Abstract
Studies have indicated that the concentration of carbon dioxide [CO2] during the dark period may influence plant dry matter accumulation. It is often suggested that these effects on growth result from effects of [CO2] on rates of respiration, but responses of respiration to [CO2] remain controversial, and connections between changes in respiration rate and altered growth rate have not always been clear. The present experiments tested whether translocation, a major consumer of energy from respiration in exporting leaves, was sensitive to [CO2]. Nineteen‐day‐old soybean plants grown initially at a constant [CO2] of 350 µmol mol–1 were exposed to three consecutive nights with a [CO2] of 220–1400 µmol mol–1, with a daytime [CO2] of 350 µmol mol–1. Change in dry mass of the individual second, third and fourth trifoliate leaves over the 3‐d period was determined, along with rates of respiration and photosynthesis of second leaves, measured by net CO2 exchange. Translocation was determined from mass balance for second leaves. Additional experiments were conducted where the [CO2] around individual leaves was controlled separately from that of the rest of the plant. Results indicated that low [CO2] at night increased both respiration and translocation and elevated [CO2] decreased both processes, to similar relative extents. The effect of [CO2] during the dark on the change in leaf mass over 3 d was largest in second leaves, where the change in mass was about 50 % greater at 1400 µmol mol–1 CO2 than at 220 µmol mol–1 CO2. The response of translocation to [CO2] was localized in individual leaves. Results indicated that effects of [CO2] on net carbon dioxide exchange rate in the dark either caused or reflected a change in a physiologically important process which is known to depend on energy supplied by respiration. Thus, it is unlikely that the observed effects of [CO2] on respiration were artefacts of the measurement process in this case.
Key words: Carbon dioxide, Glycine max, respiration, soybean, translocation
INTRODUCTION
Several studies have indicated that the concentration of carbon dioxide [CO2] during the dark period may influence plant dry matter accumulation either positively or negatively (Reuveni and Gale, 1985; Bunce, 1995, 2001; Reuveni et al., 1997; Ziska and Bunce, 1999). It is often suggested that these effects on growth result from effects of [CO2] on rates of respiration, which have been reported to decrease with increasing [CO2] (Drake et al., 1999; Gonzalez‐Meler and Seidow, 1999). However, responses of respiration to [CO2] remain controversial (Amthor, 2000; Jahnke, 2001; Tjoelker et al., 2001). A connection between changes in respiration rate and altered growth rate has not always been clear, as it is necessary to assume that a decrease in respiration at elevated [CO2] can have effects on growth ranging from positive to negative (Reuveni et al., 1997; Bunce, 2001b). The present experiments were designed to test whether translocation, a major consumer of energy from respiration (Bouma et al., 1995; Noguchi et al., 2001), was sensitive to [CO2]. By utilizing very uniform plant material, treatment effects on translocation could be inferred from changes in the rates of dry mass accumulation of plant parts, thereby reducing experimental ambiguity.
MATERIALS AND METHODS
Successive plantings of soybean (Glycine max L. Merr. ‘Kent’) were grown in a controlled environment chamber with a constant air temperature of 25 ± 0·2 °C, a dew point temperature of 18 ± 1 °C, and 12 h per day of light pro vided by a mixture of high pressure sodium and metal halide lamps at a photosynthetic photon flux density of 1·0 mmol m–2 s–1. Carbon dioxide concentration was controlled to 350 ± 5 µmol mol–1 in the daytime and 370 ± 5 µmol mol–1 at night by an infrared gas analyser that sampled chamber air continuously and controlled the injection of carbon dioxide or carbon dioxide‐free air. Two or three seeds were sown in 15 cm diameter plastic pots filled with vermiculite and flushed daily with a complete nutrient solution containing 14·5 mm nitrogen. Plants were thinned for uniformity a few days after emergence to one per pot. The coefficient of variation (standard deviation divided by the mean) of leaf dry masses among individual plants within a batch averaged 7 %.
