Abstract
In recent systematic treatments of the Cyperaceae, spikelets of all but the most primitive tribes have been considered to be indeterminate, whereas historically the number of flowers, floral sex and distribution of sexes in spikelets have been important characters in suprageneric classifications. However, descriptions of these spikelet characteristics for sawgrass, Cladium jamaicense Crantz, vary among authors. Spikelet morphology was analysed using developmental and phenological studies of sawgrass populations in south Florida, USA. Sawgrass spikelets have two flowers that expand successively. Flowers are fundamentally hermaphroditic and protogynous. The first flower to expand (F1) terminates the spikelet axis, whereas the second flower (F2), ensheathed by an addorsed prophyll, develops in the axil of the last bract produced on the axis. In 86 % of the spikelets examined from ramets of three populations, the gynoecium of the F1 flower aborted, so this flower was functionally male and the spikelet was protandrous. However, in 14 % of spikelets from these individuals, the F1 flower was hermaphroditic and could set seed. The F2 flower was typically hermaphroditic and matured stigmas, then anthers. Thus, spikelets in C. jamaicense are determinate and have two flowers that are dichogamous both within flowers and between flowers in a spikelet; spikelet sex expression can vary among plants and populations, especially in the first flower. These data for sawgrass suggest that a re‐examination of spikelet development and phenology in other genera is needed to clarify the expression of these characters in the family.
Key words: Anemophily, Cladium jamaicense, determinate spikelet, Cyperaceae, dichogamy, protandry, protogyny, indeterminate spikelet, sawgrass, spikelet morphology
INTRODUCTION
Spikelets bearing one or more reduced flowers are the basic reproductive unit in the Cyperaceae. Spikelet structure, arrangement, flower number per spikelet and floral sex have been important characters in the systematics of the family (Koyama, 1961; Eiten, 1976; Dahlgren et al., 1985; Tucker, 1987; Bruhl, 1995; Goetghebeur, 1998). Recent molecular and cladistic studies using numerous additional characters have provided suprageneric classifications and phylogenetic trees for interpretation of spikelet characters (Muasya et al., 1998, 2000). All of these studies consider cyperaceous spikelets to be indeterminate or racemose in all but the primitive tribes, in contrast to earlier workers who considered the spikelets to be determinate or cymose in at least some of the more advanced tribes (reviewed in Holttum, 1948; Mora, 1960; Eiten, 1976). Many current classifications for the family use models of spikelet structure established by Eiten (1976); yet Eiten provides only a general typology of the ultimate inflorescence units found throughout the family and does not present evidence for her conclusions other than a few verbal descriptions of mature anatomy and illustrations of dissections of mature spikelets.
Sawgrass, Cladium jamaicense Crantz, is the dominant macrophyte in the Everglades of south Florida, USA (Loveless, 1959; Davis et al., 1994; Gunderson, 1994). The most recent molecular and cladistic phylogeny of the Cyperaceae places Cladium basally in the Schoeneae (Muasya et al., 2000). However, descriptions of spikelet structure for Cladium species vary among sources, while an understanding of spikelet phenology is lacking (Mora, 1960; Steward and Ornes, 1975; Long and Lakela, 1976; Godfrey and Wooten, 1979; Tucker, 1987). Descriptions of the spikelets as indeterminate or determinate vary amongst authors, as do the number of flowers per spikelet (two or more), and the sex of the flowers in the spikelets (male, female or hermaphrodite) (Holttum, 1948; Koyama, 1961; Eiten, 1976; Long and Lakela, 1976; Godfrey and Wooten, 1979; Dahlgren et al., 1985; Tucker, 1987; Bruhl, 1995). Studies of population structure of C. jamaicense in south Florida have shown that although overall genetic diversity is low, genotypic diversity is present at a local scale (Ivey and Richards, 2001a, b). These studies indicate that seed reproduction is important to the population structure of this species. Asexual plantlet production in inflorescences and seed germination has recently been studied (Miao et al., 1997, 1998; Lorenzen et al., 2000), but little is known about the details of the mating system of C. jamaicense. Here, I describe spikelet structure in south Florida populations of C. jamaicense. I use phenological observations, dissections of mature spikelets and developmental data to define spikelet flower number and sex expression, and to determine whether the spikelet is determinate or indeterminate.
