Abstract
Morphometric analyses of vegetative and floral characters were conducted in 21 populations of five Pleurothallis (Orchidaceae) species occurring in Brazilian ‘campo rupestre’ vegetation. A phylogenetic analysis of this species group was also carried out using nuclear ribosomal DNA internal transcribed spacers (ITS1 and ITS2). Results of the ordination and cluster analyses agree with species’ delimitation revealed by taxonomic and allozyme studies. The groups formed in ordination analysis correspond to the pollinator groups determined in a previous pollination study. Relationships among the species in the cluster analysis using only vegetative characters are similar to those found in a previous allozyme study, but those indicated by cluster analysis using only floral characters differ. These results support the hypothesis that floral similarities are due to convergence driven by similar pollination mechanisms, and therefore floral traits may not be good indicators of phylogenetic relationships in this group. The results of the phylogenetic analysis support this conclusion to some extent. There is no correlation between genetic (allozyme) and morphological variability in the populations nor in the way this variability is distributed among conspecific populations. We describe a new subspecies of Pleurothallis ochreata based on differences in vegetative and chemical characters as well as geographic distribution. Absence of differentiation in floral characters, attraction of the same pollinator species, interfertility and genetic similarity support the argument for subspecific rather than specific status.
Key words: Pleurothallis, Pleurothallis ochreata subsp. cylindrifolia, Orchidaceae, morphometrics, phylogeny, convergence, morphological variability, campo rupestre
INTRODUCTION
Circumscription of orchid taxa and classification based on their possible phylogenetic relationships have mainly relied on morphological floral characters. However, some decades ago, floral biologists drew attention to pollination mechanisms that resulted from convergence and radiation events, exposing the artificial nature of classification in some groups (Dodson, 1962; van der Pijl and Dodson, 1966). Convergence and radiation in floral traits are probably widespread in this family (e.g. Johnson et al., 1998), and some cases have recently been confirmed by using macromolecular techniques (van den Berg et al., 2000; Whitten et al., 2000; Williams et al., 2001). The role of floral and vegetative characters in orchid classification has been re‐evaluated by several authors (e.g. Chase and Palmer, 1992).
Various authors (Herrera, 1996; Ollerton, 1996; Waser et al., 1996) have cast doubt on the existence of pollination syndromes [suggested by van der Pijl and Dodson (1966) and Faegri and van der Pijl (1979), among others, based on convergence due to similar specialized pollination mechanisms]. Conversely, Johnson and Steiner (2000) argued in favour of the existence of syndromes in several groups. Using more objective morphometric multivariate methods, Sakai et al. (1999) demonstrated the occurrence of convergence and radiation in floral traits and their correlation with pollination guilds in Zingiberales.
Studying five Pleurothallis spp. occurring in the Brazilian ‘campo rupestre’ vegetation, Borba et al. (2001c) found unexpectedly high genetic variability in several populations and a low to moderately high differentiation among conspecific populations. These species are pollinated by flies belonging to the Chloropidae (P. johannensis Barb.Rodr. and P. fabiobarrosii Borba & Semir by Tricimba sp.; P. adamantinensis Brade by Hippelates sp.) and Phoridae (P. teres Lindl. and P. ochreata Lindl. by Megaselia spp.). Floral similarity in Pleurothallis spp. pollinated by the same fly species appears to occur (see Figs 2–4 in Borba and Semir, 2001). Based on their allozyme results, Borba et al. (2001c) suggested that these floral similarities are due to convergence, and therefore floral traits may not be reliable indicators of phylogenetic relationships in this group. Of the five species in this study, P. johannensis and P. ochreata have recently been transferred to the genus Acianthera Scheidw. as A. johannensis (Barb.Rodr.) Pridgeon & M.W.Chase and A. ochreata (Lindl.) Pridgeon & M.W.Chase, respectively (Pridgeon and Chase, 2001). However, not all species’ epithets were assigned to Acianthera and the other segregate genera in that paper, so we here prefer to use the combinations in Pleurothallis.
