Abstract
Two cases of malignant hypertension presenting with acute kidney injury, thrombocytopenia and hemolytic anemia are presented. In both patients a prolonged duration of renal replacement therapy was required. The plasma levels of ADAMTS13 enzyme were not helpful in delineating the precise pathogenesis in both cases, as the decrements were not severe. We discuss the clinic-pathologic correlation of the biopsy findings and persistence of AKI.
Keywords: irreversible, malignant hypertension, thrombotic microangiopathy
Introduction
Malignant hypertension (MHTN) is characterized by severe hypertension and acute multi-organ ischemic complications including thrombotic microangiopathy (TMA). Renal thrombotic microangiopathy occurring with MHTN is associated with thrombosis of small vessels, intravascular hemolysis with red cell fragments (Schistocytes), platelet consumption and elevated lactic dehydrogenase (LDH) levels [1]. There may be associated oliguric acute kidney injury (AKI) which may rarely require renal replacement therapy for a variable period of time. Because of the rarity of renal TMA complicating MHTN, its clinical-pathologic features and predictors of renal prognosis are largely unknown [1–8].
The renal TMA complicating MHTN may itself resemble thrombotic thrombocytopenic purpura (TTP), but distinguishing these two entities is important because of therapeutic implications [9, 10]. Plasmapheresis is beneficial in TTP, but of no benefit in TMA associated with MHTN.
Herein, we report two cases of MHTN with biopsy-proven renal TMA, with prolonged anuria requiring dialysis, but one patient recovered renal function after 9 months. We provide a clinic-pathologic correlation of MHTN in association with TMA based on follow-up biopsy findings.
Case reports
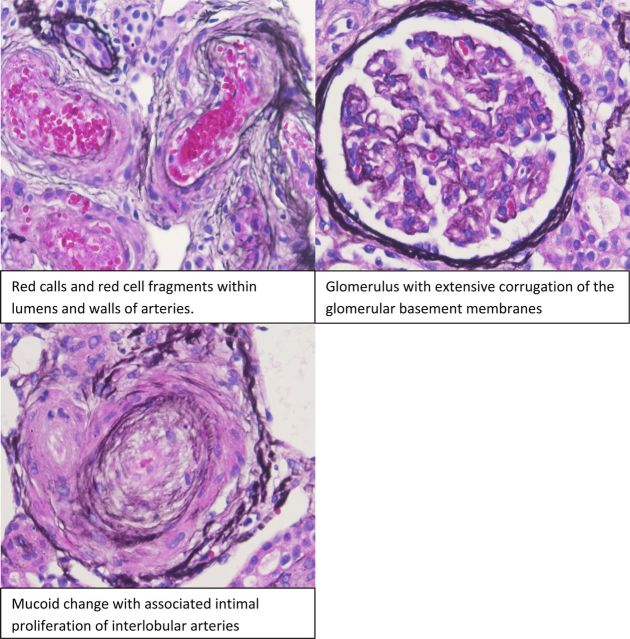
A 28-year-old black patient was admitted to hospital with headache, nausea and vomiting and an initial blood pressure (BP) of 218/120 mmHg. Physical examination showed hypertensive retinopathy with flame-shaped hemorrhages and exudates, but no papilledema. He had a serum creatinine level of 4 mg/dL (264 µmol/L), hemoglobin of 108 g/L and platelet count of 71 000. Over a 4-day period, the BP was decreased to 140/92 mmHg using Amlodipine (10 mg), Furosemide (80 mg/day), Minoxidil (10 mg/twice daily), Metoprolol (100 mg/day) and Hydrallazine (50 mg/every 8 h), but the oliguric AKI persisted with an increase in creatinine to 1144 µmol/L. Thrombocytopenia and hemolysis resolved. Coomb's test was negative. A renal ultrasound showed normal sized kidneys. Urinalysis showed hematuria, with ∼4 g/day proteinuria. There was evidence of hemolysis with schistocytes on peripheral smear, high LDH, and low haptoglobin. Serologic data such as anti-nuclear antibody, hepatitis B and C, C3, C4, human immunodeficiency virus (HIV) were all negative. The rest of the clinical data are shown in Table 1. Hemodialysis was initiated due to uremic symptoms and persistent AKI. Renal biopsy showed fibrinoid necrosis of arterioles and arteriolar thrombosis (see Figure 1). He remained dialysis-dependent for 9 months after which oliguria resolved and creatinine improved to 167.2 µmol/L. Hemodialysis was stopped and he continues to be followed up in a renal clinic.
