Abstract
Seeds were obtained from seven natural populations of Acacia dealbata, three natural populations of A. mangium and a seed orchard of A. mangium, representing the natural range of the two species. Polyploids were discovered in two of the seven populations of A. dealbata. The 2C DNA amount for diploid A. dealbata (2n = 2x = 26) was 1·74 pg, and for diploid A. mangium (2n = 2x = 26) was 1·30 pg. A naturally occurring tetraploid of A. dealbata (2n = 4x = 52) had a 2C DNA amount of 3·41 pg and a naturally occurring triploid genotype had a 2C DNA amount of 2·53 pg. The use of colchicine and oryzalin was investigated as a means of producing higher frequencies of tetraploids of both A. mangium and A. dealbata for incorporation into breeding programmes. Colchicine treatment gave tetraploid frequencies up to 29 % for A. dealbata seedlings, and up to 18 % for A. mangium seedlings. In contrast, no tetraploid A. mangium was detected following oryzalin treatment, and the low frequencies of tetraploids observed in A. dealbata could be attributed to their natural occurrence.
Key words: Acacia dealbata Link., Acacia mangium Willd., colchicine, DNA amounts, flow cytometry, polyploidy
INTRODUCTION
The genus Acacia Miller (family Leguminosae, subfamily Mimosideae, tribe Acacieae) includes 1200–1300 species distributed primarily in the dry tropics. Species range in ploidy from 2n = 2x = 26 to 2n = 8x = 104 (Bennett and Leitch, 1995). A. dealbata Link. (2n = 2x = 26) is fast growing and is believed to have considerable potential for pulp and biomass. It is being considered as a plantation species within and outside its native range, for example in South America. A. mangium Willd. (2n = 2x = 26) is a more widely grown, fast growing forest tree that is important in south‐east Asia for pulp and paper production. It has been the subject of a number of hybridization programmes (Turnbull et al., 1998). However, management of both species is difficult due to their tendency to invade native woodland and cultivated areas. The invasion of native woodlands by introduced species of Acacia is becoming a serious problem, for example in South Africa, where A. mearnsii Willd., a close relative of A. dealbata, is widely planted. The need for research to address this issue was highlighted recently in the recommendations of an international workshop in Vietnam (Turnbull et al., 1998). One solution might be to plant triploid trees if they prove to have low fertility. Such sterility might arise due to defective gamete formation or endosperm failure (Ramsey and Schemske, 1998). This could be achieved via the production of tetraploids that could be backcrossed with diploids to produce a diverse population of triploids, from which elite trees could be selected. A more direct route might be to regenerate triploid shoots or somatic embryos from immature endosperm (Garg et al., 1996; Mohamed et al., 1996), but as this is technically difficult to accomplish, this method is unlikely to provide an adequate diversity of triploids for the selection of superior phenotypes.
The strategy of producing triploids of low fertility has been exploited in the production of seedless fruit, including banana (Ortiz and Vuylsteke, 1995), a Citrus hybrid (Cavalcante et al., 2000) and melon (Ezura et al., 1993). Triploids may either provide a sterility barrier or a bridge between diploid and tetraploid species (Ramsey and Schemske, 1998). The likelihood of autotriploids in Acacia having the desired level of sterility is unpredictable because of the paucity of literature available on triploids of woody Leguminosae, and so must be tested empirically.
Mukherjee and Sharma (1993) used Feulgen microdensitometry to estimate DNA amounts in 16 species of Acacia, including A. dealbata (2C value = 2·9 pg), and Mukherjee and Sharma (1995) reported a 2C DNA amount for A. mangium of 2·3 pg. In contrast, using flow cytometry with propidium iodide (PI) as the fluorochrome, Bukhari (1997) published a 2C value for A. dealbata of 1·55 pg (s.e. 0·12 pg) accompanied by a chromosome count of 2n = 26. Across the Phyllodineae subgenus, the mean 2C value was 1·58 pg for five diploid taxa, and 3·31 pg for the tetraploid A. holosericae. No polyploids of either A. mangium or A. dealbata have been reported, but Ghimpu (1929) found examples of mixoploids (4n/8n) in four species of non‐Australian tetraploid acacias: A. arabica, A. nilotica, A. horrida and A. farnesiana. The frequency of these mixoploids in natural populations has not been assessed. Ghimpu (1929) reported that three taxa of the subgenus Phyllodineae, including A. dealbata, were diploid.
