Abstract
Background
Primary focal segmental glomerulosclerosis (FSGS) is a common glomerular disease in adults and ranks among the top causes of a primary glomerular disease causing end-stage renal disease (ESRD). Primary FSGS is, however, a diagnosis of exclusion and distinction between primary versus secondary FSGS is not always obvious, resulting in a number of patients with secondary FSGS undergoing unnecessary immunosuppressive therapy.
Methods
We reviewed the Mayo Clinic Renal Pathology Database for patients with a diagnosis of FSGS on native renal biopsy and divided the patients into nephrotic syndrome-associated (NS-associated) and non-nephrotic syndrome-associated (NNS-associated) FSGS as a first approximation followed by dividing the lesion according to the degree of foot process effacement (FPE) on electron microscopy (EM) examination.
Results
A total of 41 patients with FSGS with complete evaluation were identified. Of these, 18 were classified as having NS and 23 were classified as having NNS. Baseline characteristics (age, gender, body mass index, serum creatinine and hematuria) were not different between the groups. All of the patients with NS showed diffuse FPE ranging from 80 to 100% (mean 96%). On the other hand, of the 23 patients in the NNS group, 22 had segmental FPE and showed patchy effacement, with all cases showing 20–60% FPE (mean of 48%).
Conclusion
Adult patients presenting with NS, an FSGS lesion on LM, extensive FPE (≥80%) on EM examination and no risk factors associated with secondary FSGS are likely to have primary FSGS. Conversely, the absence of NS in a patient with segmental FPE on EM strongly suggests a secondary FSGS. Dividing FSGS into the presence or absence of NS together with the degree of FPE on EM examination is more helpful as it provides a more practical way to separate patients into cases of primary versus secondary FSGS.
Keywords: focal and segmental glomerulosclerosis, nephrotic range, nephrotic syndrome, primary, secondary
Introduction
Primary/idiopathic focal segmental glomerulosclerosis (FSGS) is a common glomerular lesion in adults and children and ranks among the most frequently encountered primary glomerular disorders causing end-stage renal disease (ESRD) [1]. Primary FSGS is, however, a diagnosis of exclusion, requiring that secondary causes be ruled out; i.e. reduced renal mass (oligonephronia), functional adaptations, infectious (e.g. HIV), drug-induced (e.g. bisphosphonates, interferons), genetic (familial or sporadic) and other primary or secondary glomerular diseases (vasculitis, IgA nephropathy and membranous nephropathy) [2]. For patients with presumed primary FSGS, a putative circulating ‘permeability factor, which is toxic to the podocyte, has been proposed as playing a role in its pathogenesis [3, 4]. The demonstration by Hoyer et al. of recurrence of FSGS soon after kidney transplantation [5] and the fact that administration of serum from FSGS patients into rats causes proteinuria supports the existence of such a factor in these patients [6]. However, no such factor has been convincingly identified, biochemically purified and tested to verify its role according to Koch's postulates for causation.
What are the clinical and morphological clues that can help differentiate primary from secondary FSGS? One major clinical feature associated with primary FSGS is the presence of nephrotic syndrome (defined as a 24-h urinary protein excretion of ≥3.5 g and serum albumin concentration of ≤3.5 g/dL) [7]. This is in contrast to nephrotic-range proteinuria defined as 24-h urinary protein excretion of ≥3.5 g in the absence of low serum albumin concentration (serum albumin <3.5 g/dL) which is commonly seen in cases of secondary FSGS [8]. On the other hand, morphological clues include the type of FSGS lesion based on light microscopy (LM) findings according to the Columbia classification (e.g. tip lesion representing primary FSGS versus perihilar lesions representing secondary FSGS) [9] as well as the degree of foot process effacement (FPE) on electron microscopy (EM) examination with diffuse and extensive FPE affecting all or nearly all of the glomerular capillaries commonly representing primary FSGS rather than secondary FSGS [10–12], the exception being cases of viral (HIV) or drug-induced FSGS lesions. However, the clinicopathological correlation is frequently missed resulting in a number of patients with secondary FSGS being mislabeled as primary FSGS and undergoing unnecessary and potentially harmful immunosuppressive therapy [13]. The absence of EM examination of the renal biopsy sample greatly diminishes the power of morphology to separate primary from secondary FSGS.
We propose that dividing the FSGS lesion into nephrotic syndrome-associated (NS-associated) and non-nephrotic syndrome-associated (NNS-associated) FSGS as a first approximation followed by dividing the lesion according to the degree of FPE will be more clinically relevant for the correct recognition of primary versus secondary FSGS. To examine this proposal, we reviewed retrospectively renal biopsies that were consistent with a lesion of FSGS performed at the Mayo Clinic in the last 14 years. The results are presented here.
Methods
Study design
After approval by the Institutional Board Review, the Mayo Clinic (Rochester) Renal Pathology Database was searched for patients with a diagnosis of FSGS on native renal biopsy from January 1999 through December 2012. A total of 167 unique patients were identified. Only biopsies that were performed at the Mayo Clinic and had complete evaluation with LM, immunofluorescence microscopy (IF) and EM were included. In addition, patients with organ transplantation, other diagnoses such as ANCA-associated vasculitis, IgA nephropathy, multiple myeloma, lymphoma, hepatitis B or C or HIV infection, FSGS secondary to medications and those with inadequate clinical data at the time of renal biopsy and at follow-up (i.e. serum albumin and proteinuria) were excluded. The clinical data were obtained from reviewing the electronic medical records. Genetic testing was not included in this analysis.