MATERIALS AND METHODS
Sawgrass inflorescences have clusters of spikelets [primary lateral inflorescence branches of Bruhl (1995)] distributed in the axils of bracts along an elongated inflorescence (Fig. 1A). The branching pattern of the inflorescence was analysed by dissecting the first secondary branch on the first lateral inflorescence branch under a Wild M3 dissect ing microscope (Wild Heerbrugg, Ltd, Heerbrugg, Switzerland). Over 200 individual spikelets were collected from ten plants in an ex situ population at Henington Pond, Florida International University, USA (25°45·648′N, 80°30·143′W). The ten plants originated from discrete ramets collected in two south Florida sawgrass populations in 1998 and planted at Henington Pond. Spikelets were dissected under a microscope to determine spikelet and flower number, and floral sex. To establish the sequence of flower maturation and carpel and stamen expansion, spikelets on primary branches 1, 4 and 7 of inflorescences on these ten plants were observed daily throughout May 2001; spikelets sampled for dissections were not taken from inflorescences that were being observed for phenology. Spikelets at different developmental stages were fixed in FAA (Ruzin, 1999), dehydrated in ethanol, CO2‐critical point dried, coated with gold–palladium and examined at 10 kV with an ISI Super IIIA scanning electron microscope (Topcon America Corporation, Paramus, NJ, USA).
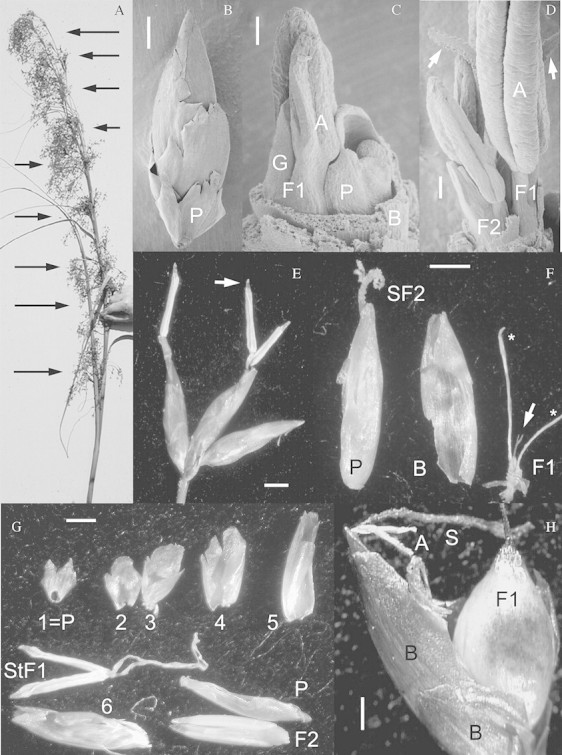
Fig. 1. A, Inflorescence of sawgrass, C. jamaicense. Flowers are produced in lateral and terminal clusters (arrows); the lateral clusters develop from buds in the axils of bracts. Approx. ×10 magnification. B, Scanning electron micrograph (SEM) of the lanceolate spikelet with a prophyll (P) and bracts of increasing length and width. Bar = 500 µm. C, SEM of the tip of a developing spikelet with ensheathing bracts removed. The base of the last‐produced bract (B) encircles the primordium of the terminal flower (F1), which has two stamens (A) and a carpel (G); B also surrounds an axillary bud with an addorsed prophyll (P). The prophyll encloses a second flower, F2, which is at an earlier developmental stage than F1 but has also initiated two anthers and a carpel. Bar = 50 µm. D, SEM of the tip of a developing spikelet with the spikelet bracts and F2 prophyll removed. The terminal flower (F1) has well developed anthers (A) and two papillate stigma branches (arrows) protrude from between the anthers. The axillary flower (F2) has developmentally younger anthers and carpel. Bar = 50 µm. E, Micrograph of three spikelets at the end of an inflorescence cluster branch; the spikelets are in the F1 male phase, with anthers exerted from the ensheathing bracts; the F1 female did not expand in these spikelets, as stigma remnants are not present. The anther apiculus is visible (arrow). Bar = 1 mm. F, Micrograph of a partially dissected spikelet. The last bract (B) on the spikelet axis has been removed from around the terminal flower (F1), which has an aborted carpel with a two‐branched stigma (arrow) and the filaments of the two stamens (asterisks), the anthers having abscised. The F2 flower, surrounded by the bract prophyll (P), is in the female phase; its stigma (SF2) is exserted but the anthers have not yet emerged from the prophyll. Bar = 1mm. G, Micrograph of a dissected spikelet with a bicarinate prophyll (1 = P), five successive bracts (2–6) of increasing length and width, the mature stamens of the terminal F1 flower (StF1), the prophyll (P) of the bud in the axil of bract 6 and the immature hermaphroditic flower (F2) that this prophyll enclosed. The two F2 anthers lie to either side of the immature F2 gynoecium. Bar = 1mm. H, Micrograph of a spikelet in which both flowers are hermaphroditic. The spikelet bracts (B) have been pulled back from the flowers. The F1 flower set fruit (F1); the F2 flower exserted both stigmas (S) and anthers (A). Bar = 500 µm.
