Abstract
This study describes the successive stages of development of branches from axillary buds in fully rooted plants of Trifolium repens grown in near optimal conditions, and the way in which this developmental pathway differs when nodal root formation is prevented as plants grow out from a rooted base. Cuttings of a single genotype were established in a glasshouse with nodal root systems on the two basal phytomers and grown on so that nodal rooting was either permitted (+R) or prevented (–R). In +R plants, axillary tissues could be assigned to one of four developmental categories: unemerged buds, emerged buds, unbranched lateral branches or secondarily branched lateral branches. In –R plants, branch development was retarded, with the retardation becoming increasingly pronounced as the number of –R phytomers on the primary stolon increased. Retarded elongation of the internodes of lateral shoots on –R plants resulted in the formation of a distinct fifth developmental category: short shoots (defined as branches with two or more leaves but with mean internode length equal to, or less than, 10 % of that of the immediately proximal internode on the parent stolon) which had reduced phytomer appearance rates but retained the potential to develop into lateral branches. Transfer of +R plants to –R conditions, and vice versa, after 66 d demonstrated that subsequent branch development was wholly under the control of the youngest nodal root present, regardless of the age and number of root systems proximal to it.
Key words: Trifolium repens (L.), white clover, branching architecture, branch development, nodal roots, short shoots
INTRODUCTION
Trifolium repens is a clonal species in which up to 2·5 years of the life of an individual plant following seed germination can be spent in a non‐clonal bipolar phase. During this period a large secondarily thickened seminal root system develops, supporting a radiating system of branching stolons that has on average five times the spread of clonally growing plants (Brock et al., 2000). Upon the death of the seminal root the plant fragments into several independent stolon systems and begins the clonal stage of its life cycle. This paper concerns the effects of the presence or absence of nodal roots along stolon axes on shoot branching architecture in this second, clonal, phase.
Comparatively recent evidence has demonstrated that branch development, and hence shoot architecture, of T. repens is influenced by the location of nodal roots within a clonal fragment. In plants with long unrooted unbranched stems, branching on a phytomer can be induced by the outgrowth of an adventitious root from one of the two root primordia present at its node (Thomas, 1987b). This finding provides experimental support for the results of earlier correlative field studies by Knight (1953), Trautner and Gibson (1966)and Chapman (1983), which suggested the possibility of a relationship between nodal rooting and lateral branch development. Subsequent evidence from both manipulative experimentation (Jones and Sackville Hamilton, 1993; Kemball and Marshall, 1994; Solangaarachchi, 1996; Lötscher and Nösberger, 1996) and correlative study (Newton and Hay, 1994) has, with few exceptions, confirmed the existence of this relationship. Broadly, it has been shown that the establishment and productivity of an individual branch are enhanced by the presence of a root at its phytomer of origin and, furthermore, that the influence of a nodal root extends over several phytomers distal to it. The opposite occurs when nodal rooting is restricted; under such conditions there is an increase in the number of branches that fail to elongate (Lötscher and Nösberger, 1996). An important objective of this study was to characterize fully the inhibition of branching that occurs upon restriction of nodal rooting and to test whether the response is stable when the number of nodal roots proximal to the youngest nodal root is varied.
Inconsistent terminology has proved to be a problem in attempting to interpret the range of responses described. The term ‘branch’ has been variously used by different authors to include all stages of the development of an axillary bud from the time its first leaf emerges from the surrounding stipular sheath, up to the stage of pronounced elongation of its stem axis. In particular, there has been a failure to recognize the distinction between axillary buds at their earliest pre‐elongation stage and those at a much later stage of development in which internode elongation is restricted by the absence of nodal roots along the length of the primary stolon. The present study was therefore undertaken to describe in detail the various stages of axillary bud outgrowth both in fully rooted plants and in those prevented from forming nodal roots along the length of their stolons so as to establish a robust developmentally based categorization of the stages of axillary bud and branch growth in T. repens.
MATERIALS AND METHODS
Definitions
Primary stolon.
The main shoot stem axis developed from a cutting.
Phytomer.
The repeated structural unit of a stolon differentiated from its apical meristem (Barlow, 1989). Each phytomer consists of a node, the internode proximal to it, an axillary bud and its subtending leaf, and two nodal root primordia.
Phytomer emergence.
The stage of phytomer development at which its developing leaf has just reached 0·3 on Carlson’s scale of leaf development (Carlson, 1966).
Plastochron.
The interval of time between the emergence of leaves at two consecutive phytomers.
Unemerged axillary bud.
A bud still completely enclosed by the stipular sheath of its subtending leaf (Fig. 1A).
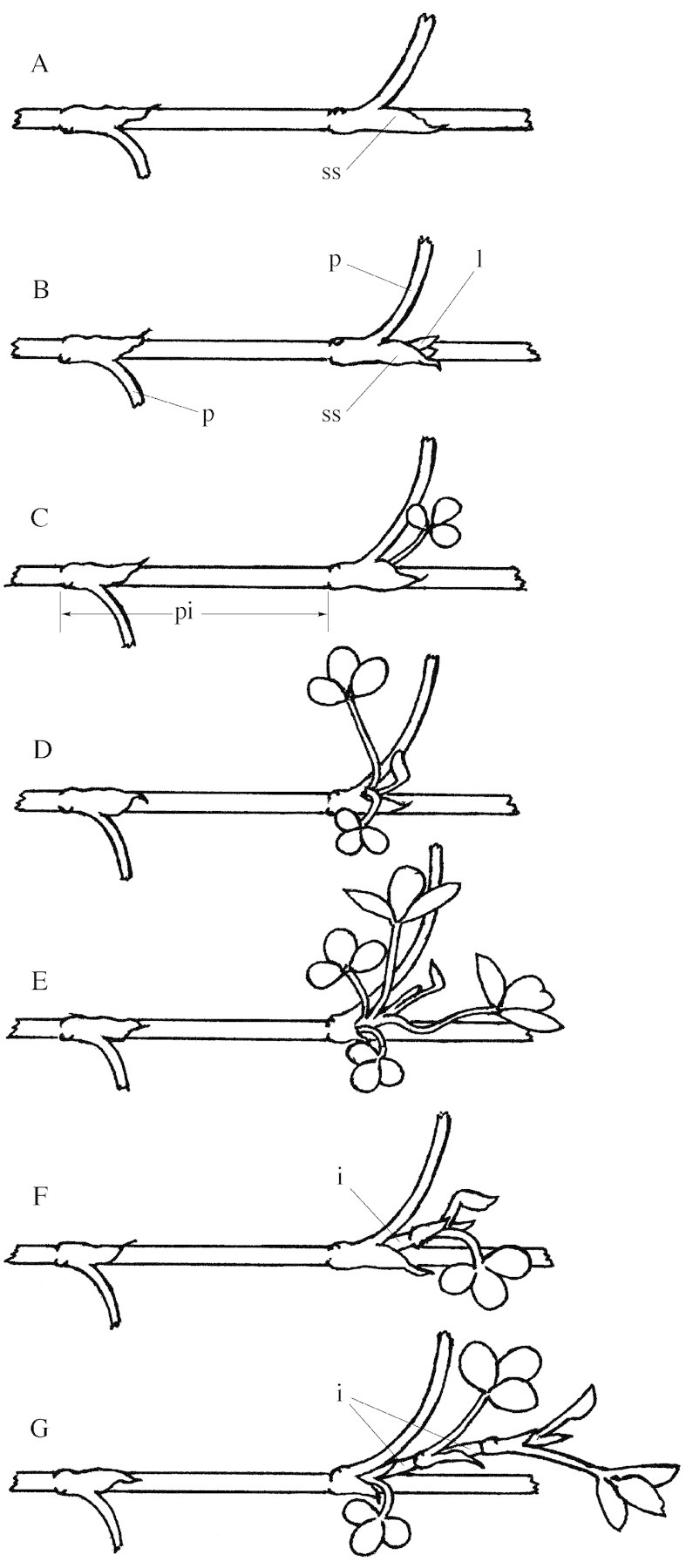
Fig. 1. Diagram illustrating the developmentally based categorization of branching in Trifolium repens. A, Unemerged axillary bud completely enclosed within the stipular sheath (ss). B, Emerged axillary bud with the lamina (l) of its first leaf visible beyond the stipular sheath. The relative size of the base of the petiole (p) of the leaf at each of two phytomers on the primary stolon is shown. C, Emerged axillary bud with a fully opened leaf and internode length less than 10 % of that of the proximal internode (pi) on the primary stolon. D, Short shoot with two opened leaves and an average internode length less than 10 % of that of the proximal internode on the primary stolon. E, Short shoot with four opened leaves showing their small stature in comparison with the size of the petiole of the leaf on the primary stolon. F, Lateral branch with one opened leaf and an internode (i) length greater than 10 % of that of the proximal internode on the primary stolon. G, Lateral branch with two opened leaves and internode lengths greater than 10 % of that of the proximal internode.