As there are few reports of polyploidy within diploid species of the subgenus Phyllodineae, and none of polyploidy in A. dealbata or A. mangium, the artificial induction of tetraploids is of interest. Doubling of the diploid chromosome number has been achieved in many other genera by the use of spindle inhibitors, which disrupt mitosis by preventing microtubule polymerization and the polar migration of chromosomes at anaphase. Colchicine, one of the most commonly used spindle inhibitors, affects microtubules in late prophase, disrupts the integrity of the nuclear envelope and attaches to chromatin during late interphase (Tambong et al., 1998). A report of colchicine‐induced tetraploidy in seedlings of A. mearnsii (Moffett and Nixon, 1960) is the only one of chromosome doubling in the Acacieae. Oryzalin, a pre‐emergent dinitroalanine herbicide, has also been used to manipulate plant ploidy levels. It has been found to be less toxic and more efficient in inducing polyploidy in Musa acuminata (van Duren et al., 1996), Gerbera (Tosca et al., 1995) and in rhododendron (Vainola 2000), and is effective in plant tissues at approx. 1000th of the concentration of colchicine (Morejohn et al., 1987).
Flow cytometry enables DNA amounts to be estimated rapidly from leaf tissue. It is therefore a means by which to determine ploidy rapidly where basic information about chromosome numbers and DNA amounts of a plant group is known. Leaf tissue is a particularly convenient source of material for the study of ploidy in mature trees from which rooted cuttings, as a source of material for chromosome counts, are not easily obtained. The fluorochrome 4′,6‐diamidino‐2‐phenylindole (DAPI) has a preference for AT bases (Doležel et al., 1992) and is, therefore, not suitable for the precise measurement of DNA amounts. However, samples for flow cytometry using DAPI are easily prepared and thus this fluorochrome is widely used in ploidy studies. PI is another fluorochrome that is widely used in flow cytometry. As it shows no base preference, it is suitable for the determination of absolute DNA amounts (Doležel et al., 1992).
The aim of this study was to obtain tetraploids of A. mangium and A. dealbata that might be incorporated into a breeding programme, leading to the production of triploids of low fertility. Data are presented on the natural occurrence of polyploids, the induction of tetraploids using colchicine and nuclear DNA amounts of diploids and polyploids of both species.
MATERIALS AND METHODS
Acacia species and genotypes
Seeds were collected by CSIRO from five to 20 individuals in each of seven natural populations of A. dealbata, three natural populations of A. mangium, and a combined seedlot from an A. mangium seed orchard in Damper, Queensland, Australia (Table 1). Sites were chosen to represent the geographic range of the two species: from Armidale, NSW (30°S) to SSE Snug, TAS (43°S) for A. dealbata, and Ceram‐Piru, IND (03°S) to Captain Billy Road, QLD (18°S) for A. mangium. All trees sampled by CSIRO were mature individuals, growing at least 50–100 m apart.
Table 1.
CSIRO seedlot numbers, location and altitude of sampled populations
| Species | Seed origin | CSIRO seedlot | Latitude°S | Longitude°E | Altitude (m a.s.l.) | Parents (no.) |
| A. dealbata | 6–15KM SSE Snug, TAS | 16385 | 43°06′ | 147°14′ | 143 | 9 |
| Jamieson‐Licola Rd, VIC | 16743 | 37°28′ | 146°24′ | 1200 | 5 | |
| Maribyrnong, VIC | 17070 | 37°46′ | 144°51′ | 40 | Unknown | |
| Diddleum Plains, TAS | 18716 | 41°20′ | 147°31′ | 400 | 20 | |
| Abercrombie River, NSW | 19767 | 34°14′ | 149°47′ | 650 | 10 | |
| Armidale, NSW | 20247 | 30°19′ | 151°41′ | 900 | 20 | |
| Kandos, NSW | 18973 | 32°56′ | 149°54′ | 600 | 10 | |
| A. mangium | Damper, QLD* | 19675 | 18°25′ | 146°01′ | 50 | 8 |
| Captain Billy Rd, QLD | 18249 | 18°57′ | 146°17′ | 100 | 5 | |
| Makapa, PNG | 19611 | 07°56′ | 142°35′ | 15 | 100 | |
| Ceram‐Piru, IND | 13621 | 03°04′ | 128°12′ | 150 | 9 |
TAS, Tasmania; VIC, Victoria; NSW, New South Wales; QLD, Queensland; IND, Indonesia; * Western Province (30 m a.s.l.); PNG, provenance orchard in QLD.
Seed germination and in vitro culture
Seeds were surface sterilized in 40 % (v/v) Domestos (Lever Brothers, Kingston‐on‐Thames, UK) for 20 min, scarified by immersion in boiling water for 1 min and germinated on half‐strength semi‐solid Murashige and Skoog (1962) basal medium supplemented with 0·06 m sucrose in 30 ml coulter pots. The youngest leaves were harvested for ploidy screening after approx. 2 months. They were retested at least once after 4 months. Representative diploid and polyploid genotypes were investigated to confirm their chromosome number and DNA amount. After 6 weeks, seedlings were sub‐cultured on a semi‐solid DKW medium (Driver and Kuniyuki, 1984), supplemented with 0·06 m sucrose, 500 µg l–1 GA3(gibberellic acid) and 500 µg l–1 BAP (benzylaminopurine). To induce roots, shoot cultures were transferred to a semi‐solid half‐strength MS (1962) medium, supplemented with 0·06 m sucrose, 5 µg l–1 IAA (indoleacetic acid) and 45 µg l–1 NAA (napthaleneacetic acid). All cultures were incubated at 25 °C with a 16 h photoperiod.