Patients were then divided into two groups: Group I: NS defined as 24-h urinary protein ≥3.5 g and serum albumin ≤3.5 g/dL and Group II: NNS (serum albumin >3.5 g/dL and/or 24-h urinary protein <3.5 g. Group II includes patients with nephrotic-range and non-nephrotic-range proteinuria.). Medical records were evaluated for whether or not the patient was treated with angiotensin-converting enzyme inhibitor (ACE-I), angiotensin receptor blockade (ARB) or immunosuppressive therapy including prednisone, calcineurin inhibitors (CNI), mycophenolate mofetil (MMF) or cyclophosphamide. None of the patients had received immunosuppressive therapy prior to the renal biopsy.
Kidney pathology evaluation
The renal biopsy of each patient was reviewed by two pathologists (S.S. and S.N.) separately to confirm the presence of an FSGS lesion. Light microscopic evaluation of renal biopsies included staining with hematoxylin and eosin, periodic acid-Schiff, Masson's trichrome and Jones methenamine silver stain. Toluidine blue stained semi-thick sections were examined, and ≥2 non-segmentally sclerosed glomeruli were identified for EM studies. When reviewing the renal biopsy, the pathologists were blinded to the clinical data (i.e. whether or not patient presented with NS or NNS). Each biopsy was classified according to the Columbia Classification and sub-classified further according to the degree of FPE [9]. Glomerulomegaly was defined if at least one glomerulus cut at or near the hilum measured >200 µm in diameter. The degree of FPE ranged from 0 to 100% when EM sections of at least two non-sclerosed glomeruli were examined. Percentage of FPE was based on loops examined. If foot processes were preserved or only partially effaced in one loop, it was excluded as diffuse effacement. 100%, all loops showed complete effacement; 90%, one of 10 loops did not show complete effacement; 80%, 2 of 10 loops did not show complete effacement. After analyzing the data and based on the cutoff of greatest significance, diffuse and widespread FPE was defined as ≥80% of the glomerular capillary surface being effaced, whereas segmental FPE was defined as <80% of the glomerular capillary being effaced. The degree of interstitial fibrosis and tubular atrophy was scored as less than or ≥25% and arteriosclerosis and arteriolar hyalinosis was scored from 0 to 3+ based on the worst affected artery.
Statistical analysis
Data are expressed as means ± SD and compared by Student's t-test. The χ2 test is used to compare proportions. P-value of <0.05 was considered statistically significant.
Results
Baseline demographics and clinical data (Table 1)
Table 1.
Baseline demographics and patient characteristics
| Nephrotic syndrome | Non-nephrotic syndrome | P-value | |
|---|---|---|---|
| n | 18 | 23 | |
| Age | 46.8 ± 21.2 | 56.0 ± 21.5 | 0.2 |
| Gender (M/F) | 14/4 | 16/7 | 0.6 |
| Race (Caucasian) | 15a | 22b | 0.3 |
| sBP (mmHg) | 137.0 ± 15.4 | 125.4 ± 18.6 | 0.04 |
| dBP (mmHg) | 82.2 ± 11.5 | 73.2 ± 15.3 | 0.04 |
| BMI (kg/m2) | 30.6 ± 8.6 | 29.8 ± 4.3 | 0.7 |
| Serum creatinine (mg/dL) | 1.91 ± 0.8 | 1.65 ± 0.6 | 0.3 |
| Serum albumin (g/dL) | 2.5 ± 0.6 | 3.9 ± 0.3 | <0.0001 |
| 24-h urinary protein (g)c | 18.8 ± 21.8 | 4.0 ± 2.9 | 0.01 |
| Total cholesterol (mg/dL)d | 367 ± 142 | 211 ± 91 | 0.001 |
| Hematuria (Y/N) | 11/6 | 8/14 | 0.08 |
aOf the remaining three patients one was African American, one American Indian and one unknown.
bOne patient was Hispanic.
cMedian was 8 g for NS and 3.2 for NNS.
dn = 16 for NS and n = 17 for NNS.
A total of 41 patients with an FSGS on renal biopsy with complete evaluation and adequate clinical data and follow-up were identified. With exception of one patient biopsied at the age of 10, all others were adults (98%), 18 years of age or older, at the time of renal biopsy. Of these, 18 were classified as having NS and 23 were classified as NNS. There were no statistically significant differences between the two groups with respect to age, gender, body mass index (BMI), baseline serum creatinine or presence of hematuria (Table 1). However, patients in the NS group had a higher systolic and diastolic blood pressure compared with NNS group (P = 0.04). As expected the mean serum albumin was significantly lower and mean 24-h urinary protein was significantly higher in the NS group compared with NNS group. Among those patients with NNS, 14 had a 24-h urine protein excretion of <3.5 g and 9 had 24-h urine excretion of ≥3.5 g. Total serum cholesterol (TC) was significantly higher in NS versus the NNS group (363 versus 222 mg/dL, P = 0.002).