Emerged axillary bud.
A bud in which one leaf is macroscopically visible beyond its stipular sheath and the mean internode length of its stem axis is less than 10 % of that of the proximal internode of its parent stolon (Fig. 1B and C).
Lateral shoot.
An outgrown axillary bud with mean internode length greater than 10 % of that of the proximal internode of the parent stolon (Fig. 1F and G) and/or two or more emerged phytomers (Fig. 1D and E). Lateral shoots are classified either as lateral branches or short shoots.
Short shoot.
A lateral shoot bearing two or more fully expanded leaves but with very short internodes, the mean length of which is equal to, or less than, 10 % of that of the proximal internode on the parent stolon (Fig. 1D and E).
Lateral branch.
A lateral shoot in which the mean internode length exceeds 10 % of that of the proximal internode on the parent stolon (Fig. 1F and G). Lateral branches are further categorized as either unbranched laterals (lateral stolons on which no higher order branching is present) or secondarily branched laterals (lateral stolons on which one or more constituent phytomers have formed a higher order secondary lateral branch).
Plant material and culture
The plant material used was a genotype with leaves of an intermediate size from an ecotypic collection of Trifolium repens L. from Spain (AgResearch Grasslands Accession number C1067). It was selected for this study because of its tendency to remain fully vegetative under warm glasshouse conditions irrespective of photoperiod (Thomas, 1982). Its branching response to an absence of locally positioned nodal roots in preliminary experiments was similar to that in five other genotypes spanning the range from a small‐leafed Swedish high‐altitude ecotype to a large‐leafed cultivar, Tamar (R. G. Thomas, pers. comm.). Glasshouse‐grown stock plants of the chosen genotype maintained at temperatures above 12 °C through the previous winter supplied all cuttings for the experiment and these grew throughout the experimental period (spring–early summer) without flowering.
Twelve stolon tip cuttings, each with an apical bud and two emerged phytomers, were taken on 23 Sep. 1998 and planted singly into 3 l polyethylene planter bags filled with a commercially obtained 60 : 40 peat moss : coarse sand mix supplemented with Osmocote fertilizer (1·5 kg m–3), dolomite (3 kg m–3), superphosphate (1 kg m–3) and trace elements (125 g m–3). Each cutting was inserted through a hole in the side of a bag made 5 mm below the soil surface so that its apical bud was positioned just outside the bag, and root systems formed at the two nodes in the potting mix. By 19 Oct. 1998 (after 26 d of growth), primary stolons had extended out by four internodes from their planter bags. At this stage any branches that had developed on either of the two basal rooted nodes were excised. Each planter bag was then placed so as to abut with trays (400 × 300 × 60 mm) containing the same potting mix so that the stolons could grow out over the soil surface.
Throughout the experiment plants were grown in a temperature‐controlled glasshouse receiving natural photoperiods and gradually increasing levels of incident photosynthetically active radiation. Mean daily maximum and minimum temperatures were 26 and 14·5 °C until the end of phase 1, and 28 and 15·3 °C, respectively, from that time until the end of phase 2.
Experimental design
The 92 d experiment was conducted in two distinct phases. Phase 1 extended over 66 d and comprised a 26‐d establishment period followed by 40 d of imposed experimental treatment beginning on 19 Oct. 1998. On this day six plants were randomly assigned to each of two treatments that either permitted (+R) or prevented (–R) nodal root formation on the stolons growing out over the soil in the trays. Treatments during this phase involved either (1) growth of the stolons in direct contact with the moist soil surface so that nodal roots formed at each node (+R), or (2) growth of the stolons out over plastic netting (20 × 20 mm grid) that elevated them approx. 3 mm above the soil surface thereby preventing the outgrowth of nodal roots (–R).
To test whether changing light and temperature environments during phase 1 influenced the developmental response of buds to the presence or absence of nodal roots, the rooting treatment of half the plants receiving each of the phase 1 treatments was changed from 28 Nov. 1998 onwards so that there were three replicate plants in each of four treatments during a 26 d second phase ending on 24 Dec. 1998 (Fig. 2). For the +R plants of phase 1, the treatments either allowed nodal rooting to continue (+R) or prevented it (+R–R). For –R plants in phase 1, the treatments either continued to prevent nodal rooting (–R), or a single nodal root was grown at the first phytomer to emerge during phase 2 (–R+1R). It is important to note that in phase 2 the +1R treatment—where a single nodal root was permitted to develop—differed from the +R treatment in which nodal roots were permitted to form at all phytomers, and was therefore closely similar to the phase 1 –R treatment.
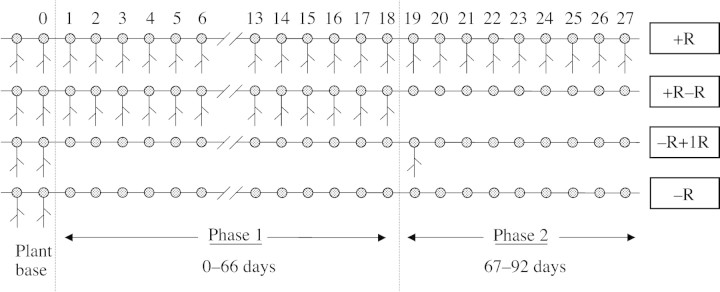
Fig. 2. A schematic representation of the position of nodal roots on the primary stolon of plants of the four nodal rooting treatments following a common establishment procedure which provided all plants with two nodal root systems at the base of the plant. The phytomer numbering system is shown and the two treatment phases of the experiment are depicted. During phase 1, plants were either allowed to form nodal roots (+R) or were prevented from doing so (–R). In phase 2, +R plants of phase 1 were either permitted (+R) or prevented (+R–R) from continuing to form nodal roots, whereas –R plants of phase 1 were either permitted to form a nodal root system at the first phytomer to emerge in phase 2 and then prevented from forming further nodal roots (–R+1R), or prevented from forming nodal roots throughout (–R).