At the beginning of the photoperiod of the 19th day after planting, a harvest of four to six plants was used to determine initial area and dry mass of terminal leaflets of the second, third and fourth trifoliolate leaves, numbered from the base. At this time, four to six plants were moved to a second ‘treatment’ chamber, while four to six other plants remained in the original ‘control’ chamber. The treatment chamber provided the same environmental conditions as the control chamber, except that [CO2] during the dark period was 220, 370, 700 or 1400 µmol mol–1 for different batches of plants. The daytime [CO2] was established 30 min before the lights were turned on to ensure that [CO2] during the light was not contaminated by the dark [CO2] treatments. At the end of the third night in the treatment chamber, area and dry mass of terminal leaflets of the second, third and fourth trifoliolate leaves were determined, and these parameters were also determined for plants kept in the control chamber. Values for the changes in mass of the second, third and fourth trifoliolate leaflets over the 3 d were determined for control and treated plants from the mean values of initial and final masses of four to six plants. Each dark [CO2] treatment was repeated using successive batches of plants. Treatment and control values of final dry mass were compared using paired t‐tests, with each batch of plants providing a single replicate in the paired comparison. There were three replicate batches of plants for each dark [CO2], except in the experiments described below to localize the dark [CO2] responses where there were three additional replicates of the 1400 µmol mol–1 [CO2] treatment.
During the 3‐d treatment periods, net carbon dioxide exchange rates of second trifoliolate leaves were determined several times each day and night for treatment and control plants (Bunce, 2000). These leaves had reached full area expansion 1 or 2 d before the treatments began. Measure ments were made under the growth conditions of light, temperature, humidity and carbon dioxide concentration, using a CIRAS‐1 portable photosynthesis system (PP Systems, Haverhill, MA, USA). The entire gas exchange system was placed inside the controlled environment chamber to minimize the impact of any leaks on carbon dioxide exchange rates. It was determined that the correction for water vapour in the CO2 analysis was accurate (Bunce, 2001). Gas exchange measurements were made only on second trifoliolate leaves because third and fourth leaves were initially too small to fill the cuvette window.
The localization of the effect of [CO2] during the dark period on the change in leaflet mass was examined by moving eight plants to the treatment chamber, but enclosing individual leaflets of second trifoliolate leaves of four of these plants in a cuvette that was continuously supplied with air at 360 µmol mol–1 CO2, while the concentration outside the cuvette during the dark period was controlled at 1400 µmol mol–1. Tests showed that enclosing leaflets in the cuvette for the 3‐d treatment period did not affect their mass compared with leaflets given the same [CO2] treatment outside the cuvette. There were three replicate batches of plants in this experiment.
Translocation from second trifoliolate leaves over the 3‐d period was estimated by mass balance, using the change in dry mass and the mean net carbon dioxide exchange rates during the light and dark (Bunce, 2000). It was assumed that no dry mass was imported, since second leaves were fully expanded in area and thus would normally no longer be importing carbohydrates (Thrower, 1962). Therefore, net photosynthesis during the day minus respiration at night defined the dry mass income for the leaflet, and translocation was taken as the difference between this income and the measured change in dry mass. In converting carbon dioxide exchange into dry mass, it was assumed that dry mass was 40 % carbon, the mean value obtained by carbon analysis of leaves.