Kidney pathology
The type of FSGS lesion was classified based on the Columbia classification (Table 2). FSGS lesions belonging to the not otherwise specified (NOS) type was the most common type of lesion in both the NS and NNS group. Perihilar FSGS was present more frequently in the Group II-NNS (31%) compared with the Group I-NS (11%), but this difference was not statistically significant. Representative LM and EM findings are shown in Figure 1.
Table 2.
Biopsy characteristics
| Nephrotic syndrome | Non-nephrotic syndrome | P-value | |
|---|---|---|---|
| n | 18 | 23 | |
| FPE | 96 ± 5.9 (80–100) | 48.3 ± 16.9 (20–90) | <0.0001 |
| FSGS lesions | 0.4 | ||
| Collapsing | 1 (5%) | 2 (9%) | |
| Tip | 3 (17%) | 1 (4%) | |
| Perihilar | 2 (11%) | 7 (31%) | |
| Cellular | 2 (11%) | 1 (4%) | |
| NOS | 10 (56%) | 12 (52%) | |
| Glomerulomegaly (Y (%)/N (%)) | 6 (33)/12 (67) | 16 (70)/7 (30) | 0.02 |
| Global glomerulosclerosis (%) | 11.4 ± 13.5 | 30.5 ± 24.1 | 0.005 |
| Tubular atrophy and interstitial fibrosis (≤25%/>25%) | 18 (100)/0 (0) | 18 (78)/5 (22) | 0.04 |
| Arteriosclerosis and arteriolar hyalinosis (<2+/≥2+)a | 13 (76)/4 (24) | 10 (50)/10 (50) | 0.09 |
aOne biopsy in the NS and three biopsies in the NNS did not have arteries on the renal biopsy.
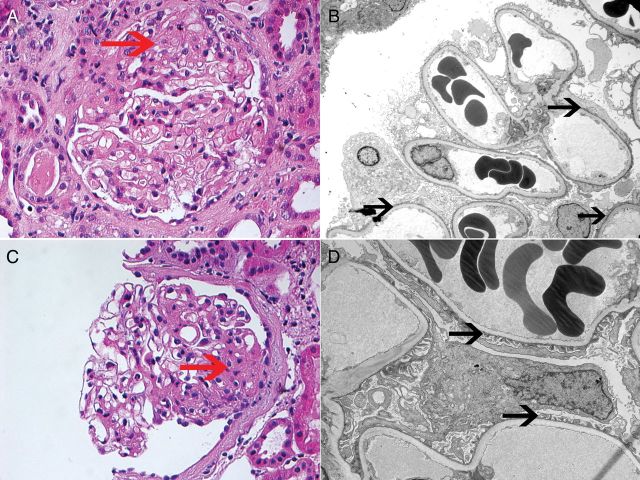
Fig. 1.
FSGS: Each panel represents one patient biopsy. The patient shown on the top panel presented with NS, while the patient shown on the bottom panel presented with NNS. Top panel: (A) LM showing FSGS, NOS type (hematoxylin and eosin ×40); (B) EM showing widespread podocyte FPE (×3100). Bottom panel: (C) LM showing FSGS, NOS type (hematoxylin and eosin ×40); (D) EM showing relatively well-preserved podocyte foot processes (×4200). Red arrow points to the FSGS lesion on LM, black arrow points at foot processes.
All of the patients with NS showed diffuse FPE ranging from 80 to 100% (mean 96%). Except for one patient with 80% FPE, the rest were 90% or above. On the other hand, of the 23 patients in the NNS group, 22 had segmental FPE and showed patchy effacement, with all cases showing 20–60% effacement (mean of 48%) except for 2 cases that showed 70% FPE and only 1 patient who had 90% FPE on EM but did not have NS. This patient was a 65-year-old female who at the time of renal biopsy had 2.5 g/24 h of urinary protein and a serum albumin of 3.2 g/dL. Serum creatinine at the time of biopsy was 1.3 mg/dL. She did not receive any immunosuppression prior to or after her renal biopsy but was on both an ACE-I and an ARB for a couple of months before evaluation at the Mayo clinic for treatment of hypertension. The FSGS lesion on renal biopsy was an NOS lesion with evidence of glomerulomegaly. In follow-up (41 months following renal biopsy), albumin improved to 3.7, 24-h urinary remained stable at 2.1 g and serum creatinine increased to 2.2 mg/dL. There were no differences in the degree of FPE when comparing patients in the NNS group with greater than or equal to or <3.5 g of urinary protein per 24 h (51.1 versus 46.6%, respectively, P = 0.5).
Of the 23 patients in Group II with NNS-associated FSGS, 5 (21%) had decreased renal mass secondary to nephrectomies, renal dysplasia, posterior urethral valves or scarring due to recurrent pyelonephritis. Ten patients (43%) were obese (BMI ≥ 30 kg/m2) and hypertensive. Of the remaining 8 patients, 6 had a BMI > 25 Kg/m2 with 4 of the patients having hypertension and hyperlipidemia in addition. There were only two patients with normal BMI in the absence of reduced renal mass in Group II. One patient had a long-standing history of hypertension and the other was diagnosed with FSGS at 18 years of age after presenting with hypertensive urgency. Genetic testing as a cause of FSGS was not performed in this patient. In the NS Group, of the 18 patients, only 2 had normal BMI, 9 were overweight with BMI ≥ 25 kg/m2 and 7 were obese with BMI ≥ 30 kg/m2.
