Abstract
Sylleptic branches grow out from lateral buds during the same growing season in which the buds are formed. This type of branching is present in poplar and in many tropical species. It results in the production of more branches, more leaves and expanded photosynthetic capacity and is thought to assist in increasing the overall growth and biomass of the tree at a young age. However, very little is known about the physiology of sylleptic branching in poplar, which is an extremely important source of fibre and fuel. In the present study of three hybrid poplar clones (11‐11, 47‐174 and 49‐177) of Populus trichocarpa × P. deltoides exhibiting contrasting degrees of sylleptic branching, an analysis was carried out on parent shoot elongation and sylleptic branching, together with a preliminary comparison of the parent shoots’ sensitivity to auxin (naphthaleneacetic acid) as a repressor of lateral bud outgrowth, and cytokinin (benzyladenine) as a promoter. Suggestive evidence was found for an inverse correlation between parent shoot sensitivity to auxin and the degree of sylleptic branching, as well as a partially positive correlation with respect to sensitivity to cytokinin. The present data are consistent with the hypothesis that auxin and cytokinin may play repressive and promotive roles, respectively, in the sylleptic branching of hybrid poplar.
Key words: Populus, hybrid poplar, sylleptic branching, auxin, cytokinin, apical dominance, lateral bud outgrowth, decapitated shoot
INTRODUCTION
Lateral buds of most temperate woody species do not grow out during the season in which they are formed. These proleptic buds overwinter and grow out during the following spring. However, in poplar and in a few other temperate species, together with many tropical species, some lateral buds grow out sylleptically, i.e. they grow out during the same season in which they are formed without an intervening rest period and usually without bud scale formation (Spath, 1912). Growth of their parent shoot also continues. It has also been observed that sylleptic branching most often occurs in vigorously growing young trees and/or juvenile shoots.
Sylleptic branching in poplar may significantly increase branch number, leaf area and the general growth of the tree, particularly in its early years (Ceulemans et al., 1990; Wu et al., 2000; T. M. Hinckley, pers. comm.). Tree crown architecture controls the density and manner of leaf arrangement and hence affects light interception and photosynthesis (Halle et al., 1978). In Larix and Tsuga, sylleptic branching is ‘an opportunist and exploitive mechanism’ that permits rapid filling of available space with photosynthetic surface and hence increases photosynthetic capacity and biomass production in the current year and ‘potential for a greater shoot complement in the following year’ (Powell, 1991).
With respect to carbon allocation in Populus, Scarascia‐Mugnozza et al. (1999) concluded from their studies that the parent shoots with sylleptic branches have a higher translocation efficiency and add more on a unit mass basis to tree growth than do shoots with only proleptic branches. Wu (1994) pointed out that tree geometry and architecture have become of major interest in determining the productivity of woody species used in short rotation intensive culture. Ceulemans et al. (1990) stated: ‘Sylleptic branches and the considerable leaf area they carry may play a critical role in the superior biomass productivity of hybrid poplars’.
Although sylleptic branching in various species has been noted many times in the general literature (Halle et al., 1978; Ceulemans et al., 1990; Genard et al., 1994; Debell et al., 1997; Cook et al., 1999), together with specific ecological (Powell, 1991) and substantial genetic studies (Bradshaw and Stettler, 1995; Howe et al., 2000), very little work has been done on the biochemical/physiological mechanisms of this interesting phenomenon. With the recent tremendous increase in the importance of hybrid poplars as a source of high quality fibre and fuel, it is vital that the physiological control mechanisms of sylleptic branching be elucidated.
From apical dominance studies in herbaceous plants, there is evidence that the auxin/cytokinin ratio may control lateral bud outgrowth, with apically derived auxin playing a repressive role and root‐derived cytokinin playing a promotive role (Cline, 1994; Bollmark et al., 1995; Sandberg et al., 1995; Tamas, 1995; Cline et al., 1997; Napoli et al., 1999). To what extent these hormones may also control sylleptic branching in woody species has yet to be established, although there is supportive evidence for repressive auxin influence (Little, 1969; Wilson, 1979; House et al., 1998; Cline, 2000) and promotive cytokinin influence (Williams and Stahly, 1968) in proleptic branching. Other reports indicate repressive effects from auxin treatments but no promotive effects from cytokinin treatments (Leaky and Longman, 1986; Cline, 2000). In studies with pea rms mutants, Beveridge et al. (2000) proposed the existence of a mobile signal that interacts with exogenous auxin in the inhibition of lateral bud outgrowth following decapitation of the shoot apex.