RESULTS
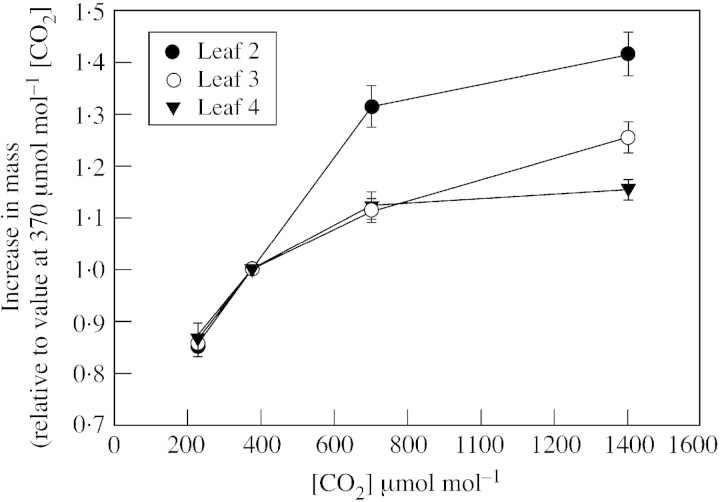
After the 3‐d treatment period, the final dry mass of second, third and fourth trifoliolate leaves increased with increasing [CO2] during the dark (Table 1). When the concentration of CO2 at night in both chambers was 370 µmol mol–1, no differences were detected between plants kept in the two chambers, using paired t‐tests. The dry mass of the second leaves was not affected by the treatment in which the second trifoliolate leaves were exposed to 360 µmol mol–1 CO2 in the dark periods of 3 d, whereas the rest of the shoot was exposed to 1400 µmol mol–1 CO2 in the dark periods (Table 1). The relative effect of high [CO2] during the dark on the accumulation of dry mass over the 3 d was greatest in second trifoliolate leaves (Fig. 1).
Table 1.
Final dry mass (± s.e.) of terminal leaflets of second, third and fourth trifoliate leaves of soybean after three 24‐h periods with [CO2] during the dark period controlled to different concentrations, with a daytime [CO2] of 350 µmol mol–1
| Final dry mass (mg per leaflet) | ||||
| Dark [CO2] (µmol mol–1) | Leaf 2 | Leaf 3 | Leaf 4 | Replicates |
| 220 | 187* ± 5 | 177* ± 4 | 98* ± 5 | 3 |
| 370 | 205 ± 6 | 186 ± 7 | 104 ± 6 | 3 |
| 700 | 224* ± 4 | 195* ± 10 | 113* ± 4 | 3 |
| 1400 | 231* ± 8 | 209* ± 7 | 126* ± 5 | 6 |
| 1400A | 208 ± 9 | 206* ± 8 | 126* ± 4 | 3 |
| Control (370) | 207 ± 5 | 185 ± 6 | 103 ± 8 | 18 |
The number of replicates is the number of batches of four to six plants. 1400A denotes the treatment in which the [CO2] around the second leaf was kept at 360 µmol mol–1, while the rest of the shoot was exposed to 1400 µmol mol–1 [CO2] during the dark. For each batch of plants, a control batch was kept at 370 µmol mol–1 CO2 during the dark.
* Value significantly different from control leaves at P = 0·05, using paired t‐tests.
Fig. 1. The increase in dry mass over three 24‐h periods for soybean leaves exposed to various [CO2] during the dark periods, but 350 µmol mol–1 [CO2] during the light periods. Values for second, third and fourth trifoliolate leaves are expressed relative to values of the same leaves exposed to 370 µmol mol–1 [CO2] during the dark. Vertical bars indicate s.e.