Plant assessments and measurements
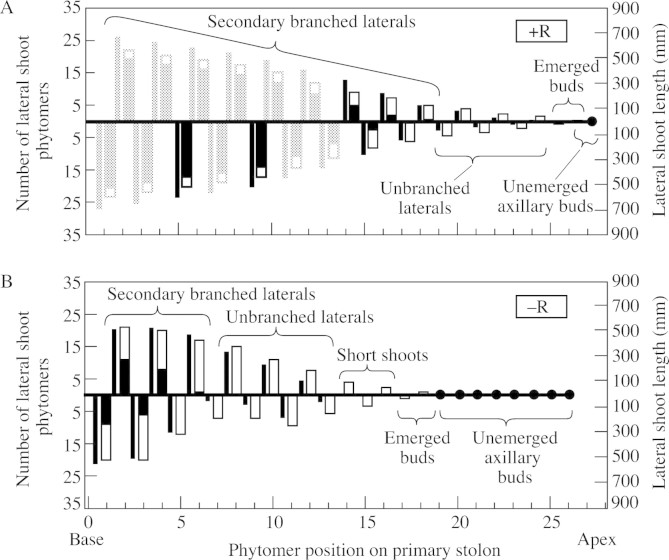
Within the primary stolon, phytomers were numbered acropetally starting with the youngest of the two basal rooted phytomers (phytomer 0), so that the first phytomer to form after the youngest rooted phytomer on the primary stolon was designated phytomer 1 and successive phytomers were termed phytomer 2, 3, etc. Branches were similarly identified by the position of their phytomer of origin on the primary stolon. Following the start of treatments on 19 Oct. 1998, development of axillary tissues was recorded weekly as follows. At each phytomer along the primary stolon, axillary bud development was assessed by counting the number of phytomers that had emerged from its apical bud and by measuring lateral shoot length. Secondary lateral shoots arising on a lateral branch were similarly assessed. These data were utilized to categorize the branch development at each phytomer position of the primary stolon according to the definitions given above, e.g. unemerged axillary bud, emerged axillary bud, lateral branch, short shoot, etc. Adjacent phytomers on the primary stolon within a given category were then grouped and identified as a developmental region (Fig. 3).
Fig. 3. Development of lateral shoots after 92 d, showing their length (narrow bars), number of unbranched phytomers (open portion of wide bars) and number of branched phytomers (solid portion of wide bars) at each phytomer position along the primary stolon of (A) plants permitted to form nodal roots (+R) and (B) plants prevented from forming nodal roots (–R). (Solid circles represent unemerged axillary buds at phytomers on the primary stolon.) On the large +R plants only positions 5 and 9 of the first 13 phytomer positions were assessed for branch development; the positions shaded lightly are estimated values. Regions of branch development along the primary stolon are indicated.
Data analysis
Means and standard errors were calculated independently for phytomer number and shoot length of the axillary tissues originating at each phytomer position on the primary stolon. Similarly, for each phytomer position, the means and standard errors of the number of secondary lateral shoots on the lateral branch and the sum of the lengths of secondary lateral shoots were calculated independently.
The curves in Fig. 6 depicting the changing rates of emergence and elongation of lateral shoots on the primary stolon and on lateral branches were fitted by Genstat (2001) to the data relating the stage of axillary bud development to its phytomer position on the primary stolon. The exponential function used (y = a + brx; where a and b are linear constants, r is a nonlinear parameter and x is the phytomer position) represented the delay and finite activity observed in the biological processes assessed and the curves accounted for a high percentage of the variance in the data (see Table 3).
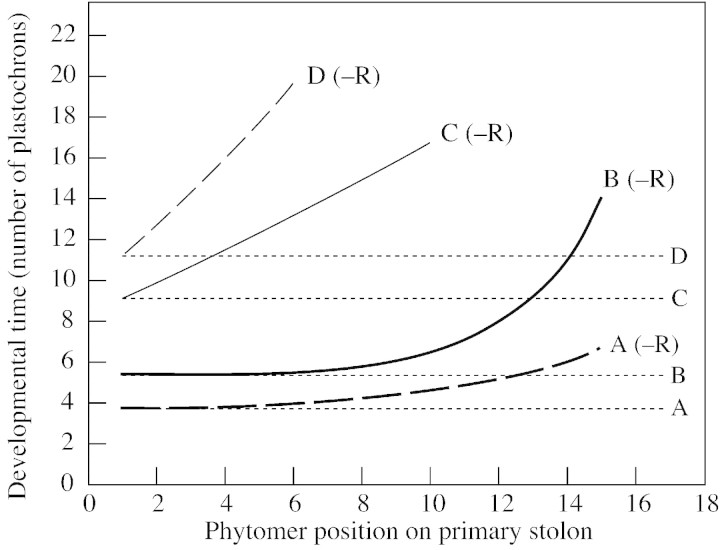
Fig. 6. The effect of the position of a phytomer on the primary stolon on the developmental time (expressed as number of plastochrons since its emergence) taken for its axillary bud to reach each of four stages of development in plants prevented from forming nodal roots (–R). The stages shown are: A, onset of axillary bud emergence from the stipular sheath (bold broken line); B, onset of lateral branch elongation (bold line), deemed to have begun when the length of its stem axis exceeded 5 mm; C, onset of emergence of secondary lateral shoots on the lateral branches (unbroken line), when leaves had emerged in the axils of two phytomers on the lateral branch; and D, onset of elongation of secondary branches on the lateral branches (broken line), when stem axes of secondary branches on a lateral branch had reached a combined length of 40 mm. Developmental times in fully rooted (+R) control plants, which were constant at all phytomer positions, are indicated by horizontal dotted lines. For each phytomer along the primary stolon axis, the number of plastochrons preceding the onset of each developmental stage was calculated from the weekly counts made of its branching status.
Table 3.
Parameter values for curves fitted to the data relating the attainment of four developmental stages of an axillary bud to its phytomer position on the primary stolon (Fig 6) using Genstat (2001)
| Parameter values | ||||
| Axillary bud developmental stage | a | b | r | Variance accounted for (%) |
| Axillary bud emergence | 3·64 | 0·09 | 1·26 | 83·2 |
| Lateral branch elongation | 5·40 | 0·02 | 1·51 | 67·7 |
| Secondary lateral shoot emergence | –25 | 33 | 1·02 | 83·4 |
| Secondary branch elongation | –15 | 25 | 1·06 | 74·6 |
All curves are of the form y = a + brx.
RESULTS
Effect of nodal roots on growth of the primary stolon
Comparison of plants of the +R and –R treatments showed that prevention of nodal root formation (–R) had no effect on the number of phytomers produced on the primary stolon by the end of phase 1 (after 66 d), although it did reduce primary stolon length (Table 1). However, by the end of phase 2 (92 d), there was a tendency (P < 0·057) for –R plants to have fewer phytomers on the primary stolon than +R plants.
Table 1.