Colchicine and oryzalin treatment and tissue culture
Oryzalin (Greyhound Chemicals, Birkenhead, UK) was dissolved in 100 % absolute ethanol and diluted using sterile water. Colchicine was dissolved in sterile water in a ventilated weighing station and subsequently diluted using sterile water in a Class 2 laminar flow cabinet to solutions of the required concentration. For each treatment, 40 seeds of A. mangium or A. dealbata were placed in 10 ml of the appropriate oryzalin or colchicine solution and agitated on a shaker at 150 r.p.m. for the relevant time period. Seeds were then removed and germinated on half‐strength Murashige and Skoog (1962) basal medium in 30‐ml coulter pots, with two seeds per container. The youngest leaves were harvested for ploidy screening after 2–3 months. Putative tetraploids were retested at least once after 6 months. Representative polyploid genotypes were subsequently investigated to confirm their chromosome number and DNA amount. After 6 weeks, seedlings were sub‐cultured on a semi‐solid DKW medium, supplemented with 0·06 msucrose, 500 µg l–1 GA3 and 500 µg l–1 BAP. To induce roots, shoot cultures were transferred to the rooting medium described above.
Measurements of fluorescence intensity of DAPI‐stained nuclei
Ten leaflets (approx. 100 mg) from an in vitro plant were chopped with a sharp razor blade in 1·0 ml ice cold nuclei isolation buffer (Arumuganathan and Earle, 1991). This buffer consisted of 5 mm Hepes, 10 mm magnesium sulfate hepahydrate, 50 mm KCl, 2 ml l–1 Triton X‐100 and 2·0 mg l–1 DAPI, pH 7·0. The suspension was filtered through a 40‐µm nylon gauze, mixed with 1·0 ml buffer, and incubated on ice for a minimum of 30 min. The suspension of nuclei was analysed using a PAS‐II flow cytometer with a 100 W high‐pressure mercury lamp, KG1, BG38 and UG1 filters, TK420 and TK569 dichroic mirrors and a GG435 barrier filter. This analysis was carried out by Flow Cytometry Services (Holland).
Measurements of fluorescence intensity for DNA quantification
Vigna radiata (L.) Wilczek ‘Berken’ (2C = 1·06 pg) (Bennett et al., 2000) was used as an internal standard to quantify the DNA amounts for all flow cytometric analyses using PI. Ten leaflets from each seedling, together with approx. 100 mg of leaf material of V. radiata, were chopped with a double‐edged razor blade in ice‐cold nuclei isolation buffer (LB01 lysis buffer; Doležel et al., 1992). This buffer consisted of 15 mm Tris, 2 mm Na EDTA, 80 mm KCl, 20 mm NaCl, 0·5 mm spermine, 15 mm β‐mercaptoethanol and 1 ml l–1 Triton X‐100, pH 7·5. Following maceration, the lysate was filtered through nylon gauze (pore size 40 µm) and made up to 2 ml with buffer. Ribonuclease A (2·9 µl of a 34 mg ml–1 solution) and PI (10 µl of a 10 mg ml–1 solution) were added and incubated on ice for 1 h in darkness. The samples were filtered through nylon gauze (pore size 20 µm) and fluorescence intensity was measured using a CA III flow cytometer (Partec GmbH, Münster, Germany) with a 40 × 0·8 quartz objective. An Argon laser light source (488 nm wavelength) was used with a TK420 dichroic mirror and an OR610 barrier filter. Estimates of the ratio of fluorescence intensities of V. radiata and each plant sample were based on the mean of five samples, each with a minimum of 104 nuclei, giving peaks with a coefficient of variation of 4–8 %.
Chromosome counts
Chromosome preparations were made following the squash preparation protocols of Schwarzacher and Heslop‐Harrison (2000). The tips (5 mm) of actively growing roots were immersed in 5 µm pronamide (Rom Has Co., Croydon, UK) for 3 h at 4 °C, fixed in a freshly prepared solution containing glacial acetic acid and absolute ethanol (1 : 3, v/v) for 1 h, then washed in 5 ml of citric acid buffer (pH 4·8) for 10 min. Root tips were transferred to a solution containing 2 % (w/v) cellulase (Calbiochem, San Diego, USA) and 3 % (w/v) pectinase (Calbiochem) in citric acid buffer (pH 4·8) and incubated in a moist chamber at 37 °C for 30 min. They were then transferred to citric acid buffer (pH 4·8) at room temperature for 15 min. Root caps were removed and the meristem was placed in a drop of 45 % acetic acid on a clean microscope slide, macerated and squashed between slide and coverslip. The slide was frozen in liquid nitrogen, the coverslip was removed and the slide was air‐dried. The slide was immersed in 1 % (w/v) orcein in 45 % acetic acid for 15 min, washed in distilled water, air‐dried and a coverslip was mounted in Histomount (National Diagnostics, Hull, UK). The chromosome numbers reported were based on consistent counts in at least ten cells.