Table 1.
Laboratory data on two patients with MHTN & TMA
| Clinical data | Mr A | Mr B |
|---|---|---|
| MAP | 158 | 180 |
| Initial creatinine | 13.4 | 9.5 |
| Peak creatinine | 13.4 | 11.1 |
| Platelet count | 85 000 | 40 000 |
| Platelet nadir | 71 000 | 29 000 |
| LDH | 1392 | 1352 |
| Haptoglobin | <30 | 69 |
| eGFR (admission) | 5.0 | 8.0 |
| ADAMTS13 | 57 | 70 |
| Bilirubin | 1.0 | 2.4 |
| Urine protein | 325 | 47 |
| Urine creatinine | 84 | 139 |
| Urine rbc | 25 | 10 |
| Renin | 38.5 | 46.5 |
| Aldosterone | 36 | 15 |
| PT | 15.1 | 13.3 |
| PTT | 31.8 | 29 |
| INR | 1.2 | 1 |
| Fibrinogen | 423 | 338 |
| Reticulocyte count | 3.4 | 1.9 |
| HIV | Non-reactive | Non-reactive |
| Hepatitis panel | Negative | Negative |
| Direct antithrombin | Negative | Negative |
| Hemodialysis | 9 months | On going |
Fig. 1.
Initial biopsy.
Case # 2: A 36-year-old black patient presented to the hospital with dyspnea, headache and visual blurring, and with a BP of 230/130 mmHg. The blood urea nitrogen was 105 mg/dL and creatinine peaked at 11 mg/dL (968 µmol/L). He had evidence of hemolysis and thrombocytopenia as shown in Table 1. Serologic data for antinuclear antibodies, Anti-double stranded DNA (Ant-DSDNA), anti scl-70 and other sclerodermal serologies were negative. The patient had evidence of hemolysis with high LDH and low platelets. Over a 3-day period, his BP control improved to 130 mmHg/80 mmHg using a similar five-drug regimen. His platelet count improved to 130 000, and LDH returned to normal. However, he required hemodialysis, since oliguric AKI persisted and a kidney biopsy was done. The biopsy showed arteriolar hyalinosis and onion-skin type changes similar to sclerodermal kidneys. This patient has remained dialysis-dependent with irreversible chronic kidney disease for 2 years.
Both patients presented with MHTN and TMA and severe oliguric AKI requiring hemodialysis. Evidence of hemolysis and thrombocytopenia resolved within 2 weeks of achieving good BP control. Oliguric AKI persisted for 9 months in Case # 1 and irreversible in Case # 2. Repeat biopsies (Figure 2A and B) showed persistent renal TMA in Case # 2, but resolution in Case 1.
Fig. 2.