Flower number, sex and position were determined for spikelets from at least 50 ramets collected from three Everglades populations: the Singeltary population (25°25·115′N, 80°28·134′ W); the Taylor Slough population (25°23·950′N, 80°36·059′W); and the northeast Everglades population (25°45·648′N, 80°30·143′W). GPS coordinates of sites were recorded with a Garmin GPS 12 (Garmin International Inc., Olathe, KS, USA). Samples were taken from plants located at least 5 m apart and were sampled from the first or second secondary branch on the first or second primary branch on each inflorescence. One spikelet per plant was analysed under a dissecting microscope. Differences among populations in the distribution of spikelet sexes were tested using the log likelihood ratio χ2 in SAS (v.8·2).
To quantify variation within and among plants, measurements of spikelet length were made using MAX‐CAL electronic digital callipers (Fowler Co., Inc., Newton, MA, USA) on three spikelets per plant for ten plants from each of the Singeltary, northeast Everglades and Henington Pond populations. Bract number per spikelet and the position and sex of flowers were recorded for each spikelet dissected. Details of floral structure were photographed on a Wild M3 dissecting microscope with a digital or video camera.
RESULTS
Inflorescence structure
Inflorescences were borne on stalks that raised them above the sawgrass leaves. Inflorescence height was similar throughout a sawgrass population. The inflorescence axis bore bracts that decreased in length up the axis; each bract subtended an axillary bud that produced a primary lateral inflorescence branch. The length of this branch also decreased at successive nodes along the main axis (Fig. 1A). The inflorescence terminated in a cluster of spikelets (Fig. 1A).
The axillary bud that produced the primary branch had a two‐keeled (bicarinate) prophyll that remained in the axil of the subtending leaf, while the internode above the prophyll (epipodium) expanded. The branch bore a series of bracts with successively shorter internodes, and terminated in a spikelet (Fig. 1E). Buds in the axils of the bracts expanded and repeated the pattern; each had a prophyll that remained in the bract axil, a long internode, then a succession of leaves with shorter internodes and a terminal spikelet. The last axillary spikelets on any order of branching had very little internodal elongation and thus appeared as a cluster surrounding the terminal spikelet (Fig. 1E). These axillary spikelets were subtended by a bract with a single tip and bore an addorsed prophyll that was generally bicarinate (Figs 1B and G). The terminal spikelet lacked this prophyll, as its prophyll remained at the base of the branch.
Spikelet structure and flower sex
Spikelets were lanceolate with an acute tip (Fig. 1B and E) and were approx. 5 mm in length (range 4·3–6·4 mm) at the time of flowering (Table 1). Spikelets produced between five and nine bracts, including the prophyll (Table 1). The bicarinate prophyll on an axillary spikelet was much shorter than the spikelet and did not encircle it completely (Fig. 1B and G). Successive bracts on axillary and terminal spikelets increased in length and width and thus in the degree to which they enclosed the younger parts of the spikelet (Fig. 1B and G).