Interestingly, all types of FSGS lesions according to the Columbia Classification were present in both Groups I (NS) and II (NNS). Patients with NNS had a significantly higher number of glomeruli that were globally sclerosed [e.g. superimposed focal global glomerulosclerosis (FGGS)] and more extensive tubular atrophy and interstitial fibrosis when compared with the NS group. Even though there was a significantly higher number of patients in the NNS group who had glomerulomegaly, 33% of patient in the NS group showed evidence of glomerulomegaly on LM. There was also a trend towards a higher degree of arteriosclerosis and hyalinosis in the NNS group compared with NS group (P = 0.09).
Treatment and follow-up
The mean duration of follow-up was 17.3 months in the NS and 27.2 months in the NNS group (P = 0.7). Of the 18 patients with NS, 14 (78%) were treated with some type of immunosuppressive therapy in addition to use of ACE-I or ARB, whereas 4 (22%) were treated conservatively with ACE-I/ARB or combination of both only. The immunosuppressive therapy included prednisone alone in three patients, CNI-based therapy in five patients, combination of CNI and MMF in three patients and cyclophosphamide-based therapy in three patients. The reasons for not treating the four patients who presented with NS included concern for side effects in two patients, advanced disease state in one patient, and assumption that patient had secondary FSGS in one patient. Of the four patients who were treated conservatively one had further progression of the disease and one had stable renal function (Cr of 1.2 g/dL) with persistent proteinuria and hypoalbuminemia. One patient showed improvement in serum creatinine from 1.2 to 1.0 mg/dL with serum albumin improving from 3.3 to 3.7 mg/dL and 24-h urinary protein improving from 6.2 to 0.6 g/24 h. The other patient showed improvement in serum creatinine from 1.7 mg/dL at time of renal biopsy to 0.9 mg/dL in follow-up. There was no serum albumin or 24-h urinary protein available in follow-up (Table 3).
Table 3.
Clinical characteristics in follow-up
| Nephrotic syndrome | Non-nephrotic syndrome | P-value | |
|---|---|---|---|
| N | 18 | 23 | |
| Mean duration of follow-up (m) (min–max) | 17.3 ± 26.3 (2.3–94.0) | 27.2 ± 17.3 (5.7–84.8) | 0.7 |
| Immunosuppressive therapy Y (%)/N (%) | 14 (78)/4 (22) | 2 (8)/21 (92) | <0.0001 |
| ESRD (dialysis or S Cr ≥5.0 mg/dL) | 3 (17%) | 6 (26%) | 0.5 |
| F/U serum creatinine (mg/dL) (min–max)a | 1.76 ± 1.4 (0.7–5.7) | 1.94 ± 1.1 (0.7–5.0) | 0.2 |
| F/U serum albumin (g/dL) | 3.7 ± 0.7 | 3.8 ± 0.5 | 0.2 |
| F/U 24-h urinary protein (g) | 3.2 ± 3.3 | 3.1 ± 3.8 | 0.9 |
| F/U total cholesterol (mg/dL) | 194 ± 51.6 | 193 ± 62.2 | 0.9 |
Cr, creatinine; F/U, follow-up.
aExcluding patients who received transplants or dialysis.
Of the 23 patients with NNS, 22 were treated conservatively with either ACE-I/ARB or combination of both and only 1 patient was treated with immunosuppression. The patient received prednisone only and decision to treat was primarily per patient's preference after discussion of the risks and benefits of therapy. The patient had a serum creatinine of 1.1, serum albumin of 3.8 g/dL and 24-h urinary protein of 5 g/24 h at presentation. She received 12 weeks of prednisone at a dose of 1 mg/kg/day which was then tapered over 6 months. In follow-up, she had a serum creatinine of 1.0, serum albumin of 4.0 and 24-h urinary protein of 2.9 g.
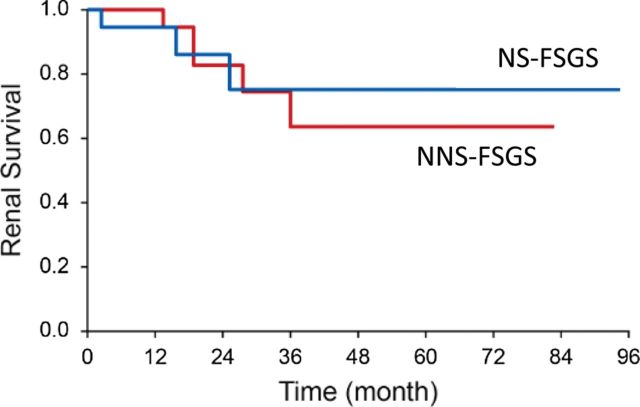
Three patients (17%) in the NS and six patients (26%) in the NNS group progressed to ESRD (P = 0.5) (requiring dialysis, transplantation or serum creatinine ≥5 mg/dL). One patient in the NS group received renal transplantation and had evidence of recurrence shortly after transplantation which was treated with plasmapheresis. The follow-up urinary protein and serum albumin level were much improved in the NS group as shown in Table 3. In the NNS group, there was a mild reduction in the 24-h urinary protein from (4.0–3.1 g) while serum albumin remained unchanged. The difference in serum albumin, TC and urinary protein was no longer significant between the NS and NNS groups in follow-up (Table 3). In follow-up, baseline serum creatinine decreased from 1.91 to 1.76 mg/dL in the NS group while increased from 1.65 mg/dL at the time of biopsy to 1.94 mg/dL in the NNS group (P = 0.2). When further dividing the patients in NNS group into those with significant glomerulosclerosis (≥33%) and interstitial fibrosis/tubular atrophy (IFTA) (≥25%) and those with less glomerulosclerosis and IFTA, those with higher degree of sclerosis and IFTA had a significantly higher serum creatinine in follow-up (3.9 ± 1.9 mg/dL) compared with those with less sclerosis (1.48 ± 0.6) (P = 0.001). There were no differences in the renal survival when comparing the NS group with NNS group (P = 0.8) as shown in Figure 2.