With respect to sylleptic branching there are several importuning questions. (1) Why do certain lateral buds of a few temperate species grow out sylleptically soon after their formation without overwintering and without sprouting until the following spring as do proleptic branches? This question is of added significance when it is recognized that sylleptic branching is more the norm for herbaceous and tropical species than the exception (Halle et al., 1978). (2) Why isn’t apical dominance sufficient to maintain the repression of these sylleptic lateral buds? (3) Since sylleptic branching is often correlated with high growth rates of the parent shoot, is sylleptic branch outgrowth therefore triggered when the growth rate of the parent shoot exceeds a certain threshold level (Halle et al., 1978)? (4) If auxin inhibits axillary bud outgrowth in apical dominance, shouldn’t the high rates of parent shoot growth characteristic of species with sylleptic branching generate sufficient auxin for the repression of this bud outgrowth? It appears paradoxical that higher parent shoot growth rates should be correlated with increased sylleptic branching, and many workers have questioned the role of auxin and/or apical dominance in sylleptic branching (Halle et al., 1978; Genard et al., 1994; Tromp, 1996; Cook et al., 1999).
The objective of the present study was to elucidate the roles of auxin and cytokinin in sylleptic branching by separately analysing the effects of exogenous treatments with these hormones in three hybrid poplar clones exhibiting contrasting degrees of sylleptic branching.
The following hypotheses were tested. (1) Reduced sensitivity to the inhibitory effects of auxin in the parent shoot is the cause of increased sylleptic branching in clones exhibiting a high degree of sylleptic branching, and vice versa in low‐syllepsis clones. This was tested by determining whether the degree of apical dominance restoration by auxin (via the classic Thimann–Skoog experiment) in a high‐syllepsis clone was lower than that in a low‐syllepsis clone. (2) Increased sensitivity to the promotive effects of cytokinin in the parent shoot is the cause of the increased degree of sylleptic branching in high‐syllepsis clones, and vice versa in low‐syllepsis clones. This was tested by determining whether there was an increase in lateral bud outgrowth in response to cytokinin treatment in a high‐syllepsis clone as compared with that in a low‐syllepsis clone
MATERIALS AND METHODS
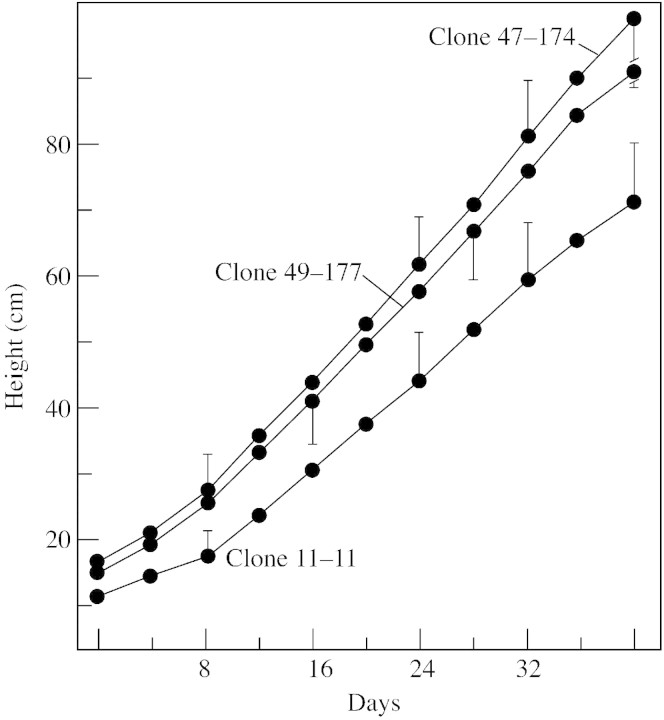
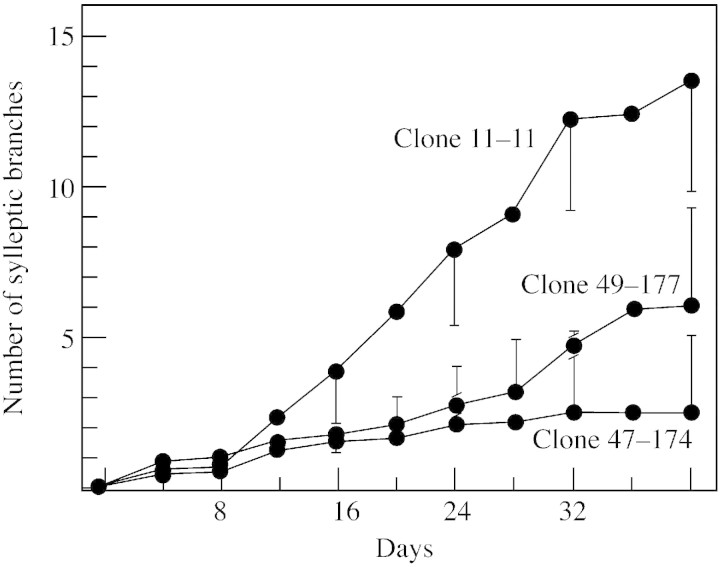
