested to explain the rapid migration rates, especially the wind‐blown transport of seeds over the frozen tundra (Hustich, 1958). The first example is from the Ayikak river in Alaska, where Salix, Alnus crispa and Picea glauca have advanced over the last 50 years (Sturm et al., 2001). Another example comes from the Urals (Moiseev and Shiyatov, 2003), where the summer temperature has increased by about 1 °C over the century and the winter temperature has increased by as much as 4 °C.
Already there are indications from satellite data relating to the status of vegetation at the northern tree line. Using optical reflectance data, Myneni et al. (1997) showed significant greening of the circumpolar vegetation over quite a brief period (1981–1991). This corresponds quite well to the inference that northern vegetation must have become more active to account for the change in the seasonal pattern of CO2 that has been observed (Keeling et al., 1996). Very recently, such data have been used to infer an increasing intensity of the terrestrial carbon sink (Myneni et al., 2001), although optical reflectance data cannot be very precise for this application. It is expected that radar remote sensing and high‐resolution optical sensing will routinely provide more exact information.
FEEDBACK WITH CLIMATE
Climate–vegetation interactions are two‐sided. On the one hand, climate determines the structure of the vegetation; on the other hand, vegetation exercises effects of climate by converting short wave energy into long wave energy, sensible heat or by driving evaporation. It is evident that coniferous forests are more effective in converting incoming energy into heat than alpine/artic vegetation. This is partly because coniferous forest canopies tend to shed their snow, whilst snow remains on the ground over treeless areas, and partly because coniferous forests have a lower albedo than almost any other vegetation type even in the summer when there is no snow. Foley et al. (1994) found that the winter albedo for coniferous stands is low, about 0·11–0·21, while tundra is snow‐covered and has a high albedo (0·6–0·8). Deciduous stands have twice the albedo of coniferous stands during winter (Chapin et al., 2000). In summertime, coniferous stands once again have the lowest albedo (around 0·8) while deciduous stands (around 0·16) and tundra (0·14–0·2) have similar albedo values (Eugster et al., 2000).
For reasons mainly connected with these albedo differences, boreal forests tend to heat up the atmosphere more than tundra or meadow systems would do. Betts (2000) argued that afforestation and expansion of forests might therefore even increase climate warming. In a model analysis, Pitman and Zhao (2000) showed that although direct effects of land cover changes on the energy balance of the earth are small, these effects are large for some areas, such as Europe, and may change climate remotely.
There are other feedbacks of tree line advances with climate that are more difficult to predict, for example, changes in the depth of the atmospheric boundary layer due to a rougher canopy, and aerosol emissions from forests (Mäkelä et al., 1997).
CONCLUSIONS
(1) Despite over a century of research, it is not clear how the tree line is determined by environmental variables. In the last few years, hypotheses based on the effect of temperature on growth limitation and resource usage have received attention, but experimental discrimination is now required to differentiate between the competing hypotheses that have been outlined by Körner (1998).
(2) There is much evidence of regeneration above both the elevational and northern tree line during periods when the summers are warm, such as at the present time.
(3) There is a strong thermal advantage of being short, which is why dwarf shrubs succeed at high elevation whilst trees do not. As seedlings grow taller, they lose this advantage, and so there may develop an extensive zone of short and stunted saplings just above the tree line, which fail to develop into tall trees.
(4) Macrofossils and palynological evidence show that tree lines have always been dynamic. Tree lines will continue to be responsive to climate change, and large advances in tree lines, with ecological and socio‐economic implications, are probable in the near future, except where prevented by human activity.
(5) The observed migration rates of tree lines do not agree well with rates estimated from simple assumptions. Models to predict the impact of climate change on tree lines need to incorporate an understanding of energy balance, physiology and reproductive biology. Even then, tree lines in mountains may be more influenced by the grazing of seedlings and saplings than most people have supposed.
ACKNOWLEDGEMENTS
We acknowledge helpful discussions with Robert Crawford and Christian Körner, and we thank Pepi Hari for permission to use the Värriö data set.