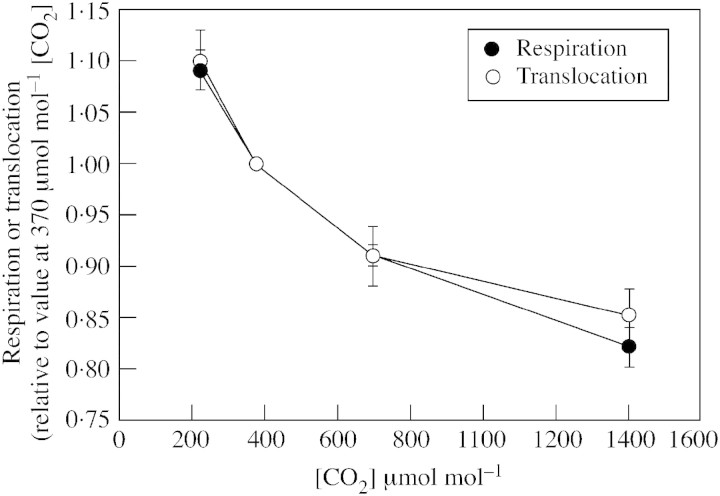
Photosynthesis was slightly higher after nights with 220 µmol mol–1 CO2 and slightly lower after nights with 1400 µmol mol–1 CO2 compared with nights at 370 µmol mol–1 (Table 2). Mean values of photosynthesis were used for the calculation of translocation rates. Statistical tests on mean values of photosynthesis were not conducted because during the first light period there was no difference between the treatments (i.e. the dark [CO2] treatments had not yet been applied). Respiration rates of second trifoliolate leaves decreased with increasing [CO2] at night (Fig. 2). When [CO2] in the dark was 370 µmol mol–1, translocation of dry mass from second trifoliolate leaves over the 3‐d period was about 84 % of the dry mass income from photosynthesis minus respiration (Table 2). Relative to these control leaves, translocation decreased with increasing [CO2] in the dark, changing nearly in parallel with the relative changes in respiration (Fig. 2).
Table 2.
Response to [CO2] during the dark periods of 3‐d on total net photosynthesis, respiration, change in dry mass, and translocation from second trifoliolate leaves of soybean over the 3‐d
| Dark [CO2] (µmol mol–1) | Net photosynthesis (mg dry mass per leaflet) | Respiration | Change in mass | Translocation |
| 220 | 411 ± 16 | 36 ± 2 | 47 ± 3 | 328 ± 13 |
| 370 | 388 ± 14 | 33 ± 3 | 56 ± 4 | 299 ± 11 |
| 700 | 376 ± 13 | 30 ± 3 | 73 ± 5 | 273 ± 9 |
| 1400 | 361 ± 9 | 27 ± 2 | 79 ± 3 | 255 ± 6 |
Values represent overall means (± s.e.) for three replicates, each consisting of four to six plants, except for the 1400 µmol mol–1 CO2 treatment when there were six replicates.
Fig. 2. Mean respiration rates during the dark periods and mean translocation rates for second trifoliolate leaves of soybeans exposed for three 24‐h periods to various [CO2] during the dark periods, but 350 µmol mol–1 [CO2] during the light periods. Values are expressed relative to values of second trifoliolate leaves exposed to 370 µmol mol–1 [CO2] during the dark. Vertical bars indicate s.e.
DISCUSSION
The low plant‐to‐plant variation among soybean seedlings was important in the detection of the dark [CO2] treatment effects on leaflet dry mass. With this uniformity and the closeness of the environmental control in the chambers, the variation among batches of seedlings was small, but still large enough that it was important that treatment and control plants came from the same batch of plants, allowing the use of paired t‐tests in testing for differences between treatment and control means. Some possible sources of variation among batches that would not occur within batches of plants were variation in time of day of planting or in the temperature of the nutrient solution. The treatments were applied for three nights so that the effects of the dark [CO2] treatments on mass become large enough to detect against the background variability in mass.
Because the dark [CO2] treatments changed net photosynthesis and translocation roughly in parallel, it might be assumed that photosynthesis limited translocation. However, this cannot account for the fact that lower photosynthesis was accompanied by larger increases in leaf mass. The dark [CO2] treatments clearly affected the partitioning of photosynthate between mass accumulation and translocation. This has not been observed for other treatments, such as light and daytime [CO2], which affected photosynthesis much more than did these dark [CO2] treatments. In those cases, changes in biomass accumulation paralleled changes in photosynthesis (Bunce, 2000), and the fraction of photosynthate translocated remained constant. The contrasting response to the dark [CO2] treatments imposed here suggests that [CO2] during the dark affected translocation rates beyond any effects due to changes in photosynthesis.