RESULTS
Naturally occurring polyploids
Seedlings of Acacia dealbata were obtained from 75 trees, growing at seven sites across the natural range of the species (Table 1). Ploidy levels of a representative population of 94–150 seedlings were determined after 2 months and again after 4 months by flow cytometry using the fluorochrome DAPI. Two populations, Maribyrnong and Kandos, included a very low percentage of triploids, essentially one or two plants in each population (Table 2). However, the Kandos population also included 6·0 % tetraploids. This confirmed an earlier screen of 120 seedlings from the Kandos population, which had estimated the frequency of tetraploids to be 8·3 %. No tetraploids were found at any other site. However, the initial ploidy screen carried out after 2 months revealed a low frequency of ‘apparent’ endopolyploidy in A. dealbata. Leaves taken from single plants from Jamieson‐Licola Rd and Abercrombie River, and two plants from Diddleum Plains had ploidy levels of 2n–8n, whereas two plants from Armidale had ploidy levels of 2n–16n (Table 3). When young leaves were subsequently analysed 2 months later, only diploid cells were detected.
Table 2.
Flow cytometry ploidy screening of A. dealbata and A. mangium seedling populations, 4 months after germination
| Ploidy (%) | |||||
| Species | Origin | Rep. no. | 2n | 3n | 4n |
| A. dealbata | 6–15KM SSE Snug | 150 | 100 | 0 | 0 |
| Jamieson‐Licola Rd | 150 | 100 | 0 | 0 | |
| Maribyrnong | 150 | 98·7 | 1·3 | 0 | |
| Diddleum Plains | 94 | 100 | 0 | 0 | |
| Abercrombie River | 150 | 100 | 0 | 0 | |
| Armidale | 124 | 100 | 0 | 0 | |
| Kandos | 150 | 93·3 | 0·7 | 6·0 | |
| A. mangium | Damper | 150 | 100 | 0 | 0 |
| Captain Billy Rd | 100 | 100 | 0 | 0 | |
| Makapa | 100 | 100 | 0 | 0 | |
| Ceram‐Piru | 100 | 100 | 0 | 0 |
Table 3.
Flow cytometric analysis of young leaves of six A. dealbata seedlings showing evidence of endopolyploidy
| Ploidy frequency (%) | ||||
| Ploidy level | ||||
| Seed origin | 2n | 4n | 8n | 16n |
| Jamieson‐Licola Rd | 60 | 30 | 10 | 0 |
| Abercrombie River | 70 | 20 | 10 | 0 |
| Diddleum Plains | 70 | 20 | 10 | 0 |
| Diddleum Plains | 60 | 30 | 10 | 0 |
| Armidale | 30 | 30 | 30 | 10 |
| Armidale | 20 | 30 | 30 | 20 |
Seedlings of A. mangium were obtained from 122 trees, growing on four sites representing the extremes of the natural range of the species. Following surface sterilization and germination in vitro, ploidy levels of a representative population of 100–150 seedlings were determined by flow cytometry using the fluorochrome DAPI. There was no evidence of polyploids or endopolyploidy in any of the seedlings tested (Table 2).
Induced polyploids
Acacia dealbata was treated with colchicine at concentrations of 0, 500, 1000, 2000 and 3000 mg l–1 for time periods of 50–150 h (Table 4). Forty seeds were sown for each treatment. The germination percentage of seeds not exposed to colchicine ranged from 70–90 % (Table 4). Regardless of concentration, colchicine appeared to have little effect on germination when applied for 50 h. However, the germination percentage tended to decline with increasing levels of colchicine, and increased lengths of exposure (Table 4). Seedlings that germinated were screened by flow cytometry using DAPI after 2 months. With just two exceptions, tetraploids were obtained in every colchicine treatment. No clear pattern emerged with respect to effectiveness of a given level of colchicine or duration of application. At every concentration of colchicine tested, between eight and 14 tetraploid plants were obtained. Six individual treatments, 500 mg l–1 for 100 and 150 h, 2000 mg l–1 for 100 and 150 h, and 3000 mg l–1 for 100 and 150 h gave high tetraploid frequencies of between 22 and 30 %. The optimum time of application varied for each concentration. As tetraploids occur naturally with a frequency of 6 % in this particular provenance of A. dealbata, it is not clear how many of the tetraploids obtained following colchicine treatment may have originated from tetraploid seeds. In fact, a single triploid plant was obtained, which is unlikely to have arisen from treatment with colchicine. All tetraploid and triploid plants were checked again 4 months later using flow cytometry with DAPI to confirm their elevated ploidy level. Some natural tetraploids of A. dealbata identified by flow cytometry using DAPI, as reported here, were confirmed by DNA quantification using PI as the fluorochrome (Table 6). A large number of mixoploids were also produced in all treatments, which included 4n and 8n cells. As non‐chimeric tetraploids were obtained, the mixoploids were discarded for the purposes of the acacia breeding programmes.