Fig. 2.
Kaplan–Meier curve showing renal survival (endpoint defined as serum creatinine >5.0 mg/dL or need for dialysis or transplantation) over time in primary and secondary FSGS. Log-rank test (P = 0.8).
Discussion
Primary FSGS is a common cause of nephrotic syndrome in adults and one of the leading primary glomerular lesions causing ESRD [1]. The morphologic FSGS lesion on LM in itself is non-specific and can be caused by a variety of different pathogenetic or etiologic mechanisms. As such, primary FSGS is a diagnosis of exclusion that is reached only after known causes of FSGS have been ruled out. However, the distinction between primary and secondary FSGS is not always obvious, resulting in a number of patients with secondary FSGS undergoing unnecessary and potential harmful immunosuppressive therapy. We propose that dividing FSGS into the presence or absence of NS together with the degree of FPE on EM examination (segmental versus diffuse) is more helpful as it provides a more practical way to separate patients into cases of primary versus secondary FSGS and in deciding appropriate treatment options.
The present study based on a predominantly adult population shows that patients with FSGS and NS have extensive FPE, ranging from 80 to 100% with a mean of 96%. On the other hand, almost all cases of NNS-associated FSGS have much less FPE with most cases showing 20–60% FPE and only exceptional cases showing up to 70% FPE. In the absence of an obvious cause (e.g. HIV, pamidronate), the presence of an FSGS lesion in a patient with NS points to a primary FSGS as the most likely diagnosis. The diffuse and widespread FPE (≥80%) is in keeping with the views that primary FSGS results from a circulating ‘permeability factor(s) that is toxic to the podocyte. Support for the hypothesis comes from experimental models where chronic administration of the aminonucleoside puromycin, which is toxic to the podocyte, results in the development of proteinuria and FSGS lesions that are similar to the glomerular lesions found in human primary FSGS [14]. In this model, EM examination shows that widespread FPE and podocyte detachment from basement membranes is the initial lesion with formation of synechiae occurring at a later stage [14–16]. Further support for a ‘permeability factor’ comes from observations of recurrent FSGS post-kidney transplant where diffuse FPE can be observed within minutes after reperfusion [17]. The diffuse FPE is followed by massive proteinuria developing within hours to days after the kidney transplantation [18]. With time, the characteristic FSGS lesion develops [5]. Thus, FPE is the earliest structural change and key initial event with an FSGS lesion developing ‘late’ in the course of the pathological process. On the other hand, in the unilateral nephrectomy post-adaptive model of secondary FSGS, however, there is glomerular tuft hypertrophy, but podocyte cell numbers do not increase [19]. Rather, podocytes are forced to hypertrophy and stretch to cover a larger surface area. This results in podocyte attenuation, but foot processes are largely preserved [20]. These data show that there are marked differences at the EM levels between models of primary (toxic, permeability factor mediated) versus secondary forms of FSGS. Indeed, Deegens et al. [12] analyzed the differences in foot process width between patients with primary versus secondary FSGS and found the effacement to be most severe in cases of primary FSGS, with foot process relatively preserved in secondary cases, with little overlap between the two. Due to the better preservation of the filtration barrier, patients with secondary FSGS are more likely to present with slowly increasing proteinuria that may be in the nephrotic range, but these patients do not develop full-blown NS (no hypoalbuminemia). To date, many large randomized trials in primary FSGS have failed to properly identify patients with primary FSGS and have included patients with secondary FSGS in these studies. In most studies, degree of FPE on EM is not evaluated and there is reliance purely on the LM findings. Similarly, nephrotic-range proteinuria has been used interchangeably with NS even though these are not necessarily synonymous [21–24]. The lack of distinction between NS and NNS in these studies may have resulted in finding an absence of clinical benefit from a specific therapy when evaluating response in patients with primary FSGS.
It is important to emphasize that in evaluating the degree of foot process integrity, it is crucial to select relatively intact glomeruli for ultrastructural studies, i.e. not segmentally sclerosed glomeruli, since a sclerosed or scarred glomerulus may show extensive FPE, regardless of the cause. Some renal pathologists claim that the percentage of FPE represents a continuum rather than a sharp threshold with respect to its value in separating a primary from a secondary from of FSGS. In this respect, it is important to correlate the timing with renal biopsy with the use of immunosuppressive therapy because a patient with primary FSGS who is or has been treated with corticosteroid therapy maybe going into remission and it should not come as a surprise if EM examination shows segmental FPE affecting variable portions of the nephron population. This certainly can be seen also in patients with MCD undergoing spontaneous remission [25].