In this study we present results of morphometric analyses using multivariate methods and a phylogenetic analysis using nuclear ribosomal DNA ITS (internal transcribed spacer) sequences for the same populations of the five Pleurothallis spp. included in our previous studies of genetic variability (Borba et al., 2001c), reproductive biology (Borba and Semir, 2001; Borba et al., 2001a) and taxonomy (Borba et al., 2000). Our aims were to: (1) confirm the delimitation of these Pleurothallis spp.; (2) verify the correlation between floral morphology and pollination guilds; (3) outline the phylogenetic relationships among these species; (4) test the hypotheses of convergence and radiation in floral traits presented by Borba et al. (2001c); and (5) verify the existence of correlation between genetic and morphological variability and determine how this variability is distributed among conspecific populations.
MATERIALS AND METHODS
Morphometric analyses
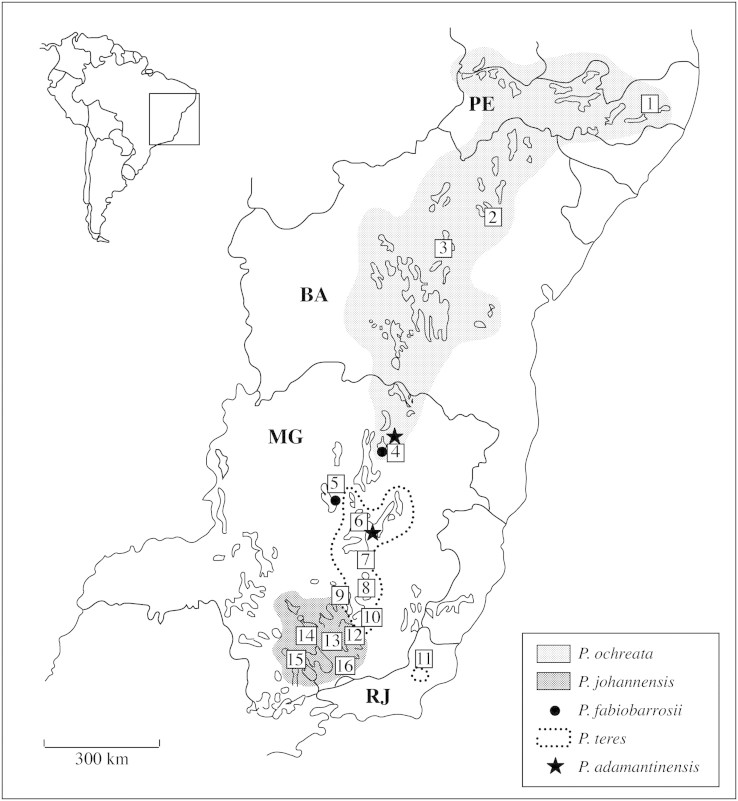
Plants of the five Pleurothallis spp. used for the morphometric analyses were collected from 21 natural populations at 16 localities in Minas Gerais, Bahia and Pernambuco states, in southeast and northeast Brazil: P. johannensis (seven populations), P. teres (six populations), P. ochreata (four populations), P. fabiobarrosii (two populations) and P. adamantinensis (two populations; Table 1; Fig. 1). Great care was taken to collect individuals from different clones, because these species can reproduce vegetatively. We sampled a total of 372 individuals of the five species. The individuals were maintained in a glasshouse at Universidade Estadual de Campinas, Campinas, São Paulo state (22°49′S, 47°06′W) for 2–3 years. Leaves, stems and flowers that developed in the glasshouse were used for measurements. In some exceptional cases, plant material collected directly in the field was used (Table 1). The climate of the areas where the individuals were collected is similar to that of Campinas, Cwb in Köeppen’s (1948) classification. Vouchers are deposited in the herbarium of Universidade Estadual de Campinas (UEC; Table 1).
Table 1.