Table 4.
Flow cytometric analysis A. dealbata seedlings following colchicine treatment of imbibing seeds
| Colchicine treatment | Percentage of seedlings | |||||
| Conc. (mg l–1) | Duration (h) | Germination (%) | 2n* | 3n† | 4n† | Mixoploid* |
| 0 | 50 | 90 | 94·4 | 0 | 5·6 | 0 |
| 0 | 75 | 70 | 100 | 0 | 0 | 0 |
| 0 | 100 | 85 | 94·1 | 0 | 0 | 5·9 |
| 500 | 50 | 60 | 83·3 | 4·2 | 0 | 12·5 |
| 500 | 75 | 60 | 66·7 | 0 | 8·3 | 25·0 |
| 500 | 100 | 60 | 37·5 | 0 | 25·0 | 37·5 |
| 500 | 150 | 53 | 19·1 | 0 | 23·8 | 57·1 |
| 1000 | 50 | 68 | 61·6 | 0 | 19·2 | 19·2 |
| 1000 | 75 | 75 | 63·3 | 0 | 10·0 | 26·7 |
| 1000 | 100 | 58 | 52·2 | 0 | 17·4 | 30·4 |
| 2000 | 50 | 75 | 70·0 | 0 | 6·7 | 23·3 |
| 2000 | 75 | 25 | 80·0 | 0 | 0 | 20·0 |
| 2000 | 100 | 45 | 61·1 | 0 | 22·2 | 16·7 |
| 2000 | 150 | 18 | 42·8 | 0 | 28·6 | 28·6 |
| 3000 | 50 | 83 | 65·6 | 0 | 3·1 | 31·3 |
| 3000 | 75 | 53 | 47·6 | 0 | 28·6 | 23·8 |
| 3000 | 100 | 25 | 30·0 | 0 | 30·0 | 40·0 |
| 3000 | 150 | 53 | 42·9 | 0 | 9·5 | 47·6 |
n = 40 for colchicine treatments, and 20 for controls.
* Identified after 2 months.
† Triploids and tetraploids confirmed after 6 months.
Table 6.
DNA amounts and chromosome counts of Acacia species of differing ploidy estimated by PI flow cytometry
| Species | Genotype | 2C DNA amount (pg ± s.d.) | 2n = | Ploidy indicated |
| A. dealbata | KDP 1 | 1·74 ± 0·05 | Diploid | |
| KDP 2 | 1·73 ± 0·04 | Diploid | ||
| KDP 3 | 1·74 ± 0·05 | 26 | Diploid | |
| KTR 1 | 2·53 ± 0·06 | Triploid | ||
| KTE 1 | 3·47 ± 0·09 | Tetraploid | ||
| KTE 2 | 3·50 ± 0·08 | Tetraploid | ||
| KTE 3 | 3·41 ± 0·08 | 52 | Tetraploid | |
| A. mangium | DDP 1 | 1·28 ± 0·03 | Diploid | |
| DDP 2 | 1·29 ± 0·02 | Diploid | ||
| DDP 3 | 1·30 ± 0·24 | 26 | Diploid | |
| DTE1 | 2·59 ± 0·07 | Tetraploid | ||
| DTE2 | 2·62 ± 0·07 | Tetraploid |
Treatments with colchicine were carried out on A. mangium at concentrations of 0, 250, 500, 750 and 1000 mg l–1 for 50–150 h (Table 5). Forty seeds were used for each treatment, with the exception of the controls, for which 20 seeds were treated. In contrast to A. dealbata, polyploids of A. mangium are not known to occur naturally. Colchicine was used at lower concentrations than for experiments with A. dealbata as concentrations over 1000 mg l–1 were found, in preliminary experiments, to inhibit germination. The germination percentage of seeds not exposed to colchicine ranged from 75–90 % (Table 5) and generally declined with increasing concentrations of colchicine and duration of exposure (Table 5). Seedlings that germinated were screened by flow cytometry using DAPI after 2 months. Tetraploids were obtained in all but two colchicine treatments. Similarly to A. dealbata, no clear pattern emerged with respect to effectiveness of a given level of colchicine or duration of application. Between seven and 17 tetraploid plants were obtained from each concentration of colchicine. Again, the optimum duration of application varied for each concentration. All tetraploid plants were checked again after 6 months by flow cytometry with DAPI to confirm their elevated ploidy level. Similarly to A. dealbata, a large number of mixoploids were also produced which included 4n and 8n cells, particularly in the lowest level of colchicine (500 mg l–1). As non‐chimeric tetraploids were obtained, the mixoploids were also discarded for the purposes of the acacia breeding programmes.