Dormant shoot cuttings of three Populus trichocarpa (black cottonwood) × P. deltoides (eastern cottonwood) Bartr. Ex Marsh F1 hybrid clones (11‐11 with a high amount of sylleptic branching; 47–174 with a low amount of sylleptic branching; and 49‐177 with a variably intermediate amount of sylleptic branching; Fig. 1) were kindly provided by Dr Jon Johnson of the Washington State University Puyallup Research and Extension Center. These clones were originated by the University of Washington–Washington State University Populus breeding programme. For vegetative propagation, greenwood cuttings were taken from the hybrid clones and planted in vermiculite under mist in a glasshouse with supplementary lighting (General Electric 400‐W mercury vapour lamps). They were transplanted into gallon pots containing Pro‐mix growing medium, and after 2 weeks were fertilized weekly. Within 1 or 2 months, natural sylleptic branching was observed in the lower and mid‐portions of the parent shoots. Measurements (made every 4 d) of parent shoot height (Fig. 2) and of natural sylleptic branching (Fig. 3) began about 2 months after transplantation of cuttings to the gallon pots and were continued for periods ranging from 28–44 d in four different trials.

Fig. 1. Three hybrid poplar clones with contrasting degrees of sylleptic branching: left, clone 47–174 with a low amount of sylleptic branching; middle, clone 49‐177 with a variably intermediate level of sylleptic branching; right, clone 11‐11 with a high amount of sylleptic branching.
Fig. 2. Parent shoot elongation (height) of the three hybrid poplar clones over 40 d. n = 28 for clone 47‐174, 18 for clone 49‐177 and 16 for clone 11‐11. Bars represent s.d.
Fig. 3. Sylleptic branching in the three hybrid poplar clones over 40 d. n = 28 for clone 47‐174, 18 for clone 49‐177 and 16 for clone 11‐11. Bars represent s.d.
Auxin treatments via the classical Thimann–Skoog test (1933) were carried out with 1 % naphthaleneacetic acid (NAA) in lanolin applied to cut stem surfaces immediately following decapitation (1–2 cm below the apex) of the parent shoots. Outgrowth (measured with a ruler) of the highest lateral bud below the point of decapitation was followed for about 2 weeks. The experiment was repeated seven times; the data in Table 1 are from one of these trials. Exogenous cytokinin [1 mm benzyladenine (BA)] in a 10 µl aqueous solution with 0·05 % Tween 20 was applied daily (on weekdays) directly to a single lateral bud in the upper region (to avoid natural sylleptic branching which occurs in the mid–lower region and which was a problem in the first two trials) of the main shoot of each sapling. Measurements of lateral bud outgrowth were made after 14–23 d. The data in Table 2 are from the last of four trials. Before treatment, the length of the inhibited lateral buds in essentially all clones in the last two trials was less than 0·2 cm and was considered to be zero in the measurements. The hormone experiments were generally carried out on 2‐ to 3‐month‐old saplings greater than 40–50 cm in height.