Populations of Pleurothallis johannensis, P. teres, P. ochreata, P. fabiobarrosii and P. adamantinensis studied, together with the number of individuals (n) used in the morphometric analysis and the number of years in cultivation in the glasshouse
| Species/population | Code | n | Years in cultivation | Location | Voucher |
| P. johannensis | |||||
| Carrancas‐MG (pop. 1) | C1 | 15 | 3·5 | 21°30′28”S; 44°36′00”W | Borba 516 |
| Carrancas‐MG (pop. 2) | C2 | 09 | 3·5 | 21°28′03”S; 44°32′56”W | Borba 517 |
| Carrancas‐MG (pop. 3) | C3 | 11 | 3·5 | 21°28′25” S; 44°37′00”W | Borba 518 |
| Itutinga‐MG | IT | 20 | 3 | 21°17′52”S; 44°42′45”W | Borba 507 |
| Santa R. Ibitipoca‐MG | IB | 24 | 2·5 | 21°36′S; 43°55′W | Borba 511 |
| São João Del Rei‐MG | SJ | 21 | 2 | 21°08′22”W; 44°17′28”W | Borba & Lucca 504 |
| Nazareno‐MG | NZ | 21 | 3 | 21°18′38”S; 44°35′27”W | Borba 519 |
| P. teres | |||||
| Serra do Rola Moça‐MG | RM | 29 | Field | 20°03′37”S; 44°01′58”W | Borba 520 |
| Serra do Cipó‐MG (pop. 1) | CA | 20 | Field | 19°17′32”S; 43°35′37”W | Semir & Vitta s.n. |
| Serra do Cipó‐MG (pop. 2) | CC | 11 | 3 | 19°19′30”S; 43°33′50”W | Borba 521 |
| Ouro Preto‐MG | OP | 20 | 2·5 | 20°24′S; 43°29′W | Borba 522 |
| Caeté‐MG | CT | 21 | 2 | 19°48′50”S; 43°40′31”W | Borba & Lucca 509 |
| Diamantina‐MG | DI | 24 | 3/Field | 18°14′36”S; 43°38′05”W | Borba 523 |
| P. ochreata | |||||
| Grão Mogol‐MG | GM | 23 | 3 | 16°33′S; 42°54′W | Borba 505 |
| Morro do Chapéu‐BA | MC | 14 | 3 | 11°33′S; 41°09′W | Solferini & Vaccarelli s.n. |
| Jacobina‐BA | JC | 09 | 2·5 | 11°40′23”S; 40°40′17”W | Romero et al. 5657 |
| Camocim de São Félix‐PE | CS | 07 | 2·5 | 8°21′S; 35°45′W | Semir s.n. |
| P. fabiobarrosii | |||||
| Joaquim Felício‐MG | SC | 21 | 2/Field | 17°43′S, 44°17′W | Borba & Felix 501 |
| Grão Mogol‐MG | GM | 20 | 2 | 16°33′S; 42°54′W | Borba & Felix 512 |
| P. adamantinensis | |||||
| Grão Mogol‐MG | GM | 18 | 2 | 16°33′S; 42°54′W | Borba & Felix 550 |
| Diamantina‐MG | DI | 14 | 2 | 18°14′S; 43°38′W | Borba 551 |
Some individuals or populations used in the morphometric studies were collected directly from the field and not cultivated prior to use (indicated as ‘field’).
Vouchers are deposited at UEC.
Fig. 1. Distribution map of Pleurothallis johannensis, P. teres, P. ochreata, P. fabiobarrosii and P. adamantinensis based on field collection and herbarium material, and location of the populations studied. The area of occurrence of ‘campo rupestre’ vegetation is outlined. Localities: 1, Camocim de São Félix (CS); 2, Jacobina (JC); 3, Morro do Chapéu (MC); 4, Grão Mogol (GM); 5, Joaquim Felício (SC); 6, Diamantina (DI); 7, Serra do Cipó (CA, CC); 8, Caeté (CT); 9, Serra do Rola Moça (RM); 10, Ouro Preto (OP); 11, Santa Maria Madalena (SM); 12, São João Del Rei (SJ); 13, Nazareno (NZ); 14, Itutinga (IT); 15, Carrancas (C1, C2, C3); 16, Santa Rita do Ibitipoca (IB); PE, Pernambuco; BA, Bahia; MG, Minas Gerais; RJ, Rio de Janeiro states.
Thirty‐five continuous morphological characters were measured, of which 26 were floral and nine vegetative (Table 2; Fig. 2). Due to the minute size of the flowers, they were dissected and then drawn with the aid of an Olympus SZH 10 stereomicroscope equipped with a camera lucida, and measurements were then made from the drawings for greater precision. Detailed descriptions and illustrations of floral and vegetative characters of these species may be found in Borba and Semir (2001) and Borba et al. (2000).
Table 2.