Supplementary Material
Received: 1 February 2002; Returned for revision: 23 April 2002; Accepted: 2 July 2002
References
- AldenJ, Mastrantonio JL,Ødum S.1993. Forest development in cold climates. New York: Plenum Press. [Google Scholar]
- BarclayAM, Crawford RMM.1984. Seedling emergence in the rowan Sorbus aucuparia from an altitudinal gradient. Journal of Ecology 72: 627–636. [Google Scholar]
- BenekeU, Davis MR.1980. Mountain environments and subalpine tree growth. Wellington, New Zealand: Forest Research Institute. [Google Scholar]
- BergB, Berg MP, Bottner P, Box E, Breymeyer A, Deanta RC, Couteaux M, Escudero A, Gallardo A, Kratz W, Madeira M, Mälkönen E, McClaugherty C, Meentemeyer V, Muñox F, Piussi P, Remacle J, Desanto AV.1993. Litter mass‐loss rates in pine forests of Europe and eastern United States – some relationships with climate and litter quality. Biogeochemistry 20: 127–159. [Google Scholar]
- BerningerF, Nikinmaa E, Hari P, Lindholm M, Meriläinen J.2002. Growth at Fennoscandian treelines is increasingly carbon saturated. Submitted to Annals of Botany . [Google Scholar]
- BernoulliM,KörnerC.1999. Dry matter allocation in treeline trees. Phyton‐Annales Rei Botanicae 39: 7–11. [Google Scholar]
- BettsRA.2000. Offset of the potential carbon sink from boreal afforestation by decreases in surface albedo. Nature 408: 187–201. [DOI] [PubMed] [Google Scholar]
- BirksHJB.1989. Holocene isochrone maps and patterns of tree spreading in the British Isles. Journal of Biogeography 16: 503–540. [Google Scholar]
- BriffaKR, Schweingruber FH, Jones PD, Osborn TJ, Harris IC, Shiyatov SG, Vaganov EA, Grudd H.1998. Trees tell of past climates but are they speaking less clearly today? Philosophical Transactions of the Royal Society 353: 65–73. [Google Scholar]
- CarlisleA, Brown AHF.1968. Biological flora of the British Isles: Pinus sylvestris L. Journal of Ecology 56: 269–307. [Google Scholar]
- ChapinIII FS, McGuire AD, Randerson JT, Pielke R, Baldocchi, DD, Hobbie SE, Roulet N, Eugster W, Kasischke ES, Rastetter EB, Zimov SA, Running S.2000. Artic and boreal ecosystems of western North America as components of the climate system. Global Change Biology 6 (suppl.): 211–223. [DOI] [PubMed] [Google Scholar]
- CrawfordRMM.1989. Studies in plant survival. Oxford: Blackwell Scientific Publications. [Google Scholar]
- CrawfordRMM.2000. Ecological hazards of oceanic environments. New Phytologist 147: 257–281. [Google Scholar]
- DuboisAD, Ferguson DK.1988. Additional evidence for the climatic history on pine in the Cairngorms, Scotland, based on radiocarbon dates and tree‐ring d/h ratios – reply. Review of Paleobotany and Palynology 54: 181–185. [Google Scholar]
- EugsterW, Rouse W, Pielke R, McFadden JP, Baldocchi DD, Kittel T, Chapin III FS, Liston GE, Vidale PL, Vaganov EA, Chambers S.2000. Land‐atmosphere energy exchange in Arctic tundra and boreal forest: available data and feedbacks to climate. Global Change Biology 6: 84–115. [DOI] [PubMed] [Google Scholar]
- FoleyJA, Kutzback JE, Coe MT, Levis S.1994. Feedbacks between climate and boreal forests during the Holocene epoch. Nature 271: 52–54. [Google Scholar]
- GearAJ, Huntley B.1991. Rapid changes in the range limits of Scots Pine 4000 years ago. Science 251: 544–547. [DOI] [PubMed] [Google Scholar]
- GrabherrG, Gotfried M, Pauli H.1994. Climate effects on mountain plants. Nature 369: 448. [DOI] [PubMed] [Google Scholar]
- GraceJ.1977. Plant response to wind. London: Academic Press. [Google Scholar]
- GraceJ.1990. Cuticular water loss unlikely to explain tree‐line in Scotland. Oecologia 84: 64–68. [DOI] [PubMed] [Google Scholar]
- GraceJ, Allen S, Wilson C.1989. Climate and meristem temperatures of plant communities near the tree‐line. Oecologia 79: 198–204. [DOI] [PubMed] [Google Scholar]
- GraybillDA, Idso SB.1986. Detecting the aerial fertilization effect of atmospheric CO2 enrichment in tree ring chronology. Global Biogeochemical Cycles 7: 81–95. [Google Scholar]
- HariP, Arovaara H.1988. Detecting CO2 enhancement in the radial increment of trees. Evidence from the northern timberline. Scandinavian Journal of Forest Research 3: 76–84. [Google Scholar]