Table 5.
Flow cytometric analysis of A. mangium seedlings following colchicine treatment of imbibing seeds
| Colchicine treatment | Number of seedlings | ||||
| Conc. (mg l–1) | Duration (h) | Germination (%) | 2n* | 4n† | Mixoploid* |
| 0 | 50 | 75 | 100 | 0 | 0 |
| 0 | 75 | 80 | 100 | 0 | 0 |
| 0 | 100 | 75 | 93·3 | 0 | 6·7 |
| 0 | 150 | 90 | 100 | 0 | 0 |
| 250 | 50 | 93 | 54·1 | 18·9 | 27·0 |
| 250 | 75 | 80 | 53·1 | 9·4 | 37·5 |
| 250 | 100 | 45 | 44·4 | 0 | 65·6 |
| 250 | 150 | 90 | 27·8 | 19·4 | 52·8 |
| 500 | 50 | 75 | 53·3 | 13·3 | 33·4 |
| 500 | 75 | 53 | 42·9 | 0 | 57·1 |
| 500 | 100 | 40 | 37·5 | 25·0 | 37·5 |
| 500 | 150 | 23 | 33·3 | 22·2 | 44·5 |
| 750 | 75 | 63 | 36·0 | 12·0 | 52·0 |
| 750 | 100 | 45 | 27·8 | 27·8 | 44·4 |
| 1000 | 50 | 45 | 83·3 | 5·6 | 11·1 |
| 1000 | 75 | 43 | 41·2 | 29·4 | 29·4 |
| 1000 | 100 | 20 | 87·5 | 12·5 | 0 |
n = 40 for colchicine treatments, and 20 for controls.
* Identified after 2 months.
† Tetraploids confirmed after 6 months.
An experiment was set up to treat seeds of A. dealbata and A. mangium with oryzalin concentrations of 0, 4, 8, 20 and 40 mg l–1 for 50 h. Forty seeds were sown for each treatment. Seedlings of both species were screened after 3 months by flow cytometry using DAPI. There was little apparent effect of oryzalin on the germination of A. mangium seeds (Table 7). No tetraploids were found, although a single putative mixoploid was obtained that had approx. 20 % 4n cells, a possibility suggested by the evidence, referred to previously, for endopolyploidy in A. dealbata. This could have been a diploid with an unusually large number of cells in the G2 stage of the cell cycle. Germination of A. dealbata was slightly reduced at higher concentrations of oryzalin (Table 7). Two tetraploids were found at concentrations of 4 and 20 mg l–1 oryzalin, although none were produced at the highest concentration tested. Mixoploids were found in all but the control treatment.
Table 7.
Flow cytometric analysis of A. dealbata and A. mangium seedlings following oryzalin treatment of imbibing seeds for 50 h
| Percentage of seedlings | |||||
| Species | Oryzalin (mg l–1) | Germination (%) | 2n* | 4n† | Mixoploid* |
| A. mangium | 0 | 65 | 100 | 0 | 0 |
| 4 | 80 | 96·9 | 0 | 3·1 | |
| 8 | 75 | 100 | 0 | 0 | |
| 20 | 85 | 100 | 0 | 0 | |
| 40 | 83 | 100 | 0 | 0 | |
| A. dealbata | 0 | 85 | 100 | 0 | 0 |
| 4 | 85 | 61·8 | 2·9 | 35·3 | |
| 8 | 75 | 73·3 | 0 | 26·7 | |
| 20 | 63 | 92·0 | 4·0 | 4·0 | |
| 40 | 60 | 75·0 | 0 | 25·0 | |
n = 40
* Identified after 3 months.
† Tetraploids confirmed after 6 months.
DNA amounts and chromosome counts
The chromosome number in root‐tip cells of diploid A. dealbata was confirmed as 26 (Fig. 1) and the DNA amount, tested by flow cytometry using PI as the fluorochrome, was estimated as 1·74 pg (s.d. ± 0·05) (Table 6). Similar DNA amounts were found in two further diploids (Table 6). In one naturally occurring tetraploid of A. dealbata, the chromosome number was confirmed as 52 (Fig. 1) and the DNA amount was 3·41 pg (s.d. ± 0·08) (Table 6). Similar DNA amounts were found in two other tetraploids (Table 6). The 2C DNA amount of a single triploid was estimated as 2·53 pg (s.d. ± 0·06) (Table 6), approx. 50 % higher than the diploid value.