Characters used in morphometric analysis of Pleurothallis johannensis, P. teres, P. ochreata, P. fabio barrosii and P. adamantinensis
| Character | Code |
| Dorsal sepal (spread) | |
| Length | 1 |
| Width | 2 |
| Apex angle | 3 |
| Fused lateral sepals (upper view) | |
| Outer width | 4 |
| Inner base width | 5 |
| Inner width | 6 |
| Inner apex angle | 7 |
| Outer apex angle | 8 |
| Petal | |
| Length | 9 |
| Width | 10 |
| Base width | 11 |
| Apex angle | 12 |
| Lip (spread) | |
| Length | 13 |
| Base width | 14 |
| Width | 15 |
| Apical lobe width | 16 |
| Isthmus length | 17 |
| Apical lobe length | 18 |
| Apex angle | 19 |
| Callus length | 20 |
| Distance between calli | 21 |
| Distance base to callus (apex) | 22 |
| Column | |
| Length | 23 |
| Foot length | 24 |
| Width | 25 |
| Base width | 26 |
| Stem | |
| Length | 27 |
| Mid‐region diameter | 28 |
| Apex diameter | 29 |
| Leaf (cross‐section at mid‐region) | |
| Lateral diameter | 30 |
| Dorsiventral diameter | 31 |
| Thickness | 32 |
| Distance between margins | 33 |
| Inner angle | 34 |
| Length | 35 |
See Fig. 2 for a diagram of the variables.
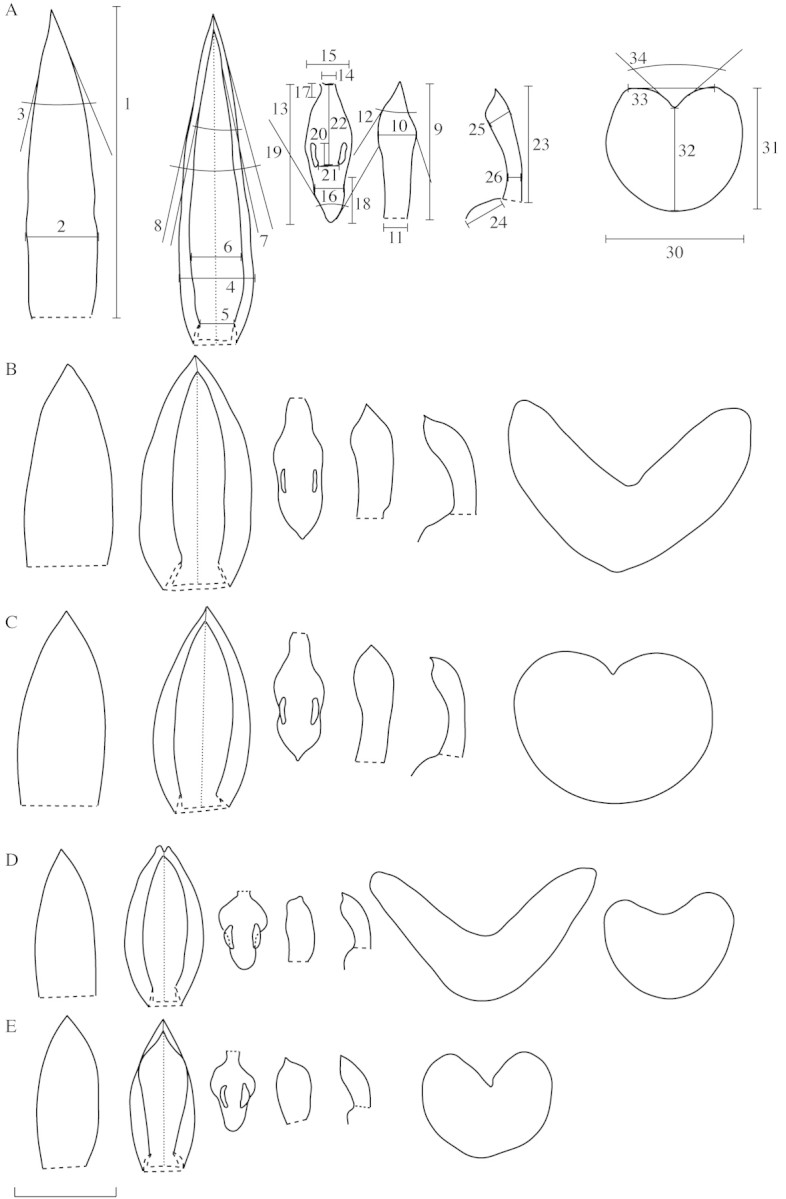
Fig. 2. Outline of flower parts and cross‐section of leaf of Pleurothallis adamantinensis (A), P. fabiobarrosii (B), P. johannensis (C), P. ochreata (D) and P. teres (E), and characters used in morphometrical analysis (except 27–29 and 35). From left to right: dorsal sepal (spread); fused lateral sepals; labellum (spread); petal; column (side view); and cross‐section of leaf at mid‐region. In P. ochreata (D), a typical leaf (left) and a leaf from the Grão Mogol population (right) are shown. See Table 2 for character codes. Scale bar = 3.5 mm (floral parts) and 5.0 mm (leaf)
We conducted principal component analysis (PCA) for all characters, including all individuals of the five species, and also for each species separately. We also carried out cluster analyses for the populations using all characters, or only floral or vegetative characters. For the population cluster analyses, we used the Mahalanobis generalized distance calculated from the pooled residual covariances within groups matrix and UPGMA (unweighted pair–group method of arithmetical averages) as the clustering algorithm. We used a multi‐response permutation procedure (MRPP) analysis to calculate the average within‐group distance (delta) for all populations and the chance‐corrected within‐group agreement (A) among populations of every species. We then carried out an analysis of correlation between genetic variability and delta values, and between genetic differentiation among populations and A values for all populations of all five species pooled. We measured genetic variability using observed mean heterozygosity (Ho) from allozyme data, and genetic differentiation