The direction of change in respiration rate with [CO2] during the dark was consistent with the effect of [CO2] on mass accumulation, i.e. lower respiration rate corresponded with a larger increase in mass. However, as is apparent in Table 2, the magnitude of the change in respiration rate with dark [CO2] was much too small to account for the change in mass accumulation directly, as it was less than 14 % of the change, in the closest case. Because respiration was such a small factor in the calculation of translocation rate, respiration and translocation can be considered essentially independent measurements. The striking similarity in the relative responses of respiration and translocation to dark [CO2] is therefore unlikely to be coincidental. It suggests that dark [CO2] affected functionally important respiration in this case. However, the similar relative responses of respiration and translocation should be interpreted with caution since the treatments affecting respiration were applied only during the dark period, whereas translocation presumably occurred in both the light and the dark.
The localization of the effect of dark [CO2] on mass accumulation in a single leaflet, as opposed to the rest of the plant, is consistent with a local effect of [CO2] on respiration and/or phloem loading, but not consistent with whole plant responses, such as, for example, an effect of dark [CO2] on plant water status, or sink regulation of phloem loading.
The method used to measure respiration eliminated two potential errors commonly cited by those suggesting that [CO2] effects on respiration are artefacts of the measuring process (cf. Bunce, 2001a). Because the whole instrument was kept at the measurement [CO2], any effect of leakage of CO2 on respiration rates would have been small, and would have been the same for all [CO2] treatments. Potential effects of water vapour dilution on respiration measurements were eliminated by checking that the particular CO2 analyser made accurate corrections of [CO2] for changes in water vapour. These precautions, coupled with observed effects of the dark [CO2] treatments on mass accumulation and translocation, suggest that the observed changes in respiration were real in this case.
The smaller relative effect of elevated [CO2] on the change in mass for leaves 3 and 4 than that for leaf 2 does not necessarily imply a difference among leaves in their relative sensitivity of translocation to [CO2]. This is because younger, developing leaves translocate relatively less of their photosynthetic income. For example, third leaves at this plant age exported about 72 % of their income (Bunce, 2000) compared with 84 % observed here for the developmentally older second leaves. If it is assumed that leaf 3 has the same values of photosynthesis and respiration as those measured for leaf 2, the relative [CO2] effect on translocation was essentially the same for leaf 2 as that given for leaf 3, despite the smaller relative effect on mass accumulation in third leaves.
Bunce (1995) observed that for soybeans grown at constant temperature, high [CO2] during the dark period produced plants with greater mean dry mass per unit leaf area, and a lower ratio of leaf area per total plant dry mass (leaf area ratio). This would be consistent with the slower rate of translocation out of leaves treated with elevated [CO2] at night seen in the present study. In situations where light interception limits growth, slower rates of translocation from leaves could potentially reduce total plant dry mass production by lowering the leaf area ratio. In situations where the efficiency of use of intercepted light limits growth, slower translocation from leaves could potentially increase total plant dry mass production by increasing leaf mass per unit of area, which is often associated with higher photosynthetic capacity (Bunce, 2000). Thus, it is not clear to what extent effects of [CO2] during the dark on translocation could be responsible for changes in total plant dry mass production in other studies. If changes in respiration caused by [CO2] during the dark influenced translocation rates, then presumably other processes dependent on energy from respiration might also be affected by dark [CO2], and could contribute to altered plant growth.
One could envision that [CO2] affected respiration, and that changes in respiration affected translocation by changing the respiratory energy available for translocation. However, it is also possible that [CO2] affected translocation directly by influencing the activity of a transport process involved in phloem loading, and that lower respiration reflected reduced use of ATP for active transport. This study cannot distinguish between these possibilities. However, studies indicating that daytime translocation is independent of [CO2] (Grodzinski et al., 1998) favour the former explanation.