- HartleyAE, Neill C, Melillo J, Crabtree R, Bowles F 1999. Plant performance and soil nitrogen mineralisation in response to simulated climate change in subartic dwarf shrub tundra. Oikos 86: 331–344. [Google Scholar]
- HäslerR.1982. Net photosynthesis and transpiration of Pinus montana on east and north facing slopes of Alpine timberline. Oecologia 54: 14–22. [DOI] [PubMed] [Google Scholar]
- HoltmeierF‐K.2000. Die Hohengrenze der Gebirgswalder. Arbeiten aus dem Institut für Landschaftsokologie, Westfalische Wilhelms‐Universitat, Munster. [Google Scholar]
- HuntleyB.1991. How plants respond to climate change: migration rates, individualism and the consequences for plants communities. Annals of Botany 67 (suppl.): 15–22. [Google Scholar]
- HustichI.1958. On the recent expansion of the Scotch pine in Northern Europe. Fennia 82: 1–25. [Google Scholar]
- IPCC.2001. Climate change2001. The scientific basis. Cambridge: Cambridge University Press. [Google Scholar]
- JamesJ, Grace J, Hoad S.1994. Growth and photosynthesis of Pinus sylvestris at its altitudinal limit in Scotland. Journal of Ecology 82: 297–306. [Google Scholar]
- JuntillaO, Nilsen J.1993. Growth and development of northern forest trees as affected by temperature and light. In: Alden J, Mastrantonio JL, Ødum S. Forest development in cold climates. New York: Plenum Press, 43–57. [Google Scholar]
- KeelingDC, Chin JFS, Whorf TP.1996. Increased activity of northern vegetation inferred from atmospheric CO2 measurements. Nature 382: 146–149. [Google Scholar]
- KöppenW.1931. Grundriss der Klimakunde. Berlin: De Gruyer. [Google Scholar]
- KörnerCh 1998. A re‐assessment of the high elevation treeline positions and their explanation. Oecologia 115: 445–459. [DOI] [PubMed] [Google Scholar]
- KullmanL.1981. Recent tree limit dynamics of Scots pine (Pinus sylvestris L) in the southern Swedish Scandes. Wahlenbergia 8: 3–67. [Google Scholar]
- KullmanL.1988. Holocene history of the forest‐alpine tundra ecotone in the Scandes Mountains (central Sweden) New Phytologist 108: 101–110. [DOI] [PubMed] [Google Scholar]
- KullmanL.1993. Pine (Pinus sylvestris L.) tree‐limit surveillance during recent decades, Central Sweden. Arctic and Alpine Research 25: 24–31. [Google Scholar]
- KullmanL.2001. 20th century warming and tree‐limit rise in the southern Scandes of Sweden. Ambi o30: 72–80. [DOI] [PubMed] [Google Scholar]
- KullmanL.2002. Rapid recent range‐margin rise of tree and shrub species in the Swedish Scandes. Journal of Ecology 90: 68–76. [Google Scholar]
- McConnellJL.1996. The history of the Pinus sylvestris treeline at Creag Fhiaclach, Invernesshire. PhD Thesis, University of Edinburgh, UK. [Google Scholar]
- MäkelaJM, Aalto P, Jokinen V, Pohja T, Nissinen A, Palmroth S, Markkanen T, Seitsonen K, Lihavainen H, Kulmala M.1997. Observations of ultrafine aerosol particle formation and growth in boreal forest. Geophysical Research Letters 24: 1219–1222. [Google Scholar]
- MäkinenH, Nöjd P, Mielikäinen K.2000. Climatic signal in annual growth variation of Norway spruce (Picea abies) along a transect from central Finland to the Arctic timberline. Canadian Journal of Forest Research 30: 769–777. [Google Scholar]
- MielikäinenK, Timonen M.1996. Growth trends of Scots pine in unmanaged and regularly managed stands in Southern and Central Finland. In: Spiecker H, Mielikäinen K, Köhl M, Skovsgaard JP, eds. Growth trends of European forests Heidelberg‐Berlin: Springer‐Verlag, 41–59. [Google Scholar]
- MillarGR, Cummins RT.1982. Regeneration of Scots pine Pinus sylvestris at the natural treeline in the Cairngrom Mountains, Scotland. Holarctic Ecology 5: 27–34. [Google Scholar]
- MoiseevPA, Shiyatov SG.2003. The use of old landscape photographs for studying vegetation dynamics at the treeline ecotone in the Ural Highlands, Russia. In: Nagy L, Grabherr G, Körner C, Thompson DBA, eds. Alpine biodiversity in Europe. Heidelberg‐Berlin: Springer‐Verlag. [Google Scholar]
- MyneniRB, Keeling CD, Tucker CJ.1997. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 386: 698–702. [Google Scholar]
- MyneniRB, Dong J, Tucker CJ, Kaufmann RK, Kauppi PE, Liski J, Zhou L, Alexeyev V, Hughes MK 2001. A large carbon sink in the woody biomass of Northern forests. Proceedings of the National Academy of Sciences of the USA 98: 14784–14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ÖquistG, Huner NPA.1991. Effects of cold acclimation on the susceptibility of photosynthesis to photoinhibition in Scots pine and in winter and spring cereals: a fluorescence analysis. Functional Ecology 5: 912–100. [Google Scholar]