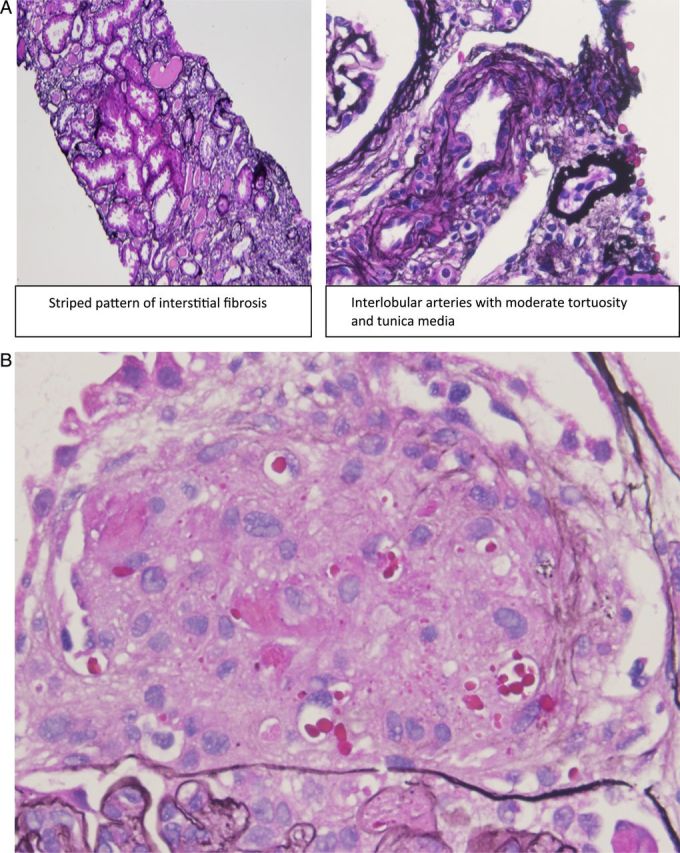
(A) Fibrosis in interstitium and persistent TMA in (B). (B) Persistent TMA in glomerulus in patient who did not recover (×400).
Discussion
TMA describes a pathologic process of microvascular thrombosis, thrombocytopenia, microangiopathic hemolysis and end-organ dysfunction [1]. Our patients presented with MHTN and renal TMA. The combination of MHTN and renal TMA is rare and ∼10 other reports of this syndrome have been reported in the literature. Renal dysfunction with persistent AKI is an important cause of morbidity and mortality in MHTN [11–13]. The prevalence of the combination of TMA and MHTN has been variously reported as 44% by Akimoto et al. [10], while van den Born reported a prevalence of 27%.
Our patients have been followed up for 18 months so far. The findings on repeat biopsy are quite striking because in patient # 1, there has been resolution of glomerular and arteriolar evidence of TMA, whereas these changes are persistent in Case # 2. Persistent TMA in Case # 2 was associated with endothelial damage, fibrinoid necrosis and an obliterative vasculopathy which may explain persistence of kidney failure with dialysis-dependence.
The precise pathogenesis of TMA occurring in the setting of MHTN is unknown, but an association between renin-angiotensin system activation has been suggested [8]. Another study has suggested that aldosterone level rather than renin activity correlated better as an indicator of vascular injury in MHTN.
Similarly, the relationship between renal prognosis and TMA when this occurs with MHTN has been a source of controversy.
Distinguishing between the MHTN with AKI and TMA from TTP is difficult due to significant overlap of presentation [7–10], although this has major therapeutic implications as plasmapheresis has a dramatic beneficial effect in TTP [1], but has no benefit in MHTN with TMA. In TTP, there is a congenital or acquired deficiency of ADAMTS13, a zinc-containing enzyme that cleaves large von Willebrand factor (VWF) multimers, thereby decreasing their thrombogenicity. Recently, it has been shown that there is an association between TMA and reduced ADAMTS13 activity in malignant hypertension [14]. Our patients had normal ADAMTS13 enzyme levels and did well without plasmapheresis. In one report, a low ADAMTS 13 level was suggested as a possible beneficial guide to possible need for plasmapheresis [7].
The issue of recoverability of renal function in MHTN with TMA remains speculative. While some studies suggest resolution of AKI resulted from resolution of TMA and tubular necrosis lesions, others suggest that TMA lesions were associated with greater likelihood of renal recovery [15].
Teaching points
MHTN may be associated with TMA and persistent AKI with prolonged need for renal replacement therapy.
Good blood pressure control leads to resolution of hemolysis and thrombocytopenia, although AKI may persist for a variable period.
Use of ACE- inhibitors and vasodilators are beneficial in this setting.
Plasmapheresis is not necessary unless severe thrombocytopenia (<25 000/mm3) or severe deficiency of ADAMTS13 is documented.