Table 1.
Restoration of apical dominance by auxin (NAA) treatment
| Clone | 47‐174 (n = 11) | 49‐177 (n = 7) | 11‐11 (n = 4) |
| HLB (water) | 5·1 ± 1·3 | 5·0 ± 1·5 | 5·3 ± 0·8 |
| HLB (NAA) | 1·9 ± 0·9 | 3·9 ± 0·6 | 4·6 ± 0·7 |
| % repression | 63 | 22 | 13 |
Repression of outgrowth of the highest lateral bud (HLB) below the point of decapitation by immediate application of 1 % NAA in lanolin to the cut stem surface.
Measured after 12 d.
Data are lengths in cm ± s.d.
Table 2.
The promotion of lateral bud outgrowth by daily weekday cytokinin treatments of 10 µl BA (1 mm) in 0·05 % Tween 20 to a single lateral bud/plant
| Clone | 47‐174 (n = 21) | 49‐177 (n = 20) | 11‐11 (n = 19) |
| Water | 0 | 0 | 0·05 ± 0·1 |
| BA | 0·4 ± 0·9 | 5·2 ± 1·7 | 1·6 ± 1·3 |
Measured after 23 d.
Data are lengths in cm ± s.d.
RESULTS
A comparison of total parent shoot growth of the three clones in a 40 d trial indicated very rapid growth rates, particularly for clones 47‐174 (20·5 mm d–1) and 49‐177 (18·8 mm d–1) (Fig. 2), whereas that of clone 11‐11 was somewhat lower (14·8 mm d–1). Measurements of the degree of sylleptic branching by day 40 (Fig. 3) indicated that clone 11‐11 had the highest number of branches, with a mean of 13·3, whereas clone 49‐177 had 5·7 and clone 47‐174 had only 2·6. The results of this trial were generally representative of those of the other three trials, with some variation. In most trials there appeared to be an inverse correlation between the rate of parent shoot growth and the amount of sylleptic branching among the three clones.
When auxin (1 % NAA in lanolin) was applied to the cut stem surface of the main shoot immediately following decapitation 1 or 2 cm below the apex, the resulting percentage repression of outgrowth of the highest lateral bud after about 2 weeks was greatest in clone 47‐174 (Table 1), the fastest growing clone with the least amount of natural sylleptic branching (Figs 2 and 3). This was true in all seven experiments even though there was considerable variation within and between trials. Hence, these results were consistent with the first hypothesis, namely, that reduced sensitivity to the inhibitory effects of auxin in the parent shoot may be the cause of the increased sylleptic branching in the high‐syllepsis clone and vice versa in the low‐syllepsis clone.
It was noticed that it was only the highest lateral bud below the point of decapitation that was significantly repressed by the NAA, and even this bud’s outgrowth was not completely repressed, particularly in clones 11‐11 and 49‐177. The buds lower on the shoot were not repressed and often grew out vigorously (data not shown).
When cytokinin (1 mm BA) was applied daily directly to a single inhibited lateral bud on the upper part of the parent shoot of each sapling, the initiation of BA‐induced bud outgrowth was often observed within 5 or 6 d in all three clones. The most vigorous lateral outgrowth occurred in clone 49‐177 (Table 2), which, in our studies, exhibited an intermediate to high level (data not shown) of sylleptic branching. Clone 47‐174 with the lowest syllepsis responded least to BA, while the response of clone 11‐11 with the highest syllepsis was intermediate. There was essentially no outgrowth in the control buds in any clone. Overall, these results were partially consistent with the second hypothesis, namely, that increased sensitivity to the promotive effects of cytokinin in the parent shoot is the cause of increased syllepsis in the higher syllepsis clones.