Table 1.
Spikelet structure and sex in three south Florida populations of sawgrass Cladium jamaicense
| Population | Spikelet length (mm) | Number of bracts | Number of flowers | % Spikelets F1 M; F2 H | N (n)‡ |
| Henington Pond | 5·3 ± 0·4 | 5–9 | 2 | 100 | 10 (30) |
| Singeltary | 4·9 ± 0·4 | 5–9 | 2 | 93* | 10 (30) |
| NE Everglades | 5·4 ± 0·4 | 5–9 | 2 | 40† | 10 (30) |
| Total | 5·2 ± 0·4 | 5–9 | 2 | 78 | 30 (90) |
F1 and F2 are the first and second flowers to mature in the spikelet. M, Male flower; H, hermaphroditic flower.
* Alternative sex was F1 M; F2 M; spikelet sex varied within a plant.
† Alternative sex was F1 H; F2 H; spikelet sex was constant within a plant.
‡N, Number of plants sampled; (n), number of spikelets sampled (three per plant).
All of the spikelets examined in both in situ and ex situ populations produced two flowers (Fig. 1C, D, F and G; Table 1). The first flower (F1) was completely enclosed by an ensheathing bract whose edges overlapped (Fig. 1C, F and G). This flower terminated the spikelet axis (Fig. 1C). There was no prophyll associated with the F1 flower and no evidence of a remnant apex produced beyond this flower (Fig. 1C). The bract that encircled the terminal flower subtended an axillary bud with an addorsed prophyll (Fig. 1C). This prophyll ensheathed a flower (F2) that terminated the axillary bud axis (Fig. 1C, F and G). Both flowers were developmentally hermaphroditic and produced two anthers and a single gynoecium (Fig. 1D, F and G).
The anthers of both F1 and F2 were four locular and had an apiculus or sterile projection at the tip (Fig. 1E). Typically, the gynoecium of F1 had two stylar branches (Fig. 1D and F). The gynoecium of F1 flowers aborted in 86 % of samples from the three Everglades populations (Fig. 1F; Table 2). Styles on aborted carpels turned reddish‐brown and the stigmatic papillae elongated. After carpel abortion, F1 matured as a male flower (Fig. 1E). However, in 14 % of the combined sample the gynoecium did not abort, and the F1 flower was hermaphroditic (Table 2) and could set fruit (Fig. 1H). When the gynoecium did not abort, F1 flowers were protogynous with the stigma maturing prior to the anthers.
Table 2.
Percentage of spikelets with flowers of different sexes in three south Florida populations of sawgrass, Cladium jamaicense
| Population | F1 M; F2 M | F1 M; F2 H | F1 H; F2 M | F1 H; F2 H | N* |
| NE Everglades | 0 | 97 | 0 | 3 | 61 |
| Taylor Slough | 0 | 64 | 0 | 36 | 50 |
| Singeltary | 4 | 92 | 0 | 4 | 50 |
| Total | 1 | 85 | 0 | 14 | 161 |
F1 and F2 are the first and second flowers to mature in the spikelet. M, Male flower; H, hermaphroditic flower.
* N, Number of ramets per population; one spikelet per ramet was sampled.
The F2 flower, which developed in the axil of the bract surrounding F1, was developmentally delayed compared with F1 (Fig. 1C, D and G). In all but two plants examined, this flower matured as a hermaphrodite; in the exceptions, the carpel aborted, producing a male flower (Table 2). The F2 carpel typically had three or four style branches, although in a few cases only two were produced. Like F1 hermaphrodites, F2 was protogynous (Fig. 1F).