Supplementary Material
Received: 4 March 2002; Returned for revision: 20 May 2002; Accepted: 17 June 2002 Published electronically: 5 August 2002
References
- AmthorJS.2000. Direct effects of elevated CO2 on nocturnal in situ leaf respiration in nine temperate deciduous tree species is small. Tree Physiology 20: 139–144. [DOI] [PubMed] [Google Scholar]
- BoumaTJ, De Visser R, Van Leeuwen PH, De Kock MJ, Lambers H.1995. The respiratory energy requirements involved in nocturnal carbohydrate export from starch‐storing mature source leaves and their contribution to leaf dark respiration. Journal of Experimental Botany 46: 1185–1194. [Google Scholar]
- BunceJA.1995. Effects of elevated carbon dioxide concentration in the dark on the growth of soybean seedlings. Annals of Botany 75: 365–368. [Google Scholar]
- BunceJA.2000. Contrasting effects of carbon dioxide and irradiance on the acclimation of photosynthesis in developing soybean leaves. Photosynthetica 38: 83–89. [Google Scholar]
- BunceJA.2001a Effects of prolonged darkness on the sensitivity of leaf respiration to carbon dioxide concentration in C3 and C4 species. Annals of Botany 87: 463–468. [Google Scholar]
- BunceJA.2001b The response of soybean seedling growth to carbon dioxide concentration at night in different thermal regimes. Biotronics 30: 15–26. [Google Scholar]
- DrakeBG, Azcon‐Bieto J, Berry J, Bunce J, Dijkstra P, Farrar J, Gifford RM, Gonzalez‐Meler MA, Koch G, Lambers H, Siedow J, Wullschleger S.1999. Does elevated atmospheric CO2 concentration inhibit mitochondrial respiration in green plants? Plant, Cell and Environment 22: 649–658. [Google Scholar]
- Gonzalez‐MelerMA, Siedow JN.1999. Direct inhibition of mitochondrial respiratory enzymes by elevated CO2: does it matter at the tissue or whole‐plant level? Tree Physiology 19: 253–259. [DOI] [PubMed] [Google Scholar]
- GrodzinskiB, Jiao J, Leonardos ED.1998. Estimating photosynthesis and concurrent export rates in C3 and C4 species at ambient and elevated CO2 Plant Physiology 117: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JahnkeS.2001. Atmospheric CO2 concentration does not directly affect leaf respiration in bean or poplar. Plant, Cell and Environment 24: 1139–1151. [Google Scholar]
- NoguchiK, Go C‐S, Miyazawa S‐I, Terashima I, Ueda S, Yoshinari T.2001. Cost of protein turnover and carbohydrate export in leaves of sun and shade species. Australian Journal of Plant Physiology 28: 37–47. [Google Scholar]
- ReuveniJ, Gale J.1985. The effects of high levels of carbon dioxide on dark respiration and growth of plants. Plant, Cell and Environment 8: 623–628. [Google Scholar]
- ReuveniJ, Gale J, Zeroni M.1997. Differentiating day from night effects of high ambient [CO2] on the gas exchange and growth of Xanthium strumarium L. exposed to salinity stress. Annals of Botany 79: 191–196. [Google Scholar]
- ThrowerSL.1962. Translocation of labelled assimilates in the soybean. II. The pattern of translocation in intact and defoliolated plants. Australian Journal of Biological Sciences 15: 629–649. [Google Scholar]
- TjoelkerMG, Oleksyn J, Lee TD, Reich PB.2001. Direct inhibition of leaf respiration by elevated CO2 is minor in 12 grassland species. New Phytologist 150: 419–424. [Google Scholar]
- ZiskaLH, Bunce JA.1999. Effect of elevated carbon dioxide concentration at night on the growth and gas exchange of selected C4 species. Australian Journal of Plant Physiology 26: 71–77. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.