DISCUSSION
Our results suggested that an inverse correlation may exist among the three clones between total parent shoot elongation and the degree of sylleptic branching. In terms of hormonal control mechanisms, it might be surmised that clone 147‐174 with the fastest growing parent shoot is the most sensitive to the inhibitory effect of auxin and/or may produce more auxin resulting in less branching. In contrast, clone 11‐11 with the slowest growing parent shoot is least sensitive to auxin and/or produces less auxin resulting in more sylleptic branching.
The results of our experiments to restore apical dominance using exogenous auxin (NAA) suggested a generally greater sensitivity to auxin (with more repression of lateral bud outgrowth) in clone 147‐174, and are consistent with this explanation and, hence, support the first hypothesis. However, these results are not consistent with the speculation of Sachs and Thimann (1967) that more rapid shoot growth is accompanied by less sensitivity to auxin. Because the growth rate of even the slowest growing of the three clones (11–1; Fig. 2) is quite high, it is justified to question whether the differences in parent shoot growth rate among these three very rapidly growing hybrid clones are large enough to be considered as a possible causal factor for the observed differences in sylleptic branching.
Although there are a number of reports in the literature concerning the use of cytokinins in organogenesis and in micropropagation with Populus and some with transgenic ipt (isopentyl transferase) overproduction, as far as can be determined, this study is the first to report the promotive effects of exogenous cytokinin on lateral bud outgrowth in hybrid poplar as well as the effects of auxin/cytokinin particularly with regard to their role in sylleptic branching.
The results with BA appear to be partially consistent with the second hypothesis that increased sensitivity to the promotive effects of cytokinin in the parent shoot is the cause of increased syllepsis in the high‐sylleptic clone. The fact that clone 49‐177, with a variably intermediate level of sylleptic branching, appeared somewhat more sensitive to BA than the high‐sylleptic clone, 11‐11, may suggest the influence of other factors and perhaps even an underestimation in our measurement of sylleptic branching in clone 49‐177. However, the second hypothesis is supported with respect to the reduced bud outgrowth following BA treatment obtained in the low‐sylleptic clone, 147‐174 (Table 2).
The results of the present preliminary study with hybrid poplar clones exhibiting contrasting degrees of sylleptic branching are consistent with the hypothesis that auxin and cytokinin may play repressive and promotive roles, respectively, in the control of this branching. However, mobile signals in addition to auxin and cytokinin may also be involved and should be considered (Beveridge et al., 2000). Further research is needed to elucidate more fully the controlling mechanisms.
Supplementary Material
Received: 28 January 2002; Returned for revision: 3 March 2002; Accepted: 29 May 2002 Published electronically: 5 August 2002
References
- BeveridgeC, Symons G, Turnbull C.2000. Auxin inhibition of decapitation‐induced branching is dependent on graft‐transmissible signals regulated by genes Rms1 and Rms2.Plant Physiology 123: 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BollmarkM, Chen H‐J, Moritz T, Eliasson.1995. Relations between cytokinin level, bud development and apical control in Norway spruce, Picea abies.Physiologia Plantarum 95: 563–568. [Google Scholar]
- BradshawH, Stettler R.1995. Molecular genetics of growth and development in Populus IV Mapping QTLs with large effects on growth, form and phenology traits in a forest tree. Genetics 139: 963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CeulemansR, Stettler R, Hinckley T, Isebrands J, Heilman P.1990. Crown architecture of Populus clones as determined by branch orientation and branch characteristics. Tree Physiology 7: 157–167. [DOI] [PubMed] [Google Scholar]
- ClineM.1994. The role of hormones and apical dominance. New approaches to an old problem in plant development. Physiologia Plantarum 90: 230–237. [Google Scholar]