Spikelets of plants observed daily at Henington Pond all had F1 male flowers and F2 hermaphroditic flowers (Table 1). All of the spikelets on these plants expanded F1 anthers first (Fig. 1E), followed 2–4 d later by F2 stigmas, then by F2 anthers 1–3 d later. Anthers of both types of flowers lasted a single day; they abscised on the day that they expanded or the day after, leaving the filaments in the spikelet (Fig. 1F). Initially, stigmas were white and papillate (Fig. 1F), but they then dried, becoming brown and shrivelled, 1 or 2 d after expansion (Fig. 1H).
Of the four arrangements of male and hermaphroditic flowers possible in two‐flowered spikelets, spikelets that matured a male flower first, then a hermaphroditic flower, were most common (Tables 1 and 2). Spikelets that matured two hermaphroditic flowers were the second most frequent case. Spikelets with two male flowers were infrequent, and no spikelets that matured a hermaphroditic flower and then a male flower were observed (Table 2). The frequency of spikelet sex types varied significantly among populations (Table 2; log likelihood ratio χ2 = 30·52, P < 0·0001).
DISCUSSION
Determinate vs. indeterminate spikelet structure
Most recent illustrations of spikelet morphology in Cladium show an axillary male flower below an axillary hermaphroditic flower in a racemose spikelet, with sterile bracts both below and above the fertile bracts (Koyama, 1961; Eiten, 1976; Dahlgren et al., 1985). Descriptions of the spikelet depict a ‘distal’ or ‘upper’ perfect flower and a ‘subdistal’ or ‘lower’ staminate flower (Tucker, 1987; Goetghebeur, 1998). Bruhl (1995) described the spikelets of most Cyperaceae as axillary and those of Cladium as lacking a terminal flower. This current interpretation of spikelet structure differs from earlier interpretations of the spikelet of at least some Cyperaceae as a determinate, sympodial or cymose structure [Mora, 1960; Kern, 1974; see discussions in Eiten (1976) and Holttum (1948)]. The most recent developmental study (Mora, 1960) describes the spikelet of C. mariscus, as well as that of species in a number of other genera, as determinate.
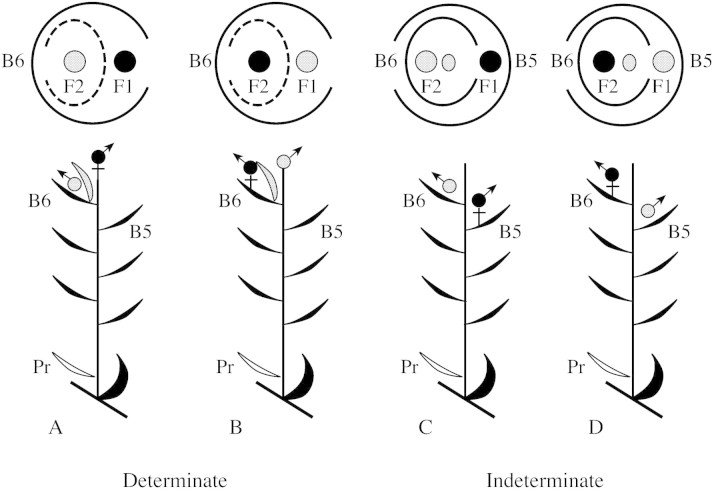
The arrangement of openings of the ensheathing bracts and the position of flowers with respect to both bracts and prophylls differ substantially under determinate and indeterminate interpretations of the spikelet (Fig. 2). The data presented here show that the spikelet has: (1) a typically male flower encircled by a bract with no evidence of an axis or leaves produced beyond that flower or of a remnant apex and with no associated prophyll; and (2) a hermaphroditic flower that is developmentally delayed in relation to the male flower and that is produced in the axil of the bract encircling the male flower. In addition, the hermaphroditic flower is surrounded by a prophyll whose sheath opens away from the male flower, as is typical of a monocotyledonous prophyll on an axillary bud. There is no evidence for additional leaves or a remnant apex produced beyond the hermaphroditic flower. Thus, the spikelet of C. jamaicense has a terminal male flower and an axillary hermaphroditic flower subtended by the last bract produced on the spikelet axis (Fig. 2B).