- PaulsenJ, Weber UB, Körner Ch.2000. Tree growth near treeline: abrupt or gradual reduction with altitude? Arctic, Antarctic and Alpine Research 32: 14–20. [Google Scholar]
- PearsNV.1968. Post glacial tree‐lines of the Cairgorm Mountains, Scotland. Transactions of the Botanical Society of Edinburgh 40: 361–394. [Google Scholar]
- PeterssonD.1998. Climate, limiting factors and environmental change in high‐altitude forests of Western North Americxa. In: Beniston M, Innes LJ, eds. The impact of climate variability on forests New York: Springer, 191–208. [Google Scholar]
- PitcairnCER, Fowler D, Grace J.1995. Deposition of fixed atmospheric nitrogen and foliar nitrogen content of bryophytes and Calluna vulgaris (L) Hull. Environmental Pollution 88: 193–205. [DOI] [PubMed] [Google Scholar]
- PitmanAJ, Zhao M.2000. The relative impact of observed change in land cover and carbon dioxide as simulated by a climate model. Geophysical Research Letters 27: 1267–1270. [Google Scholar]
- PressMC, Potter JA, Burke MJW, Callaghan TV, Lee JA 1998. Response of a subartic dwarf shrub heath community to simulated environmental change. Journal of Ecology 86: 315–327. [Google Scholar]
- RathgeberC, Guiot J, Eduard JJ.2000. Utilisation d’un modèle biogéochimique en dendroécologie. Application au pin Cembro. Communcations de Recherche, Academie des Sciences Paris 323: 489–497. [DOI] [PubMed] [Google Scholar]
- SchulzeED, Chapin FS, Gebauer G.1994. Nitrogen nutrition and isotope differences among life forms at the northern treeline of Alaska. Oecologia 100: 406–412. [DOI] [PubMed] [Google Scholar]
- ScuderiLA, Schaaf CB, Orth KU, Band LE.1993. Alpine growth variability – simulation using an ecosystem process model. Arctic and Alpine Research 25: 175–182. [Google Scholar]
- SkreO.1993. Growth of mountain birch (Betula pubescens Ehrh) in response to changing temperature. In: Alden J, Mastrantonio JL, Ødum S. Forest development in cold climates.New York: Plenum Press, 65–78. [Google Scholar]
- StanhillG, Cohen S.2001. Global dimming: a review of the evidence for a widespread and significant reduction in global radiation with discussion of its probable causes and possible agricultural consequences. Agricultural and Forest Meteorology 107: 255–278. [Google Scholar]
- StevensGC, Fox JF.1991. The causes of treeline. Annual Review of Ecology and Systematics 22: 177–191. [Google Scholar]
- SturmM, Racine C, Tape K.2001. Climate change – increasing shrub abundance in the Arctic. Nature 411: 546–547. [DOI] [PubMed] [Google Scholar]
- SuniT, Berninger F, Markkanen T, Keronen P, Rannik U, Vesala T.2002. Interannual variability and timing of growing‐season CO2 exchange in a boreal forest. Submitted to Journal of Geophysical Research . [Google Scholar]
- SveinbjörnssonB.2000. North American and European treelines: external forces and internal processes controlling position. Ambio 29: 388–395. [Google Scholar]
- SveinbjörnssonB, Nordell O, Hauhanen H.1992. Nutrient relations of mountain birch growth at and below the elevational tree line in Swedish Lapland. Functional Ecology 6: 213–220. [Google Scholar]
- TimoneyK.1995. Tree and tundra cover anomalies in the subarctic forest‐tundra of northwest Canada. Arctic 48: 13–21. [Google Scholar]
- TranquilliniW.1979. Physiological ecology of the alpine treeline. Berlin: Springer. [Google Scholar]
- TuhkanenS.1993. Treeline in relation to climate, with special reference to oceanic areas. In: Alden J, Mastrantonio JL, Ødum S. Forest development in cold climates. New York: Plenum Press, 115–134. [Google Scholar]
- Warren‐WilsonJ.1966. An analysis of plant growth and its control in arctic environments. Annals of Botany 30: 383–402. [Google Scholar]
- WieserG.2000. Seasonal variation of leaf conductance in a subalpine Pinus cembra during the winter months. Phyton 40: 185–190. [Google Scholar]
- WilsonC, Grace J, Allen S, Slack F.1987. Temperature and stature, a study of temperatures in montane vegetation. Functional Ecology 1: 405–414. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.