One of the interesting findings in our study was the absence of correlation between the subvariant (type) of FSGS lesion on LM and the degree of proteinuria. Based on the Columbia criteria, FSGS lesions are classified based on LM as NOS, perihilar, cellular, tip and collapsing variant [9]. Typically tip, cellular and collapsing FSGS have widespread FPE, while in NOS, FPE is variable and in the perihilar subtype effacement is relatively mild and segmental [26–28]. In our study, the majority of patients had NOS lesions but the presence of an NOS lesion could not separate patients into NS or NNS-associated FSGS. In addition, even though glomerulomegaly has been reported in patients with obesity-related FSGS [10] and was most commonly seen in patients with NNS-associated FSGS (70%), 33% of patients with NS-associated FSGS had finding of glomerulomegaly on LM and therefore the presence of glomerulomegaly is not always indicative of secondary FSGS. It should be noted that there were only 41 patients included in the current study and it is possible a significant difference may have been present that was not detected due to the small number of patients.
Traditionally, secondary FSGS has been attributed to being hemodynamically mediated or due to functional adaptation, drugs, infections or genetic mutations. In our study, 65% of the patients in the NNS group had either reduced renal mass or obesity. Our results are consistent with the findings of Praga et al. who showed that 88% of patients with a diagnosis of FSGS in the absence of NS had an identifiable secondary cause [8]. On the other hand, while hypertension, hyperlipidemia, tobacco use and sleep apnea were common, we could not clearly identify risk factor(s) in the remaining 35% of the patients. We did not test for an underlying genetic mutation as the cause of FSGS so we cannot exclude that some patients had FSGS due to a genetic mutation. On the other hand, none of the patients had a family history of renal diseases or other extra-renal manifestation and unless there is family history of FSGS or clinical evidence of a syndromic process, routine genetic testing in adults with FSGS is not recommended as it is rarely attributed to a specific mutation (<15% of all adult cases) [29, 30]. The finding of a genetic mutation, however, would be compatible with our own unpublished observations that adults with genetic mutations may have nephrotic-range proteinuria but do not develop NS. Nevertheless, a search for potential etiology of secondary FSGS should be pursued in all cases.
In the present study, the majority of the patients (79%) with NS and widespread FPE were treated with immunosuppression with overall a favorable response resulting in a reduction in the mean serum creatinine, improvement in serum albumin, reduction in proteinuria and serum total cholesterol; in keeping with previous reports [31]. The observation of the patients in this group who were not treated with immunosuppressive therapy either progress to ESRD or remained nephrotic supports the diagnosis of primary FSGS. On the other hand, patients with NNS and segmental FPE on EM had a progressive course with increase in the mean serum creatinine and no change in the serum albumin, TC or proteinuria. The fact that proteinuria did not increase in this group suggests that the segmental FPE observed on renal biopsy was not part of an ‘early’ phase of primary FSGS. Further support for considering these patients as having secondary FSGS is the trivial proteinuria response to high-dose corticosteroids in the one patient that was treated with prednisone therapy. In the subgroup analysis, patients who had >33% glomerulosclerosis and >25% IFTA were the patients who showed progression of kidney disease compared with those with less glomerulosclerosis and IFTA. This suggests that the main reason for the increase in serum creatinine over time is likely the higher degree of sclerosis, fibrosis and atrophy rather than absence of treatment with immunosuppression in this group. The overall loss of renal function in the NNS group overtime is consistent with the finding by Praga et al. who similarly showed a poor prognosis in patients with obesity-induced FSGS with almost half the patients developing ESRD [32].
In our cohort, over 90% of the patients were Caucasian and only one patient was <18 years of age at the time of renal biopsy; therefore, the results presented in this study do not apply to AA population or children. However, it should be emphasized that in great majority of hypertensive AA patients, renal biopsy does not show FSGS but rather FGGS, and EM often shows only segmental FPE [33]. The majority of AA patients included in these studies do not have nephrotic syndrome, but rather sub-nephrotic-range proteinuria [34] and respond poorly to treatment with corticosteroids [35]. These data suggest that many AA patients have a disease that is different from the primary FSGS seen in Caucasians.
Patients with presumed primary FSGS treatment are candidates for immunosuppressive treatment. Patients with secondary FSGS should be treated conservatively, with the goal of maximizing blood pressure control with the use of angiotensin II blockade (hypertension is more common in patients with secondary than primary FSGS), low salt diet (<4 g/day), low protein diet (0.8–1 g/kg/day), lipid control with the use of a statin, smoking cessation, weight control and avoidance of nephrotoxic medications. The position to recommend conservative therapy in patients with secondary FSGS is based on the fact that angiotensin II blockade is usually able to reduce proteinuria to sub-nephrotic levels in these patients. Furthermore, patients with primary FSGS in whom proteinuria has been reduced to <3.5 g/24 h associated with good long-term renal prognosis (<15% progress to ESRD over a course of 10 years) making it difficult to justify high-dose corticosteroids in these patients [23, 36]. Recent KDIGO guidelines state that ‘There is no evidence to suggest corticosteroid or immunosuppressive therapy in secondary FSGS’ [37].