among populations by Fst [Wright, 1978; data from Borba et al. (2001c), in which the same individuals of these populations were used]. All data were arcsin‐transformed before calculating the correlation coefficient, and its significance was tested using Table B.17 in Zar (1999). The Mahalanobis generalized distance matrices (all variables, only floral variables and only vegetative variables) were compared with matrices of Nei’s (1978) genetic distance [from Borba et al. (2001c), data not shown] and geographic distance (see Appendix 1 of Borba et al., 2001c) of populations of P. johannensis and P. teres using the Mantel test, with the Monte Carlo option of PCOrd (1000 randomizations). The procedure was not used for the other species because of the low number of populations. PCA, the MRPP analysis and the Mantel test were carried out using PCOrd 4·10 (McCune and Mefford, 1999), and the cluster analyses were run using the Statistica for Windows software package (StatSoft, 1995).
Phylogenetic analysis
Material from the five species (eight populations) used in the morphometric studies and another five samples of different lineages of Pleurothallidinae were selected (Table 3). DNA was extracted from fresh leaves using a modified 2 × CTAB protocol (Doyle and Doyle, 1987) and purified using QIAquick silica columns (QIAgen, Ltd, Crawley, UK). ITS1, 5·8S and ITS2 regions were amplified as a single piece using the primers 17SE and 26SE (Sun et al., 1994). The PCR programme consisted of 4 min at 94 °C (initial denaturation), 28 cycles of 1 min at 94 °C (denaturation), 1 min at 50 °C (annealing) and 3 min at 72 °C (extension), and 7 min at 72 °C final extension. Resulting PCR products were purified using Concert (Gibco BRL, now Invitrogen, Ltd, Paisley, UK) silica columns. Cycle sequencing was performed in both directions with the Big Dye Terminator Kit (PE Applied Biosystems, Inc., Warrington, UK) and run on an ABI 377 automated sequencer following the manufacturer’s protocols. Electropherograms were superimposed and edited with Sequencher 3·1·1 (Genecodes Inc., Ann Arbor, MI, USA), and edited sequences were aligned by eye. The data were analysed using PAUP 4·0 (Swofford, 1998), with Fitch parsimony (Fitch, 1971; equally weighted, unordered character states) as the optimality criterion and a branch‐and‐bound search to find all shortest trees. Support was assessed using 1000 replicates of character bootstrapping (Felsenstein, 1985). Trees were rooted using Octomeria gracilis Lodd. ex Lindl. because this genus was identified as sister to the remaining Pleurothallidinae (Pridgeon et al., 2001). All sequences have been submitted to GenBank (accession numbers in Table 3). The aligned matrix is available from the authors (E.L.B., borba@uefs.br and C.V.D.B., vcassio@gmx.de).
Table 3.