Mean (± s.e.) number of phytomers after 66 (end of phase 1) and 92 d (end of phase 2), and length (after 66 d) of primary stolons of plants that either formed nodal roots (+R) or were prevented from doing so (–R)
| Number of phytomers on primary stolon | Stolon length (mm) | ||
| Root treatment | 66 d (n = 6) | 92 d (n = 3) | 66 d (n = 6) |
| +R | 18·2 ± 0·75 | 26·3 ± 0·58 | 392 ± 7·9 |
| –R | 17·7 ± 0·47 | 24·7 ± 0·58 | 360 ± 18·7 |
| t‐value | 1·34 | 3·02 | 3·80 |
| Probability of significance | 0·217 | 0·057 | 0·009 |
Means were compared by two‐sample t‐tests and the t‐value and its probability of significance are presented.
Effect of nodal roots on lateral shoot development
Plants grown with all phytomers rooted exhibited unrestricted growth of lateral branches, the rate of growth of each being approximately the same as that of the primary stolon (Fig. 3A). For instance, at phytomer position 18 on the primary stolon, which bore the youngest secondarily branched lateral branch at the end of the experiment, there were five emerged lateral branch phytomers, whereas at phytomer position 1, which had formed 18 phytomers earlier, there were 23 emerged lateral branch phytomers. Thus, for every new phytomer emerging from the primary stolon apical bud, one new one also emerged on each of the lateral branches.
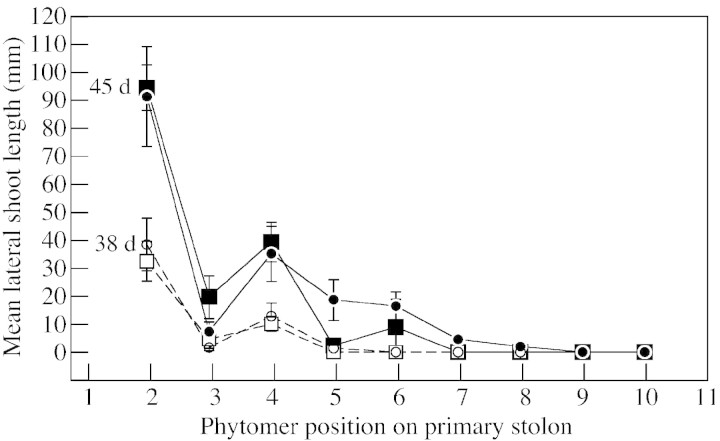
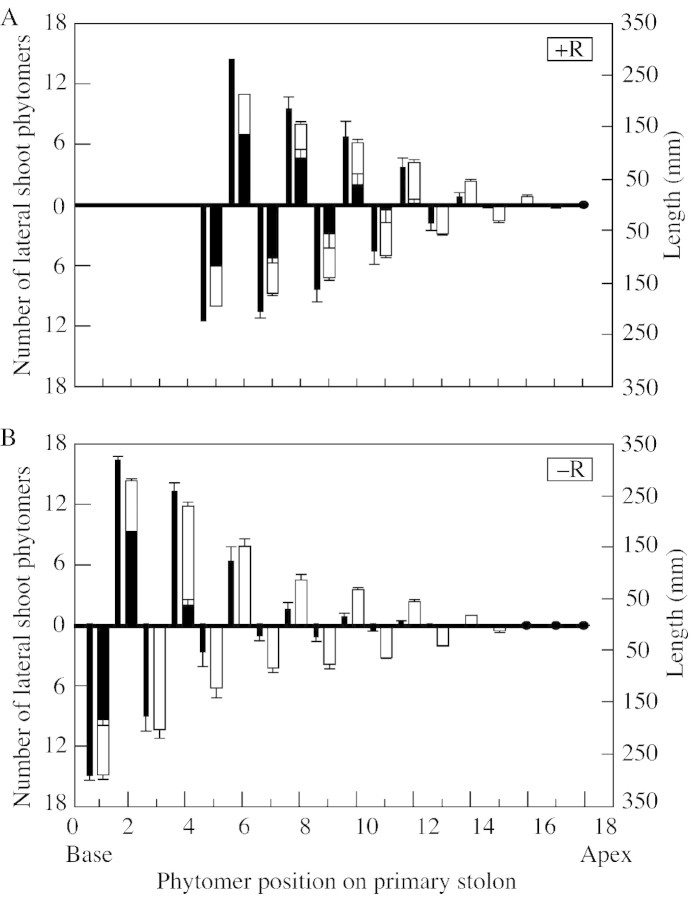
Lack of locally positioned nodal roots strongly retarded the growth of lateral shoots (Fig. 3B), reducing both their phytomer number and length, with the effect being greatest for length. Reduced internode elongation of lateral shoots following emergence became measurable from phytomer position 5 onwards, after 45 d, when the primary stolon had ten non‐rooted emerged phytomers, but was not detectable at 38 d when only eight phytomers had emerged (Fig. 4). By day 66, a region of short shoots was forming between the ‘emerged axillary bud’ and ‘unbranched lateral’ regions, the lateral shoots at positions 13 and 14 showing heavily reduced internode extension (Fig. 5). Three aspects of the developmental trajectory of short shoots in relation to that of comparably positioned lateral branches in rooted plants are shown in Table 2. First, as the phytomer position on the primary stolon increased, the delay in outgrowth of axillary buds to form lateral shoots increased in –R plants relative to +R plants, there being no delay at position 7 but over a week’s delay at position 15. Secondly, short shoots had a rate of phytomer production that was less than half that of lateral branches at comparable phytomers of the +R treatment. Thirdly, the data demonstrate that a short shoot can develop into an unbranched lateral stolon: by day 92 the short shoots previously present on primary stolon phytomers 7 and 9 were 40 and 70 mm long, respectively.
Fig. 4. The length of the lateral shoot at each phytomer position along the primary stolon of plants after 45 (solid symbols) and 38 (open symbols) d growth. Primary stolons were either permitted to form nodal roots (circles) or prevented from doing so (squares). Vertical bars indicate ± s.e.m.
Fig. 5. Development of lateral shoots at the end of phase 1 (66 d), showing their length (narrow bars), number of unbranched phytomers (open portion of wide bars) and number of branched phytomers (solid portion of wide bars) at each phytomer position along the primary stolon of (A) plants permitted to form nodal roots (+R) and (B) plants prevented from doing so (–R). (Solid circles represent unemerged axillary buds at phytomers on the primary stolon.) Bars indicate s.e.m. (The first‐formed four large branches of the +R plants were not assessed at this time.)
Table 2.
Demonstration of the development of short shoot characteristics by comparison of the number of emerged phytomers on lateral shoots at phytomer positions 7, 9 and 15 on the primary stolon of plants either freely rooting (+R) or prevented from forming nodal roots (–R) at a range of assessment times
| Assessment time (days after plant establishment) | |||||||||
| 38 | 45 | 52 | 59 | 66 | 72 | 79 | 86 | 92 | |
| Phytomer 7 | |||||||||
| +R | 0 | 1(0) | 3(22) | 8(190) | |||||
| –R | 0 | 1(0) | 2(0) | 3(0) | 7(40) | ||||
| Phytomer 9 | |||||||||
| +R | – | 0 | 2(10) | 6(162) | |||||
| –R | – | 0 | 1(0) | 3(0) | 7(70) | ||||
| Phytomer 15 | |||||||||
| +R | – | – | – | 0 | 2(7) | 3(27) | 4(116) | 7(215) | 9(290) |
| –R | – | – | – | 0 | 0 | 1(0) | 2(0) | 3(0) | 3(0) |
The length (mm) of lateral shoots developing at each phytomer position is shown in parentheses for each assessment time. (– indicates that the phytomer had as yet not emerged from the apical bud.)