Based on this retrospective analysis, we propose that adult patients presenting with NS, an FSGS lesion on LM, extensive FPE (≥80%) on EM examination and no readily available factors associated with secondary FSGS (such as viral infection or drugs) are very likely to have primary FSGS. Conversely, the presence of sub-nephrotic or nephrotic-range proteinuria and a normal serum albumin concentration, in a patient with segmental FPE on EM strongly suggests a secondary FSGS, although the underlying cause may not be obvious with the available diagnostic tools.
It could be argued that in the presence of NS and a renal biopsy showing FSGS on LM, with negative IF, the results from EM are irrelevant since widespread FPE is likely to be present in all patients. However, we would like to emphasize that the basic concept in the definition of primary FSGS is of a ‘primary podocytopathy’. Thus, the finding of widespread FPE effacement on EM confirms the clinical impression that we are indeed dealing with a pathological process that affects primarily the podocyte. Second, in cases where the IF microscopy is unavailable, results from EM can help establish the diagnosis. Third, EM is also of help in ruling out superimposed pathology, e.g. Fabry or Alport.
We would also like to point out that extensive FPE can occur without NS. Thus, although the majority of non-nephrotic cases had FPE <80%, this was not true in all cases. In a patient who is non-nephrotic, we would recommend conservative treatment only, with close follow-up in those who present widespread FPE as they may represent early phase of FSGS. We would only consider immunosuppressive therapy if there is progression to full nephrotic syndrome. In an even rarer scenario of a patient with a full nephrotic syndrome and segmental FPE on EM, it is important to rule out the use of immunosuppressive therapy prior or concomitant to the time of the renal biopsy because the EM findings may represent a resolving process.
It should be understood that morphology alone (e.g. without any clinical information) cannot reliably distinguish a primary from a secondary form of FSGS. Thus, primary and secondary FSGS need to be viewed as clinico-pathological entities and it is the clinician's role to integrate the pathological findings with clinical information in order to make the most precise separation of primary from secondary FSGS. Long-term follow-up is needed because some patients whose clinical presentation may suggest a secondary FSGS may progress to full NS. In this last group, a repeat renal biopsy should be considered.
Funding
None.
Conflict of interest statement
None declared.
References
- 1.Maisonneuve P, Agodoa L, Gellert R, et al. Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: results from an international comparative study. Am J Kidney Dis. 2000;35:157–165. doi: 10.1016/S0272-6386(00)70316-7. [DOI] [PubMed] [Google Scholar]
- 2.Korbet SM. Primary focal segmental glomerulosclerosis. J Am Soc Nephrol. 1998;9:1333–1340. doi: 10.1681/ASN.V971333. [DOI] [PubMed] [Google Scholar]
- 3.Savin VJ, Sharma R, Sharma M, et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334:878–883. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 4.Wei C, El Hindi S, Li J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoyer JR, Vernier RL, Najarian JS, et al. Recurrence of idiopathic nephrotic syndrome after renal transplantation. Lancet. 1972;2:343–348. doi: 10.1016/s0140-6736(72)91734-5. [DOI] [PubMed] [Google Scholar]
- 6.Le Berre L, Godfrin Y, Lafond-Puyet L, et al. Effect of plasma fractions from patients with focal and segmental glomerulosclerosis on rat proteinuria. Kidney Int. 2000;58:502–511. doi: 10.1046/j.1523-1755.2000.00434.x. [DOI] [PubMed] [Google Scholar]
- 7.Glassock RJ, Fervenza FC, Hebert L, et al. Nephrotic syndrome redux. Nephrol Dial Transplant. 2014 doi: 10.1093/ndt/gfu077. doi:10.1093/ndt/gfu077. [DOI] [PubMed] [Google Scholar]
- 8.Praga M, Morales E, Herrero JC, et al. Absence of hypoalbuminemia despite massive proteinuria in focal segmental glomerulosclerosis secondary to hyperfiltration. Am J Kidney Dis. 1999;33:52–58. doi: 10.1016/s0272-6386(99)70257-x. [DOI] [PubMed] [Google Scholar]
- 9.D'Agati VD, Fogo AB, Bruijn JA, et al. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43:368–382. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 11.D'Agati V. The many masks of focal segmental glomerulosclerosis. Kidney Int. 1994;46:1223–1241. doi: 10.1038/ki.1994.388. [DOI] [PubMed] [Google Scholar]
- 12.Deegens JK, Dijkman HB, Borm GF, et al. Podocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosis. Kidney Int. 2008;74:1568–1576. doi: 10.1038/ki.2008.413. [DOI] [PubMed] [Google Scholar]
- 13.Sethi S, Glassock RJ, Fervenza FC. Focal segmental glomerulosclerosis: towards a better understanding for the practicing nephrologist. Nephrol Dial Transplant. 2014 doi: 10.1093/ndt/gfu035. doi:10.1093/ndt/gfu035. [DOI] [PubMed] [Google Scholar]