- ClineM.2000. Execution of the auxin replacement apical dominance experiment in temperate woody species. American Journal of Botany 87: 182–190. [PubMed] [Google Scholar]
- ClineM, Wessel T, Iwamura H.1997. Cytokinin/auxin control of apical dominance in Ipomoea nil.Plant and Cell Physiology 38: 659–667. [Google Scholar]
- CookC, Rabe E, Jacobs G.1999. Early expression of apical control regulates length and crotch angle of sylleptic shoots in peach and nectarine. HortScience 34: 604–606. [Google Scholar]
- DebellD, Harrington C, Clendenen G, Zenda J.1997. Tree growth and stand development of four Populus clones in large monoclonal plots. New Forests 14: 1–18. [Google Scholar]
- GenardM, Pages L, Kervella.1994. Relationship between sylleptic branching and components of parent shoot development in peach tree. Annals of Botany 74: 465–470. [Google Scholar]
- HalleF, Oldeman R, Tomlinson P.1978. Tropical trees and forests: an architectural analysis. Heidelberg: Springer‐Verlag. [Google Scholar]
- HouseS, Dieters M, Johnson M, Haines R.1998. Inhibition of orthotropic replacement shoots with auxin treatment on decapitated hoop pine (Araucaria cunninghamii) for seed orchard management. New Forests 16: 221–230. [Google Scholar]
- HoweG, Saruul P, Davis J, Chen T.2000. Quantitative genetics of bud phenology, frost damage and winter survival in an R2 family of hybrid poplars. Theoretical Applied Genetics 101: 632–642. [Google Scholar]
- LeakyR, Longman K.1986. Physiological, environmental and genetic variation in apical dominance as determined by decapitation in Triplochiton scleroxylon.Tree Physiology 1: 193–207. [DOI] [PubMed] [Google Scholar]
- LittleC.1969. Apical dominance in long shoots of white pine (Pinus strobes). Canadian Journal of Botany 48: 239–253. [Google Scholar]
- NapoliC, Beveridge C, Snowden K.1999. Re‐evaluating concepts of apical dominance and the control of axillary bud outgrowth. Current Topics in Developmental Biology 44: 127–169. [DOI] [PubMed] [Google Scholar]
- PowellG.1991. Preformed and neoformed extension of shoots and sylleptic branching in relation to shoot length in Tsuga canadensis Trees 5: 107–116. [Google Scholar]
- SachsT, Thimann K.1967. The role of auxins and cytokinins in the release of buds from dominance. American Journal of Botany 54: 136–144. [Google Scholar]
- SandbergG, Sitbon F, Eklof S, Edlund A, Astot C, Blackwell J, Moritz T, Sundberg B, Olsson O.1995. Regulation of auxin and cytokinin turnover in plants. 15th International Conference on Plant Growth Substances, Minneapolis, Minnesota, July 14–18. [Google Scholar]
- Scarascia‐MugnozzaG, Hinckley T, Stettler R, Heilman P, Isebrands J.1999. Production physiology and morphology of Populus species and their hybrids grown under short rotation. III. Season carbon allocation patterns from branches. Canadian Journal of Forest Research 29: 1419–1432. [Google Scholar]
- SpathH.1912. Der Johannistriebe. Berlin: Parey. [Google Scholar]
- TamasI.1995. Hormonal regulation of apical dominance. In: Davies P, ed. Plant hormones, physiology, biochemistry and molecular biology. 2nd edn Boston, MA: Kluwer Academic Publishers, 572–597. [Google Scholar]
- ThimannK, Skoog F.1933. Studies on the growth hormone of plants. III. The inhibition of growth substance on bud development. Proceedings of the National Academy of Sciences of the USA 19: 714–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TrompJ.1996. Sylleptic shoot formation in young apple trees exposed to various soil temperature and air humidity regimes in three successive periods of the growing season. Annals of Botany 77: 63–70. [Google Scholar]
- WilliamsM, Stahly E.1968. Effect of cytokinins on apple shoot development from axillary buds. HortScience 3: 68–69. [Google Scholar]
- WilsonB.1979. Epicormic shoot growth in striped maple. Canadian Journal ofForest Research 9: 110–113. [Google Scholar]
- WuR‐L.1994. Qualitative genetics of yield breeding for Populus short rotation culture. II. Genetic determination and expected selection response of tree geometry. Canadian Journal of Forest Research 24: 155–165. [Google Scholar]
- WuR‐L, Hu X, Han Y.2000. Molecular genetics and developmental physiology: implications for designing better forest crops. Critical Reviews in Plant Science 19: 377–393. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.