Fig. 2. Models of spikelet structure in C. jamaicense, showing the arrangement of bracts and of male and hermaphroditic flowers as seen in condensed transverse (top row) and longitudinal (bottom row) views, under determinate (A and B) and indeterminate (C and D) hypotheses of spikelet development. The interior ensheathing bract is a spikelet bract (B6) under models C and D, but is the prophyll (dotted line) of a bud axillary to B6 under models A and B. Prophylls are not associated with axillary flowers in C and D. C. jamaicense has a spikelet with the arrangement of bracts, flowers, and prophylls presented in B. Black circles, hermaphroditic flowers; grey circles, male flowers; grey oval, spikelet apex; black leaves, bracts on the spikelet main axis; grey leaves, prophylls on spikelet axillary branches; Pr, Prophyll on main spikelet axis; B5, B6, fifth and sixth bracts on the main spikelet axis; F1, first flower to mature; F2, second flower to mature.
An argument against this interpretation is that the F2 bract, which is in the prophyllar position, does not have the bicarinate prophyll morphology typical of prophylls on spikelets (Koyama, 1961; Eiten, 1976). However, Cyper aceae prophylls can be very diverse in their form (Blaser, 1944), whereas prophylls on sylleptic branches, such as the F2 branch, often have a relatively unmodified morphology (Bell, 1991). A determinate interpretation of the spikelet is consistent with the determinate structure of the inflorescence branches and the inflorescence itself. All of these branches terminate in spikelets; the spikelets, in turn, terminate in flowers.
This description of spikelet structure in C. jamaicense contradicts current interpretations of spikelet morphology in the Cyperaceae, which recognize the odd spikelet in the primitive Hypolytreae as determinate, but consider more advanced Cyperaceae to have indeterminate spikelets (Eiten, 1976; Dahlgren et al., 1985; Bruhl, 1991, 1995; Goetghebeur, 1998). Studies of South African Carpha, Epischoenus and Schoenus species show that the spikelets in these genera are not indeterminate (Browning and Guthrie, 1994; Browning and Gordon‐Gray, 1995). The data and illustrations of spikelets in these papers depict a determinate developmental pattern similar to that described here for C. jamaicense. Recent phylogenies put Carpha as a sister group to Cladium and both genera basal in the Schoeneae (Muasya et al., 2000). More intriguing is the illustration of a spikelet of Rhynchospora corymbosa (Fig. 39A, Goetghebeur, 1998). The Rhynchosporeae are sister to the Schoeneae but are more advanced in the family (Muasya et al., 2000). This illustration shows a spikelet with a flower described as axillary to bract 4 but subsequently ensheathed by bract 5. This spikelet would be more parsimoniously interpreted as having a determinate developmental pattern in which bract 4 does not subtend a flower, and bract 5 is the last leaf on the spikelet, followed by a terminal flower; subsequent development is from a bud axillary to bract 5, similar to the pattern seen in C. jamaicense. Based on his examinations of developing spikelets, Mora (1960) included Rhynchospora among genera with determinate spikelets.
Developmental and phenological studies of living material across a broader range of species are needed to determine the distribution of spikelet types and sexes among tribes in the Cyperaceae. The data presented here raise the question of whether the determinate structure in the C. jamaicense spikelet is plesiomorphic, reflecting sawgrass’s basal position in the relatively primitive Schoeneae, or whether insufficient data on spikelet development in more advanced Cyperaceae prevent us from understanding the distribution of this character in the family.
Spikelet flower number and sex
Previous descriptions of the number of flowers in the spikelet of Cladium have varied (Mora, 1960; Long and Lakela, 1976; Godfrey and Wooten, 1979), although many authors have agreed that there are two (Koyama, 1961; Tucker, 1987; Bruhl, 1995; Goetghebeur, 1998). Similarly, the description of floral sex has ranged from bisexual to male and female (Mora, 1960; Koyama, 1961; Eiten, 1976; Long and Lakela, 1976; Godfrey and Wooten, 1979; Tucker, 1987; Bruhl, 1995; Goetghebeur, 1998). All sawgrass spikelets examined in this study produced two flowers, although the timing of flower maturation differed among these flowers. Both flower primordia were bisexual, and both flowers could function as hermaphrodites and set fruit. In most spikelets, however, F1 was functionally male, while F2 was hermaphroditic. Thus, flowers in a spikelet can, but do not always, differ in sex expression. The variable expression of floral sex means that spikelet sex can vary among plants within a single population and across a geographical region. These data provide a background for interpreting the diverse descriptions of spikelet structure in this genus and emphasize the need for population studies of living material to understand floral and spikelet sex expression, which have been important systematic characters at the suprageneric level in the family (Koyama, 1961; Eiten, 1976; Dahlgren et al., 1985; Bruhl, 1995; Goetghebeur, 1998; Muasya et al., 2000).