- 14.Glasser RJ, Velosa JA, Michael AF. Experimental model of focal sclerosis. I. Relationship to protein excretion in aminonucleoside nephrosis. Lab Invest. 1977;36:519–526. [PubMed] [Google Scholar]
- 15.Inokuchi S, Shirato I, Kobayashi N, et al. Re-evaluation of foot process effacement in acute puromycin aminonucleoside nephrosis. Kidney Int. 1996;50:1278–1287. doi: 10.1038/ki.1996.439. [DOI] [PubMed] [Google Scholar]
- 16.Vernier RL, Papermaster BW, Good RA. Aminonucleoside nephrosis. I. Electron microscopic study of the renal lesion in rats. J Exp Med. 1959;109:115–126. doi: 10.1084/jem.109.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JW, Pardo V, Sageshima J, et al. Podocyte foot process effacement in postreperfusion allograft biopsies correlates with early recurrence of proteinuria in focal segmental glomerulosclerosis. Transplantation. 2012;93:1238–1244. doi: 10.1097/TP.0b013e318250234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheong HI, Han HW, Park HW, et al. Early recurrent nephrotic syndrome after renal transplantation in children with focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2000;15:78–81. doi: 10.1093/ndt/15.1.78. [DOI] [PubMed] [Google Scholar]
- 19.D'Agati VD. Podocyte injury in focal segmental glomerulosclerosis: lessons from animal models (a play in five acts) Kidney Int. 2008;73:399–406. doi: 10.1038/sj.ki.5002655. [DOI] [PubMed] [Google Scholar]
- 20.Nagata M, Kriz W. Glomerular damage after uninephrectomy in young rats. II. Mechanical stress on podocytes as a pathway to sclerosis. Kidney Int. 1992;42:148–160. doi: 10.1038/ki.1992.272. [DOI] [PubMed] [Google Scholar]
- 21.Gipson DS, Trachtman H, Kaskel FJ, et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 2011;80:868–878. doi: 10.1038/ki.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trachtman H, Vento S, Gipson D, et al. Novel therapies for resistant focal segmental glomerulosclerosis (FONT) phase II clinical trial: study design. BMC Nephrol. 2011;12:8. doi: 10.1186/1471-2369-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troyanov S, Wall CA, Miller JA, et al. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16:1061–1068. doi: 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 24.Ramachandran R, Kumar V, Rathi M, et al. Tacrolimus therapy in adult-onset steroid-resistant nephrotic syndrome due to a focal segmental glomerulosclerosis single-center experience. Nephrol Dial Transplant. 2014;29:1918–1924. doi: 10.1093/ndt/gfu097. [DOI] [PubMed] [Google Scholar]
- 25.Black DA, Rose G, Brewer DB. Controlled trial of prednisone in adult patients with the nephrotic syndrome. Br Med J. 1970;3:421–426. doi: 10.1136/bmj.3.5720.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 27.Thomas DB, Franceschini N, Hogan SL, et al. Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int. 2006;69:920–926. doi: 10.1038/sj.ki.5000160. [DOI] [PubMed] [Google Scholar]
- 28.Stokes MB, Valeri AM, Markowitz GS, et al. Cellular focal segmental glomerulosclerosis: clinical and pathologic features. Kidney Int. 2006;70:1783–1792. doi: 10.1038/sj.ki.5001903. [DOI] [PubMed] [Google Scholar]
- 29.Santin S, Bullich G, Tazon-Vega B, et al. Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2011;6:1139–1148. doi: 10.2215/CJN.05260610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rood IM, Deegens JK, Wetzels JF. Genetic causes of focal segmental glomerulosclerosis: implications for clinical practice. Nephrol Dial Transplant. 2012;27:882–890. doi: 10.1093/ndt/gfr771. [DOI] [PubMed] [Google Scholar]
- 31.Chun MJ, Korbet SM, Schwartz MM, et al. Focal segmental glomerulosclerosis in nephrotic adults: presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol. 2004;15:2169–2177. doi: 10.1097/01.ASN.0000135051.62500.97. [DOI] [PubMed] [Google Scholar]
- 32.Praga M, Hernandez E, Morales E, et al. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2001;16:1790–1798. doi: 10.1093/ndt/16.9.1790. [DOI] [PubMed] [Google Scholar]
- 33.Fogo A, Breyer JA, Smith MC, et al. Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: a report from the African American Study of Kidney Disease (AASK) Trial. AASK Pilot Study Investigators. Kidney Int. 1997;51:244–252. doi: 10.1038/ki.1997.29. [DOI] [PubMed] [Google Scholar]
- 34.Toto RD. Proteinuria and hypertensive nephrosclerosis in African Americans. Kidney Int Suppl. 2004;66:S102–S104. doi: 10.1111/j.1523-1755.2004.09224.x. [DOI] [PubMed] [Google Scholar]
- 35.Crook ED, Habeeb D, Gowdy O, et al. Effects of steroids in focal segmental glomerulosclerosis in a predominantly African-American population. Am J Med Sci. 2005;330:19–24. doi: 10.1097/00000441-200507000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Velosa JA, Holley KE, Torres VE, et al. Significance of proteinuria on the outcome of renal function in patients with focal segmental glomerulosclerosis. Mayo Clin Proc. 1983;58:568–577. [PubMed] [Google Scholar]
- 37.Kidney Disease: Improving Global Outcomes (KDIGO) glomerulonephritis Work Group. KDIGO Clinical Practice Guidelines for Glomerulonephritis. Kidney Int. 2012;2(Suppl 2):139–274. [Google Scholar]