Effect of phytomer position relative to the youngest root on lateral shoot development
In fully rooted plants (+R), the developmental time (expressed in plastochrons) taken to attain each stage of bud development was the same at each phytomer position on the primary stolon regardless of its distance from the basal rooting system (Fig. 6). The interval between phytomer emergence on the primary stolon and emergence of its axillary bud was 3·8 plastochrons; the interval between phytomer emergence and the onset of elongation of its lateral branch (see Fig. 6 caption) was 5·5 plastochrons; the interval between phytomer emergence and emergence of secondary branches on its lateral branch was 9·1 plastochrons; and that between phytomer emergence and the start of elongation of its secondary branches was 11·2 plastochrons.
In plants lacking nodal roots (–R), the interval between the emergence of a phytomer and the attainment of the various stages of development of its axillary tissues increased with distance from the basal root systems. This resulted in exponential relationships between each of the four defined developmental stages of the axillary bud tissues (see Fig. 6) and phytomer position of the bud along the primary stolon, the characteristics of which are detailed in Table 3. For example, the interval between the emergence of a phytomer and the start of elongation of its lateral branch (Fig. 6, fitted line B) increased from 5·5 plastochrons at phytomer position 1 to 5·7 plastochrons at phytomer position 6, 6·3 at position 10 and 10·9 at position 14. This effect was smallest for the earliest stage, axillary bud emergence, and increasingly great for the later stages. For instance, compared with +R plants, the delay in attainment of each stage in –R plants at phytomer position 6 was 0·2 (4·0 in –R plants minus 3·8 in +R plants) plastochrons for axillary bud emergence, 0·2 (5·7 – 5·5) plastochrons for lateral branch elongation, 3·9 (13·0 – 9·1) for secondary branch emergence and 8·1 (19·3 – 11·2) for secondary branch elongation.
Figure 6 demonstrates that secondary branching of the lateral branches was much more severely affected than was lateral branching of the primary stolon. Within the time span of the experiment, in which 27 phytomers emerged on the primary stolon over a period of 92 d, the stage of secondary branch elongation (Fig. 6, fitted line D) was reached only at the first six phytomers to emerge on the primary stolon, whereas elongation of primary branches (fitted line B), although severely delayed (by nine plastochrons), took place at all phytomer positions up to and including position 15. Failure of secondary lateral shoots on lateral branches to elongate into secondary branches led to their development as short shoots at phytomers on the primary stolon from position 7 onwards, thereby maintaining the lateral branches at these positions in the unbranched category.
Effect of phase 2 crossover treatments
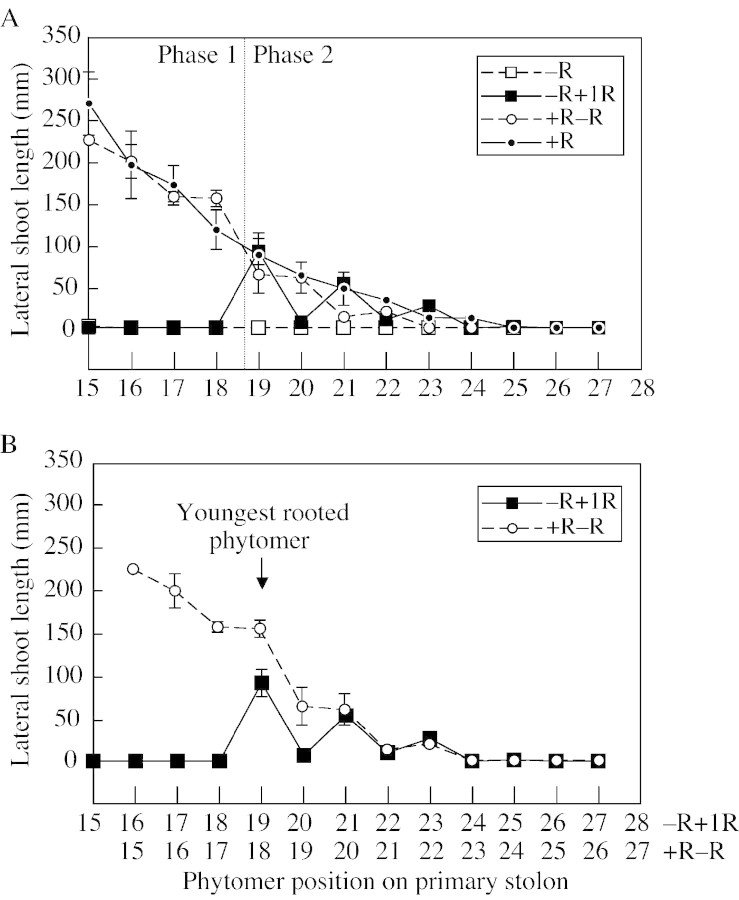
Changing the rooting treatment of plants at the end of phase 1 had a measurable effect both on the branch of the first treated phytomer (number 19) of phase 2 growth and on branches of the more distal phytomers by day 92 at the end of phase 2 (Fig. 7A). The response in +R–R plants was a retardation in lateral branch development compared with that in the +R treatment, the sum of all mean branch lengths at phytomers 19 to 25 being 161 and 264 mm, respectively. In the –R+1R treatment, the sum of all branch lengths at phytomers 19 to 25 increased to 197 mm from 0 mm in the –R treatment. Comparison of phase 2 branch development of +R–R and–R+1R plants shown in Fig. 7A is made difficult by the fact that the youngest rooted phytomer in the +R–R treatment was at phytomer 18, whereas the single rooted node on the –R+1R plants formed at phytomer 19 (see Fig. 2). To facilitate comparison, the branch lengths are presented in Fig. 7B with the youngest rooted phytomers aligned on the x‐axis. This realignment shows that branch development distal to the youngest nodal root of the +R–R and –R+1R plants was strikingly similar in phase 2.
Fig. 7. Length of lateral shoots at the completion of the experiment (day 92) at the last four phytomer positions on the primary stolon formed in phase 1 and all phytomer positions formed in phase 2. A, Length for each of the four rooting treatments (see Fig. 2 for treatment descriptions). B, Length for the –R+1R and +R–R treatments shown with the position of their youngest nodal root on the primary stolon (at phytomer positions 19 and 18, respectively) aligned on the x‐axis so as to facilitate comparison of the length of shoots formed at distal phytomers. Vertical bars represent ± s.e.m.