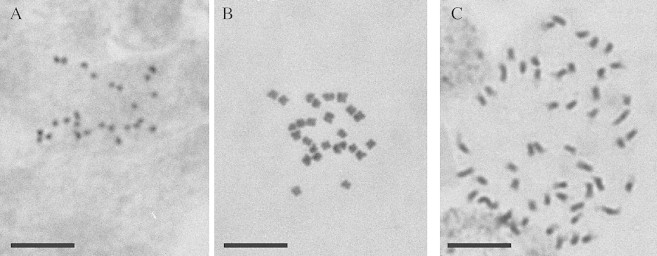
Fig. 1. Chromosomes of diploid Acacia mangium (2n = 26) (A), diploid A. dealbata (2n = 26) (B) and tetraploid A. dealbata (2n = 52) (C). Bar = 10 µm.
The chromosome number in root‐tip cells of diploid A. mangium was confirmed as 26 (Fig. 1) and the 2C DNA amount was estimated as 1·30 pg (s.d. ± 0·02) (Table 6). The 2C DNA amounts of two other diploids were found to be similar (Table 6). The 2C DNA amounts of two colchicine‐induced tetraploids were approximately double those of the diploids (Table 6).
Herbarium specimens of immature plants used for chromosome counts and estimates of DNA amounts are retained at Horticulture Research International.
DISCUSSION
Our mean estimate of 1·74 pg for the 2C DNA amount for A. dealbata is slightly higher than that of 1·55 pg (± 0·12 s.e) obtained by Bukhari (1997) for a specimen of A. dealbata, of unspecified origin, using chicken erythrocytes (CE) as a standard. However, C values for CE vary among authorities and breeds (Bennett et al., 2000), and CE show different hydrolysis curves from those of plants (Johnston et al., 1999). These problems identified in the use of CE as calibration standards have led to a recommendation that they should not be used for the estimation of plant C values (Bennett et al., 2000), and may indeed be responsible for this small discrepancy. The estimates by Mukherjee and Sharma (1993, 1995) of the 2C DNA amounts of A. dealbata and A. mangium (2·9 and 2·3 pg, respectively) are much higher than our estimates (1·74 and 1·30, respectively). However, our data, like those of Mukherjee and Sharma (1993, 1995), indicate that the DNA amount of A. dealbata is greater than that of A. mangium by a factor of about 1·3. Thus, the discrepancies may relate to differences in the use of calibration standards. The 2C DNA amount of Allium cepa (33·5 pg), which Mukherjee and Sharma (1993, 1995) used as their standard, exceeds that of Acacia by more than one order of magnitude. This disparity would have accentuated any system errors, such as non‐linearity in the measurement of transmitted light. Bukhari (1997) found major, taxonomically related differences in genome size in Acacia, the 2C DNA amounts of diploids in the subgenus Heterophyllum being larger, for example, than those of most tetraploids in the subgenus Acacia. In the present study, differences between the 2C DNA amounts of A. dealbata and A. mangium may reflect their taxonomic status as species of different sections.
The occurrence of A. dealbata triploids and tetraploids along with diploids at Kandos and triploids in the Maribyrnong population is of particular interest because there are no previous reports of intraspecific ploidy variation within the Acacieae. The triploids may have arisen as hybrids between diploids and tetraploids or from combinations of reduced and unreduced gametes of diploids. Their natural occurrence presents an opportunity for studying the fertility and vigour of triploids of varied genotypes in the wild, and the possibility of testing the hypothesis that they could be used in plantations to minimize the invasion of woodlands in exotic locations. This report has established that colchicine can be used successfully to produce tetraploid plants of both A. mangium and A. dealbata. The frequency is believed to be adequate for the requirements of a breeding programme. It is known that colchicine can induce a high percentage of chimeras (Wan et al., 1989), and this was also the case with the two acacia species. Although oryzalin has been successful in inducing polyploidy in other species, it was ineffective in the production of tetraploid A. mangium and A. dealbata. Although tetraploid individuals of A. dealbata do occur in nature, their frequency is very low, and they may be confined to particular provenances. Furthermore, propagation of mature A. dealbata can be problematical. Consequently, to provide the material necessary for breeding purposes, induction of tetraploidy is a necessary technique.
ACKNOWLEDGEMENTS
The authors thank Fiona Wilson, Karen Barrett, Helen Robertson‐Turner and Grantley Adams for their support during this study. We also thank Shell Forestry Limited for their support of this work.