Both flowers in the spikelet of C. jamaicense are protogynous, as has been attributed to the Cyperaceae generally (Goetghebeur, 1998). Abortion of the F1 gynoecium may be an extreme expression of this protogyny, as the stigma appears to mature in the aborted carpel prior to ovary development. What controls abortion of the F1 female is unknown, but when multiple flowers from a single plant were examined, the F1 flowers were similar in their sex expression, so this character may vary at the level of the individual.
Because the two flowers in a spikelet mature at different times, spikelets, as well as flowers, are dichogamous. Male/hermaphroditic spikelets go through a male phase (F1 anthers mature), a female phase (F2 stigma matures), then another male phase (F2 anthers mature). Thus, although C. jamaicense flowers are protogynous, most spikelets are functionally protandrous. Temporal separation of the sexes, or dichogamy, is common in anemophilous plants (Ackerman, 2000), where it may reduce self‐pollination. The efficacy of spikelet dichogamy in preventing geitenogamy, however, depends on a number of other factors, such as the phenology of spikelets within an inflorescence, the timing of expansion of different inflorescences on a genet, the dynamics of wind pollen dispersal and the presence or absence of a self‐incompatibility system. How sawgrass flower phenology and variation in flower sex expression function in relation to these other parameters should be the subject of future research.
ACKNOWLEDGEMENTS
I thank Drs R. L. Jones and D. W. Lee for their support and Dr T. Philippi for help with statistical analyses. This study was completed as an ancillary project to ongoing research on wetland ecosystem processes and wetland restoration at Florida International University’s Southeast Environmental Research Center (FIU SERC). Everglades plants were collected under USDA National Park Service collecting permit number 201003. This paper is SERC Contributed Paper No. 180.
Supplementary Material
Received: 3 January 2002; Returned for revision: 10 May 2002; Accepted: 6 June 2002 Published electronically: 5 August 2002
References
- AckermanJD.2000. Abiotic pollen and pollination: ecological, functional, and evolutionary perspectives. Plant Systematics and Evolution 222: 167–185. [Google Scholar]
- BellAD.1991. Plant form: an illustrated guide to flowering plant morphology. Oxford: Oxford University Press. [Google Scholar]
- BlaserHW.1944. Morphology of the Cyperaceae. II. The prophyll. American Journal of Botany 31: 53–64. [Google Scholar]
- BrowningJ, Gordon‐Gray KD.1995. Studies in Cyperaceae in southern Africa 27: a contribution to knowledge of spikelet morphology in Epischoenus and the relationship of this genus to Schoenus South African Journal of Botany 61: 147–152. [Google Scholar]
- BrowningJ, Guthrie I.1994. Studies in Cyperaceae in southern Africa 22: spikelet structure in African species of Carpha R. Br. South African Journal of Botany 60: 148–151. [Google Scholar]
- BruhlJJ.1991. Comparative development of some taxonomically critical floral/inflorescence features in Cyperaceae. Australian Journal of Botany 39: 119–127. [Google Scholar]
- BruhlJJ.1995. Sedge genera of the world: relationships and a new classification of the Cyperaceae. Australian Systematic Botany 8: 125–305. [Google Scholar]
- DahlgrenR, Clifford HT, Yeo PF.1985. The families of the monocotyledons. Berlin, Heidelberg, New York: Springer. [Google Scholar]