Comparison of the pattern of lateral shoot development in phases 1 and 2
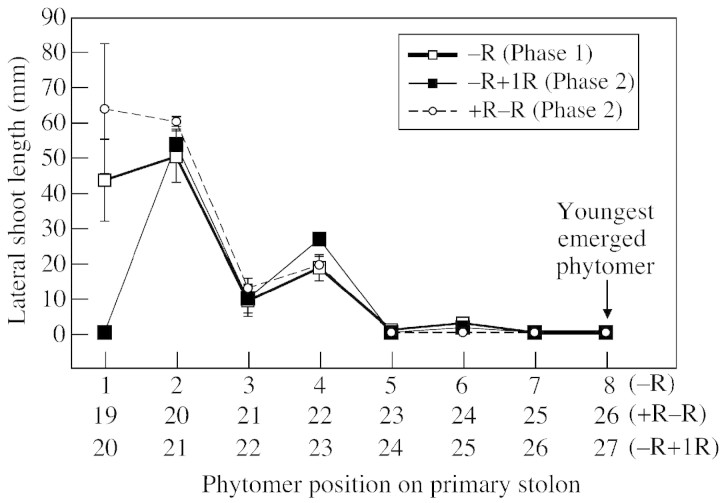
Branch development in plants at an equivalent developmental stage, with eight phytomers emerged distal to the youngest nodal root on the primary stolon, was compared in –R plants in phase 1 and +R–R and –R+1R plants at the end of phase 2 (Fig. 8). Because the rate of growth was higher in phase 2 than in phase 1, this stage, which was attained after 26 d in phase 2, was not reached until day 40 in phase 1. Despite this difference, the response to the lack of nodal roots was similar in phase 1 and in both phase 2 treatments. The difference in age of the basal nodal root systems (40 vs. 92 d) and a similar difference in age of basal stem tissue, in addition to an increase in the quantity of stem tissue, had little effect on branching architecture.
Fig. 8. Lengths of lateral shoots after emergence of the first eight non‐rooted phytomers of the primary stolon distal to the youngest nodal root in phase 1 for the –R treatment and after comparable development of eight non‐rooted phytomers in phase 2 for the +R–R and –R+1R treatments. Vertical bars represent ± s.e.m.
DISCUSSION
This study describes the successive stages of development of branches from axillary buds in fully rooted plants grown in near optimal light, temperature, water and nutrient conditions without interplant competition, defoliation or intra‐plant competition from branch systems associated with basal nodal roots (Solangaarachchi, 1996). It then describes the way in which this developmental pathway differs when nodal root formation is prevented as plants grow out from a rooted base (Fig. 3).
Although the information presented is restricted to the responses obtained from a single genotype with leaves of an intermediate size, similar responses have been obtained in preliminary experiments with a range of diverse genotypes. Hence, it is likely that the responses reported in this study are generic for the species.
Branching architecture
Under the favourable conditions of this experiment, the primary stolon of fully rooted plants produced approx. 2·5 phytomers per week and maintained a stable branching architecture. This consisted of a relatively constant distal zone of eight or nine phytomers of developing axillary buds and lateral branches and an ever‐extending basal region of phytomers with secondarily branched lateral branches. Immediately proximal to the apical bud was one phytomer with an unemerged axillary bud, and proximal to that a region of two phytomers with emerged buds (see Fig. 3A). Proximal again there was a region of five or six phytomers supporting unbranched lateral branches, and at the base of the primary stolon a region of phytomers supporting secondarily branched lateral branches that increased by one phytomer for each plastochron. Thus, in fully rooted plants, following their emergence from the apical bud, phytomers passed successively from the unemerged axillary bud region into the emerged axillary bud region after one plastochron, then after two more plastochrons into the unbranched lateral branch region and thence, after a further five to six plastochrons, into the secondarily branched lateral branch region. During this developmental sequence, the number of phytomers along each lateral shoot increased at approximately the same rate as did the number on the primary stolon, with the result that the branching architecture of the plants gave them the appearance of two‐dimensional fir trees. This developmental pattern suggests that apical dominance plays no role in the regulation of lateral shoot development in fully rooted plants.
An absence of nodal roots along the length of the primary stolon resulted in very significant modification of the pattern of branch development described above for rooted stolons, with the differences becoming increasingly pronounced as the number of phytomers between the basal root system and the apical bud of the primary stolon increased. Prevention of further nodal rooting in a plant induced the development of a distinctive branching pattern that was characterized by the formation of five clearly defined regions of branch development along the primary stolon (see Fig. 3B). In 92‐d‐old plants that had produced 25 phytomers distal to the youngest root system, the first region of development (unemerged axillary buds) extended over the eight phytomers proximal to the apical bud. In rooted plants there was only a single phytomer in this region.
The second region (emerged axillary buds), immediately proximal to the unemerged axillary buds, consisted of those phytomers on which buds had less than two fully expanded leaves and the mean internode length on their stem axes was less than 10 % of that of the proximal internode on the primary stolon. In plants where nodal root formation was prevented, there were two or three phytomers in this region at day 92.
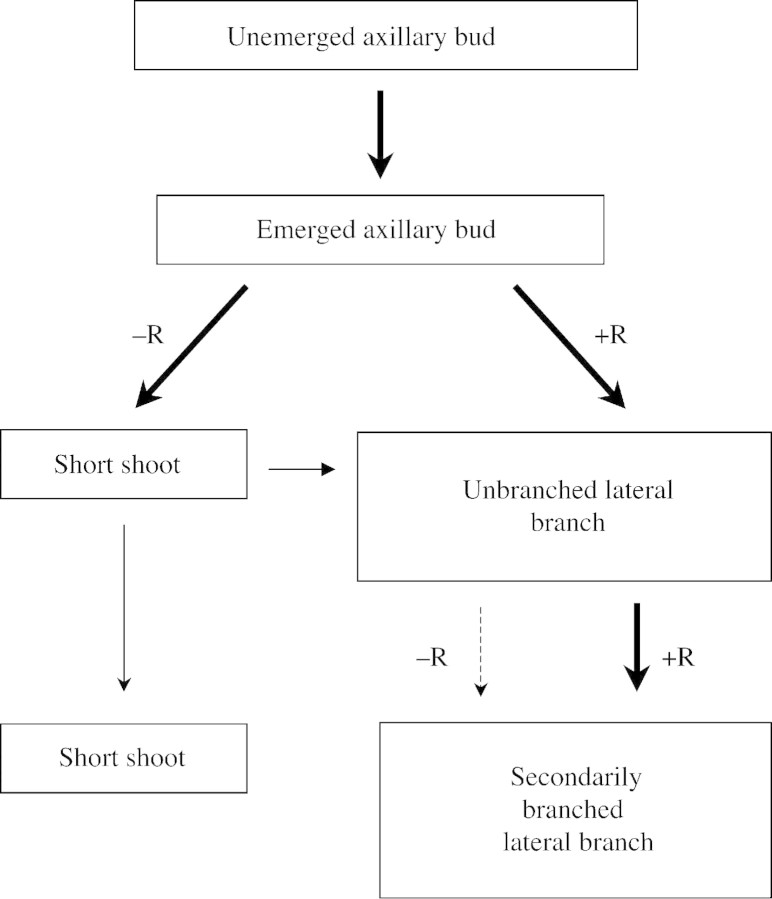
The third region (short shoots), which comprised three to six phytomers of the primary stolon in 92‐d‐old plants, was located proximal to the emerged axillary bud region (Fig. 3B). As in the case of the short shoots typical of so many woody plants (e.g. Goebel, 1905; Hallé et al., 1978), with which they appear homologous, the defining characteristic of shoots of T. repens in this category is their extremely reduced internode elongation. In addition, the leaves on short shoots, commonly up to five, had reduced lamina area and petiole length compared with those on the primary stolon or lateral branches (Fig. 1D and E). Short shoots also had a phytomer appearance rate that was approximately half that of elongating lateral branches (Table 2). The first short shoots to form, those at phytomer positions 7–12 on the primary stolon, remained as such for a period of 2 weeks or more, but then developed into lateral branches (see Table 2). However, their rate of development into lateral branches decreased as the number of phytomers between them and the youngest nodal root increased (Fig. 6). The recognition of the existence of this short shoot category as a distinct stage of retarded lateral shoot development in T. repens is important. It has not been reported before. In one previous study, Lötscher and Nösberger (1996) separated the category of ‘axillary branch’, in which there was ‘no elongation’ of internodes, from that of lateral branches in which elongation of internodes did occur. In that case, however, ‘axillary branch’ was used as an all‐embracing term to cover both emerged axillary buds and short shoots. Failure to distinguish between these two developmental categories led to fully rooted plants being described as producing ‘axillary branches’ even though they would not have possessed short shoots. The present study shows short shoots to represent a very distinct category of retarded axillary shoot elongation, occurring between axillary bud emergence and lateral branch elongation. This occurs in plants lacking nodal roots along their primary stolon but is absent in fully rooted plants grown with unlimited resources.