Supplementary Material
Received: 1 March 2002; Returned for revision: 1 May 2002; Accepted: 18 June 2002
References
- ArumuganathanK, Earle ED.1991. Estimation of nuclear DNA content of plants by flow cytometry. Plant Molecular Biology Reporter 9: 229–233. [Google Scholar]
- BennettD, Leitch IJ.1995. Nuclear DNA amounts in angiosperms. Annals of Botany 76: 113–176. [Google Scholar]
- BennettMD, Bhandol P, Leitch IJ.2000. Nuclear DNA amounts in angiosperms and their modern uses – 807 new estimates. Annals of Botany 86: 859–909. [Google Scholar]
- BukhariM.1997. Nuclear DNA amounts in Acacia and Prosopis (Mimosaceae) and their evolutionary implications. Heriditas 126: 45–51. [Google Scholar]
- CavalcanteHC, Schifino‐Wittmann MT, Dornelles ALC.2000. Meiotic behaviours and pollen fertility in an open‐pollinated population of ‘Lee’ mandarin [Citrus clemantina × (C. paradisi × C. tangerina)]. Scientia Horticulturae 86: 103–114. [Google Scholar]
- DoleželJ, Sgorbati S, Lucretti S.1992. Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiologia Plantarum 85: 625–631. [Google Scholar]
- DriverJA, Kuniyuki AH.1984. In vitro propagation of paradox walnut rootstock. Hortscience 19: 507–509. [Google Scholar]
- EzuraH, Amagai H, Oosawa AK.1993. Efficient production of triploid melon plants by in vitro culture of abnormal embryos excised from dried seeds of dipolid × tetraploid crosses and their characteristics. Japanese Journal of Breeding 43: 193–199. [Google Scholar]
- GargL, Bhandari NN, Rani V, Bhojwani SS.1996. Somatic embryogenesis and regeneration of triploid plants in endosperm cultures of Acacia nilotica Plant Cell Reports 15: 855–858. [DOI] [PubMed] [Google Scholar]
- GhimpuMV.1929. Contribution a l’etude chromosomique des Acacia. Comptes‐Rendu Acadamie Science Paris 188: 1429–1431. [Google Scholar]
- JohnstonJS, Bennett MD, Rayburn AL, Galbraith DW, Price HJ.1999. Reference standards for determination of DNA content of plant nuclei. American Journal of Botany 86: 609–613. [PubMed] [Google Scholar]
- MoffettAA, Nixon KM.1960. Induced tetraploidy in Black Wattle (Acacia mearnsii De Willd). Wattle Research Institute Report 1959–60. [Google Scholar]
- MohamedME, Hicks RGT, Blakesley D.1996. Shoot regeneration on mature endosperm of Passiflora caerulea Plant Cell Tissue Organ Culture 46: 161–164. [Google Scholar]
- MorejohnLC, Bureau TE, Mole‐Bajer T, Bajer AS, Fosket DE.1987. Oryzalin, a dinitro‐aniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro Planta 172: 252–264. [DOI] [PubMed] [Google Scholar]
- MukherjeeS, Sharma AK.1993. In situ nuclear DNA content in perennial fast and slow growing acacias from arid zones. Cytobios 75: 33–36. [Google Scholar]
- MukherjeeS, Sharma AK.1995. In situ nuclear DNA variation in Australian species of Acacia Cytobios 83: 59–64. [Google Scholar]
- MurishigeT, Skoog F.1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiologia Plantarum 15: 473–497. [Google Scholar]
- OrtizR, Vuylsteke D.1995. Factors influencing seed set in triploid Musa spp. L. and production of euploid hybrids. Annals of Botany 75: 151–155. [Google Scholar]
- RamseyJ, Schemske DW.1998. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Reviews of Ecology and Systematics 29: 467–501. [Google Scholar]
- SchwarzacherT, Heslop‐Harrison P.2000. Practical in situ hybridization. Oxford: Bios Scientific Publishers Ltd. [Google Scholar]
- TambongJT, Sapra VT, Garton S.1998. In vitro induction of tetraploids in colchicine‐treated cocoyam plants. Euphytica 104: 191–197. [Google Scholar]
- ToscaA, Pandolfi R, Citterio S, Fasoli A, Sgorbati S.1995. Determination by flow‐cytometry of the chromosome doubling capacity of colchicine and oryzalin in gynogenetic haploids of Gerbera Plant Cell Reports 14: 455–458. [DOI] [PubMed] [Google Scholar]
- TurnbullJW, Midgley SJ, Cossalter C.1998. Tropical acacias planted in Asia: an overview. In: Turnbull JW, Compton HR, Pinyopusarerk K, eds. Recent developments in acacia planting: proceedings of an international workshop held in Hanoi, Vietnam,27–30 October 1997 ACIAR Proceedings, 82. ACIAR, Canberra. [Google Scholar]
- VainolaA.2000. Polyploidization and early screening of Rhododendron hybrids. Euphytica 112: 239–244. [Google Scholar]
- VanDurenM, Morpurgo R, Doležel J, Afza R.1996. Induction and verification of autotetraploids in diploid banana (Musa acuminata) by in vitro techniques. Euphytica 88: 25–34. [Google Scholar]
- WanY, Petolino JF, Widholm JM.1989. Efficient production of doubled haploid plants through colchicine treatment of anther‐derived maize callus. Theoretical and Applied Genetics 77: 889–892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