Conflict of interest statement
None declared.
References
- 1.Barbour T, Johnson S, Cohney S, et al. Thrombotic Microangiopathy and associated renal disorders. Nephrol Dial Transplant. 2012;27:2673–2685. doi: 10.1093/ndt/gfs279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao J, Zhou L, Wei J, et al. Uremia and thrombotic microangiopathy: conditions that have the same manifestations. Ren Fail. 2013;35:286–288. doi: 10.3109/0886022X.2012.743913. [DOI] [PubMed] [Google Scholar]
- 3.Gowda M, Nainani N, Lohr J, et al. A patient with malignant hypertension, thrombotic microangiopathy, and nephrotic-range proteinuria. Am J Kidney Dis. 2014;63:A23–A25. doi: 10.1053/j.ajkd.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 4.Deguchi I, Uchino A, Suzuki H, et al. Malignant hypertension with reversible brain stem hypertensive encephalopathy and thrombotic microangiopathy. J Stroke Cerebrovasc Dis. 2012;21:915.e17–915.e20. doi: 10.1016/j.jstrokecerebrovasdis.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Van den Born BJ, Honnebier UP, Koopmans RP, et al. Microangiopathic hemolysis and renal failure in malignant hypertension. Hypertension. 2005;45:246–251. doi: 10.1161/01.HYP.0000151620.17905.ee. [DOI] [PubMed] [Google Scholar]
- 6.Guerin C, Gonthier R, Berthoux FC. Long-term prognosis in malignant or accelerated hypertension. Nephrol Dial Transplant. 1988;3:33–37. [PubMed] [Google Scholar]
- 7.Shibagaki Y, Fujita T. Thrombotic microangiopathy in malignant hypertension and hemolytic uremic syndrome (HUS)/thrombotic thrombocytopenic purpura (TTP): can we differentiate one from the other? Hypertens Res. 2005;28:89–95. doi: 10.1291/hypres.28.89. [DOI] [PubMed] [Google Scholar]
- 8.Clark WF, Hildebrand A. Attending rounds: microangiopathic hemolytic anemia with renal insufficiency. Clin J Am Soc Nephrol. 2012;7:342–347. doi: 10.2215/CJN.07230711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egan JA, Bandarenko N, Hay SN, et al. Differentiating thrombotic microangiopathies induced by severe hypertension from anemia and thrombocytopenia seen in thrombotic thrombocytopenic purpura. J Clin Apher. 2004;19:125–129. doi: 10.1002/jca.20016. [DOI] [PubMed] [Google Scholar]
- 10.Boctor FN, Prichard JW. Kidney involvement in thrombotic thrombocytopenic purpura and malignant hypertension. Transfusion. 2009;49:1783–1784. doi: 10.1111/j.1537-2995.2009.02250.x. [DOI] [PubMed] [Google Scholar]
- 11.Higashi AY, Nogaki F, Ono T, et al. Case of kidney failure with thrombotic microangiopathy lesions in renal biopsy caused by accelerated hypertension in a young adult. Nihon JinzoGakkai Shi. 2009;51:878–883. [PubMed] [Google Scholar]
- 12.Malignant hypertension presenting as hemolysis, thrombocytopenia, and renal failure. Rev Cardiovasc Med. 2003;4:255–259. [PubMed] [Google Scholar]
- 13.Akimoto T, Muto S, Ito C, et al. Clinical Features of Malignant hypertension with thrombotic microangiopathy. Clin Exp Hypertens. 2011;33:77–83. doi: 10.3109/10641963.2010.503303. [DOI] [PubMed] [Google Scholar]
- 14.Remuzzi G. Is ADAMTS13 deficiency specific for thrombotic thrombocytopenic purpura? No. J Thromb Haemost. 2003;1:632–634. doi: 10.1046/j.1538-7836.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- 15.Khanna A, McCullough PA. Malignant hypertension presenting as hemolysis, thrombocytopenia, and renal failure. Rev Cardiovasc Med. 2003;4:255–259. [PubMed] [Google Scholar]