Thus the development of an axillary bud into a lateral branch follows one of two pathways as shown in Fig. 9. In plants with all phytomers fully rooted, the short shoot stage is bypassed and emerged axillary buds develop directly into elongating lateral branches. In the absence of nodal roots on the primary stolon, buds develop to the short shoot stage from which, under some conditions, they proceed slowly to elongate into lateral branches or where they can remain for at least several weeks. Such failure of short shoots to grow into lateral branches was demonstrated in –R plants retained in the glasshouse for 3 months after the completion of the present experiment.
Fig. 9. Possible developmental pathways of lateral shoot formation from a vegetative axillary bud in Trifolium repens, as affected by the presence (+R) or absence (–R) of nearby nodal roots, illustrating pathways involving short shoots.
The number of phytomers in the fourth (unbranched lateral) and fifth (secondarily branched lateral) regions on the primary stolon of –R plants differed from those in the corresponding regions of fully rooted plants. Unbranched lateral branches were positioned 6–13 phytomers distal to the youngest nodal root and, in comparison with similarly positioned branches on rooted plants, formed slightly fewer phytomers and were shorter (Fig. 3). The four to six phytomers bearing secondarily branched lateral branches immediately distal to the youngest nodal root constituted the fifth region in the 92‐d‐old –R plants. The degree of secondary branching on these lateral branches was less than that on comparable branches on freely rooted plants (see Fig. 3).
In –R plants, the branching regions described showed some overlap with neighbouring regions. For instance, a short shoot was sometimes present between two unbranched lateral branches at the distal end of that region. In general, branch development was more retarded at phytomers orientated to the side of the primary stolon opposite to that bearing the youngest basally positioned nodal root, and more vigorous at those phytomers orientated to the same side. This pattern of alternate high and low values for successive phytomers was particularly evident with respect to length of lateral branches, and occurred in both phase 1 (Fig. 5) and phase 2 (Fig. 7). Such a response pattern is consistent with a sectorial response associated with the known xylem vascular connections of a nodal root to the lower vascular bundle sympodium on the same side as the root (Thomas, 1987a) and a limited capability to move xylem translocates to the opposite side of the stolon (Sackville Hamilton and Hay, 1998).
Comparison of the branching architecture of plants either fully rooted or totally without nodal roots along the lengths of their primary stolons during growth in phase 2 revealed differences in the flux of phytomers between the branching regions. In established rooted plants there was a steady basipetal flow of phytomers through the branching regions such that, with each new emerging phytomer, one new phytomer was added to the distal end of each region and the basalmost phytomer of each region progressed developmentally into the next branching region. This resulted in the region of secondarily branched lateral branches increasing in size by one phytomer upon the emergence of each new phytomer from the primary stolon apical bud, whereas all other regions maintained a constant number of phytomers. Over a period of several weeks, all of the increase in overall phytomer number occurred in the region of branched lateral branches. In contrast, when nodal rooting was absent along the primary stolon, this developmental flux was slowed, with the result that during phase 2, over 60 % of the increase in phytomer number of the primary stolon accumulated in the short shoot and axillary bud regions, 35 % in the unbranched lateral region and only 5 % in the branched lateral region.
Chronology of the responses of the processes of branch development to an absence of nodal roots
Theoretically, manipulation of nodal root frequency of plants could affect branching architecture via effects on any combination of the processes affecting branching, namely maintenance of axillary bud viability (Newton and Hay, 1995), emergence of axillary buds, lateral shoot elongation rate and rate of phytomer production on lateral shoots. As all axillary buds remained viable under the near optimal glasshouse conditions used in this experiment (see Newton and Hay, 1996), maintenance of axillary bud viability could be ruled out as a process influencing branching architecture. Similarly, as the rate of phytomer production on lateral shoots was retarded only when they were present as short shoots, and all elongating lateral branches had a phytomer production rate equal to that of the primary stolon (see Fig. 3), this also could be eliminated as a major factor determining branching architecture. Thus, axillary bud emergence and lateral shoot elongation were deemed to be the most significant processes determining branching architecture, and their changes over time were evaluated using sequential assessments of branching (Fig. 6).
The first response to growth of the primary stolon apex away from the basal root system in –R plants, identifiable at phytomer position 5 after 45 d when the primary stolon had produced ten unrooted phytomers (Fig. 4), was reduced elongation of newly formed lateral branches. The second response, a delay in axillary bud emergence at newly emerged phytomers on the primary stolon, became detectable shortly after this. By 52 d, the primary stolon had 11–12 unrooted phytomers and short shoots had formed at phytomers 7 and 8. Delayed secondary branch emergence on lateral branches was observed after 13–14 unrooted phytomers had formed on the primary stolon. This response was followed by retarded elongation of secondary branches and short shoot formation on the lateral branches. Thus, lateral branches in the region of the secondarily branched laterals on the primary stolon had a reduced proportion of phytomers bearing secondary branches compared with those of rooted plants, and they ultimately exhibited the five branching regions described for the primary stolon. The largest unbranched lateral branches had two or three times the number of phytomers occurring on those of rooted plants. Overall there was increasing sensitivity to lack of nodal roots as axillary bud development progressed from emergence to lateral shoot elongation and, ultimately, to the formation of secondary branches (Fig. 6). The presence of nearby nodal roots was more important for the completion of the later stages of branch development than for the early stages.
Predictability of responses
Several principles can be derived from the treatments that involved transfer of plants from +R to –R conditions and vice versa. First, for plants in the –R+1R treatment, the similarity of branching architecture in phase 1 (when they were –R plants) and in phase 2 after the same number of plastochrons of non‐rooted growth distal to a root system (Fig. 8) establishes that it is the presence or absence of nodal roots at any phytomer that leads to modification of shoot branching architecture at neighbouring distal phytomers. Similar, very repeatable, patterns of modified branching in response to restriction of nodal rooting to every sixth or 12th phytomer of the primary stolon described by Lötscher and Nösberger (1996) also clearly linked an absence of local nodal roots to reduced branching potential.
Secondly, the similarity of the branching architecture of +R–R and –R+1R plants during phase 2 growth (Fig. 8) indicates that the number of basally positioned root systems has little effect on the branching architecture of the subsequently formed non‐rooted shoot system. The contrast between the treatments in the number of nodal root systems was profound. In addition to the two root systems at their base, the +R–R plants had root systems at each of the 18 phytomers along their primary stolons as well as on their lateral branch systems, whereas the –R+1R plants had only the single nodal root system at phytomer 19 on their primary stolons.