- DavisSM, Gunderson LH, Park WA, Richardson JR, Mattson JE.1994. Landscape dimension, composition, and function in a changing Everglades ecosystem. In: Davis SM, Ogden JC, eds. Everglades, the ecosystem and its restoration Delray Beach, FL: St Lucie Press, 419–444. [Google Scholar]
- EitenLT.1976. Inflorescence units in the Cyperaceae. Annals of the Missouri Botanical Garden 63: 81–112. [Google Scholar]
- GodfreyRK, Wooten JW.1979. Aquatic and wetland plants of southeastern United States: Monocotyledons. Athens, GA: The University of Georgia Press. [Google Scholar]
- GoetghebeurP.1998. Cyperaceae. In: Kubitzki K, Huber H, Rudall PJ, Stevens PS, Stutzel T, eds. The families and genera of vascular plants Berlin: Springer‐Verlag, 141–190. [Google Scholar]
- GundersonLH.1994. Vegetation of the Everglades: determinants of community composition. In: Davis SM, Ogden JC, eds. Everglades, the ecosystem and its restoration Delray Beach, FL: St Lucie Press, 323–340. [Google Scholar]
- HolttumRE.1948. The spikelet in Cyperaceae. Botanical Review 14: 525–541. [Google Scholar]
- IveyCT, Richards JH.2001a Genetic diversity of Everglades sawgrass, Cladium jamaicense (Cyperaceae). International Journal of Plant Science 162: 817–825. [Google Scholar]
- IveyCT, Richards JH.2001b Genotypic diversity and clonal structure of Everglades sawgrass, Cladium jamaicense (Cyperaceae). Inter national Journal of Plant Science 162: 1327–1335. [Google Scholar]
- KernJH.1974. Cyperaceae. In: van Steenis CGGJ, ed. Flora Malesiana, series I, Spermatophyta Leyden, The Netherlands: Noordhoff Inter national Publishing, 435–753. [Google Scholar]
- KoyamaT.1961. Classification of the family Cyperaceae (1). Journal of the Faculty of Science, University of Tokyo, Section III, Botany 8: 37–148. [Google Scholar]
- LongRW, Lakela O.1976. A flora of tropical Florida. Miami, Florida: Banyan Press. [Google Scholar]
- LorenzenB, Brix H, McKee KL, Mendelssohn IA, Miao SL.2000. Seed germination of two Everglades species, Cladium jamaicense and Typha domingensis Aquatic Botany 66: 169–180. [Google Scholar]
- LovelessCM.1959. A study of the vegetation in the Florida Everglades. Ecology 40: 1–9. [Google Scholar]
- MiaoSL, Borer RE, Sklar FH.1997. Sawgrass seedling responses to transplanting and nutrient additions. Restoration Ecology 5: 162–168. [Google Scholar]
- MiaoSL, Kong L, Lorenzen B, Johnson RR.1998. Versatile modes of propagation in Cladium jamaicense in the Florida Everglades. Annals of Botany 82: 285–290. [Google Scholar]
- MoraLE.1960. Beiträge zur Entwicklungsgeschichte und vergleichenden Morphologie der Cyperaceen. Beiträge zur Biologie der Pflanzen 35: 253–341. [Google Scholar]
- MuasyaAM, Simpson DA, Chase MW, Culham A.1998. An assessment of suprageneric phylogeny in Cyperaceae using rbcL DNA sequences. Plant Systematics and Evolution 211: 257–271. [Google Scholar]
- MuasyaAM, Bruhl JJ, Simpson DA, Culham A, Chase MW.2000. Suprageneric phylogeny of Cyperaceae: a combined analysis. In: Wilson KL, Morrison DA, eds. Monocots: systematics and evolution Collingwood, Australia: CSIRO Publishing, 593–601. [Google Scholar]
- RuzinSE.1999. Plant microtechniques and microscopy. New York: Oxford University Press. [Google Scholar]
- StewardKK, Ornes WH.1975. The autecology of sawgrass in the Florida Everglades. Ecology 56: 162–171. [Google Scholar]
- TuckerGC.1987. The genera of Cyperaceae in the southeastern United States. Journal of the Arnold Arboretum 68: 361–445. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.