Thirdly, the branching architecture of the first eight phytomers of the primary stolon formed in the absence of nodal roots in phase 1 was similar to that of the comparable eight phytomers formed in phase 2 (see Fig. 8). The combination of very much larger basal root and stolon systems and improved growth conditions (higher temperatures and longer days) during phase 2 had little effect on branching architecture. A similar, very repeatable, pattern of branching architecture along stolons in response to treatments varying the frequency of nodal rooting under conditions of constant daylength and temperature was reported earlier by Lötscher and Nösberger (1996). The reason that the differences in size of basal root systems and environmental growth conditions between phases 1 and 2 had little effect on branching architecture in this study was therefore probably that neither factor had an influence, rather than that there was an interactive effect of the two factors.
Mechanisms controlling architectural modification
The rapid and very repeatable positive branching response to the outgrowth of a single nodal root system (–R+1R) and the subsequent diminution in response with increasing phytomer number distal to the root suggests that factor(s) necessary for branch development are supplied by nodal roots. It also suggests that this supply is limited and will support only a restricted but quantifiable extent of branch development. Two further sets of observations support this suggestion and point, in addition, to the probability that branch growth promoters necessary for axillary bud outgrowth and lateral shoot elongation are transported acropetally in the xylem from roots to shoot sinks. First, the stimulation of branching associated with the growth of a nodal root occurs only distal to the root. In the present study there was no evidence of outgrowth of axillary buds proximal to the root in plants of the –R+1R treatment (Fig. 7), and in the previous study by Lötscher and Nösberger (1996) a small increase in probability of lateral shoot development was recorded only at the single phytomer immediately proximal to a nodal root. Secondly, there was a sectorial pattern of branching response, with distal phytomers orientated to the same side of the stolon as the youngest root having more pronounced branch development than those orientated to the other side. Similar distributional characteristics measured in studies of the distribution of nutrients or dye from nodal roots have been shown to be associated with the intra‐plant xylem architecture of T. repens (Hay and Sackville Hamilton, 1996; Lötscher and Hay, 1996a, b; Sackville Hamilton and Hay, 1998).
The cause of the restriction of branch development distal to a nodal root, regardless of the age and number of root systems proximal to it, remains to be elucidated. The inference drawn from the above results is that there is limited capability of roots to supply any substances necessary for branching, either basipetally or acropetally, from ‘sufficiently’ to ‘insufficiently’ supplied sectors within plants. Acropetal movement of branch growth promoters from roots may be restricted either because the next distal nodal root system on the same side of the stolon effectively blocks further acropetal transport, or because the lateral branch originating at the same phytomer as the root is a very strong sink. The latter is a likely scenario given that the proportion of phosphate exported from a nodal root to its associated branch increases markedly as branch size increases (see Sackville Hamilton and Hay, 1998).
ACKNOWLEDGEMENTS
The authors thank Jocelyn Tilbrook and Fred Potter of AgResearch Grasslands for technical assistance and statistical advice, respectively. We thank Harry Clark for constructive comments on an earlier draft. Funding from the New Zealand Foundation for Research and Technology (Contract C10826) is gratefully acknowledged.
Supplementary Material
Received: 25 March 2002; Returned for revision: 20 May 2002; Accepted: 11 June 2002 Published electronically: 5 August 2002
References
- BarlowPW.1989. Meristems, metamers and modules and the development of shoot and root systems. Botanical Journal of the Linnean Society 100: 255–279. [Google Scholar]
- BrockJL, Albrecht KA, Tilbrook JC, Hay MJM.2000. Morphology of white clover during development from seed to clonal populations in grazed pastures. Journal of Agricultural Science 135: 103–111. [Google Scholar]
- CarlsonGE.1966. Growth of white clover leaves – developmental morphology and parameters at ten stages. Crop Science 6: 293–294. [Google Scholar]
- ChapmanDF.1983. Growth and demography of Trifolium repens stolons in grazed hill pastures. Journal of Applied Ecology 20: 597–608. [Google Scholar]
- Genstat.2001. Version 5·42. Lawes Agricultural Trust, Rothamsted Experimental Station.
- GoebelK.1905. Organography of plants. Part II. Special organography. Oxford: Oxford University Press. [Google Scholar]
- HalléF, Oldeman RAA, Tomlinson PB.1978. Tropical trees and forests, an architectural analysis. Berlin: Springer‐Verlag. [Google Scholar]
- HayMJM, Sackville Hamilton NR.1996. Influence of xylem vascular architecture on the translocation of phosphorus from nodal roots in a genotype of Trifolium repens during undisturbed growth. New Phytologist 132: 575–582. [DOI] [PubMed] [Google Scholar]
- JonesM, Sackville Hamilton NR.1993. Influence of rooting on stolon branching in white clover. Proceedings of the XVII International Grassland Congress, 161–162. [Google Scholar]
- KemballWD, Marshall C.1994. The significance of nodal rooting in Trifolium repens L.: 32P distribution and local growth responses. New Phytologist 127: 83–91. [DOI] [PubMed] [Google Scholar]
- KnightWE.1953. Interrelationships of some morphological and physiological characteristics of Ladino clover. Agronomy Journal 45: 197–199. [Google Scholar]
- LötscherM, Hay MJM.1996a Distribution of mineral nutrient from nodal roots of Trifolium repens: genotypic variation in intra‐plant allocation of 32P and 45Ca. Physiologia Plantarum 97: 269–276. [Google Scholar]
- LötscherM, Hay MJM.1996b Distribution of phosphorus and calcium from nodal roots of Trifolium repens: the relative importance of transport via xylem or phloem. New Phytologist 133: 445–452. [Google Scholar]
- LötscherM, Nösberger J.1996. Influence of position and number of nodal roots on outgrowth of axillary buds and development of branches in Trifolium repens L. Annals of Botany 78: 459–465. [Google Scholar]
- NewtonPCD, Hay MJM.1994. Patterns of nodal rooting in Trifolium repens L. and correlations with stages in the development of axillary buds. Grass and Forage Science 49: 270–276. [Google Scholar]
- NewtonPCD, Hay MJM.1995. Non‐viability of axillary buds as a possible constraint on effective foraging of Trifolium repens L. Abstracta Botanica 19: 83–88. [Google Scholar]
- NewtonPCD, Hay MJM.1996. Clonal growth of white clover: factors influencing the viability of axillary buds and outgrowth of a viable bud to form a branch. Annals of Botany 78: 111–115. [Google Scholar]
- Sackville HamiltonNR, Hay MJM.1998. Vascular architecture of a large‐leafed genotype of Trifolium repens Annals of Botany 81: 441–448. [Google Scholar]
- SolangaarachchiSM.1996. The influence of nodal roots on the growth and branching of white clover (Trifolium repens L.). Journal of the National Science Council of Sri Lanka 24: 27–36. [Google Scholar]
- ThomasRG.1982. A comparison of the effects of environment on inflorescence initiation in nine lines of white clover (Trifolium repens L.). New Zealand Journal of Botany 20: 151–162. [Google Scholar]
- ThomasRG.1987a The structure of the mature plant. In: Baker MJ, Williams WM, eds. White clover Wallingford: CABI International, 1–29. [Google Scholar]
- ThomasRG.1987b Vegetative growth and development. In: Baker MJ, Williams WM, eds. White clover Wallingford: CABI International, 31–62. [Google Scholar]
- TrautnerJL, Gibson PB.1966. Fate of white clover axillary buds at five intensities of sunlight. Agronomy Journal 58: 557–558. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.