Species of Pleurothallidinae sequenced for ITS
| Species/population | Voucher | GenBank no. |
| P. johannensis Barb. Rodr. (São João Del Rei‐MG) | Borba & Lucca 504 (UEC) | AF366939 |
| P. teres Lindl. (Ouro Preto‐MG) | Borba 522 (UEC) | AF366935 |
| P. teres Lindl. (Santa Maria Madalena‐RJ) | Borba503 (UEC) | AF366937 |
| P. ochreata Lindl. (Grão Mogol‐MG) | Borba505 (UEC) | AF366934 |
| P. ochreata Lindl. (Morro do Chapéu‐BA) | Solferini & Vaccarelli s.n (UEC) | AF366933 |
| P. ochreata Lindl. (Pernambuco state) | Kew 1974–1034 (K) | AF262858 |
| P. fabiobarrosii Borba & Semir (Grão Mogol‐MG) | Borba & Felix512 (UEC) | AF366938 |
| P. adamantinensis Brade (Diamantina‐MG) | Borba 551 (UEC) | AF366936 |
| Octomeria gracilis Lodd. ex Lindl. | Hermans 2334 (K spirit 58256) | AF262911 |
| Pleurothallis grandiflora Lindl. | Hermans 2654 (K) | AF368320 |
| Dracula chimaera (Rchb.f.) Luer | Chase O‐967 (K) | AF262966 |
| Masdevallia floribunda Lindl. | Chase O‐296 (K) | AF260146 |
| Restrepiella ophiocephala (Lindl.) Garay & Dunst. | Chase O‐291 (K) | AF262909 |
The populations of Pleurothallis johannensis, P. teres, P. ochreata, P. fabiobarrosii and P. adamantinensis used are indicated in parentheses. Vouchers are deposited at UEC and K.
RESULTS
Interspecific morphological variability
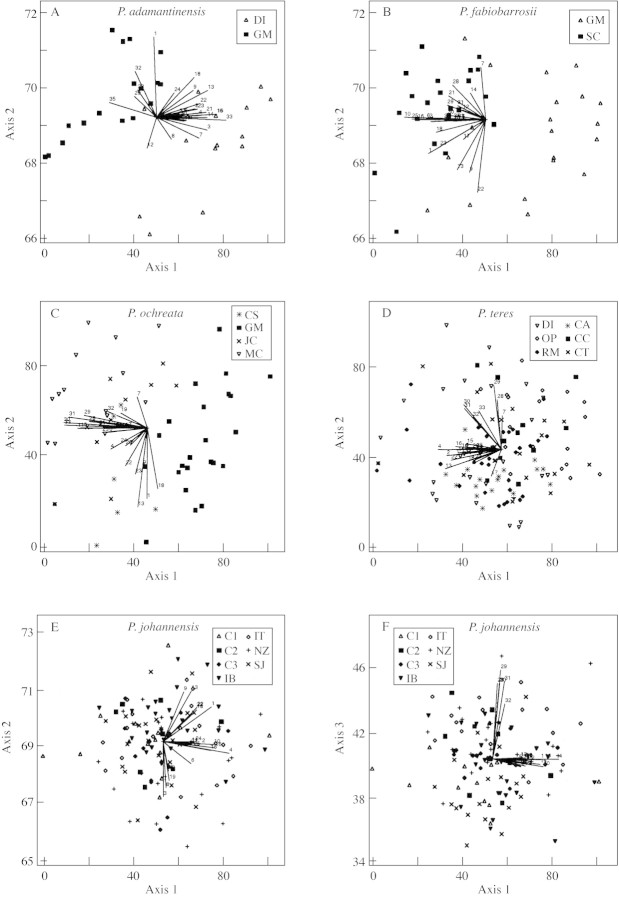
In the ordination analysis (PCA), three groups were delimited on the first two axes (Fig. 3A). One of these groups was formed exclusively by all individuals of P. adamantinensis and was separated from the other groups mainly by the angles of floral parts and sepal length. The other groups were formed by individuals of P. johannensis with P. fabiobarrosii, and P. teres with P. ochreata. These two groups were separated by the majority of floral characters, almost all of them well represented on the first axis. The three groups were coincident with the three pollinator groups (Fig. 3A) found by Borba and Semir (2001a). The species within the two species pairs (P. johannensis–P. fabiobarrosii and P. teres–P. ochreata) were separated on the third axis, formed mainly by vegetative characters (Fig. 3B). However, there was some overlap on the third axis between individuals of P. teres and P. ochreata, due to the similarity in leaf characters of the Grão Mogol population of P. ochreata to P. teres (Fig. 2D and E).
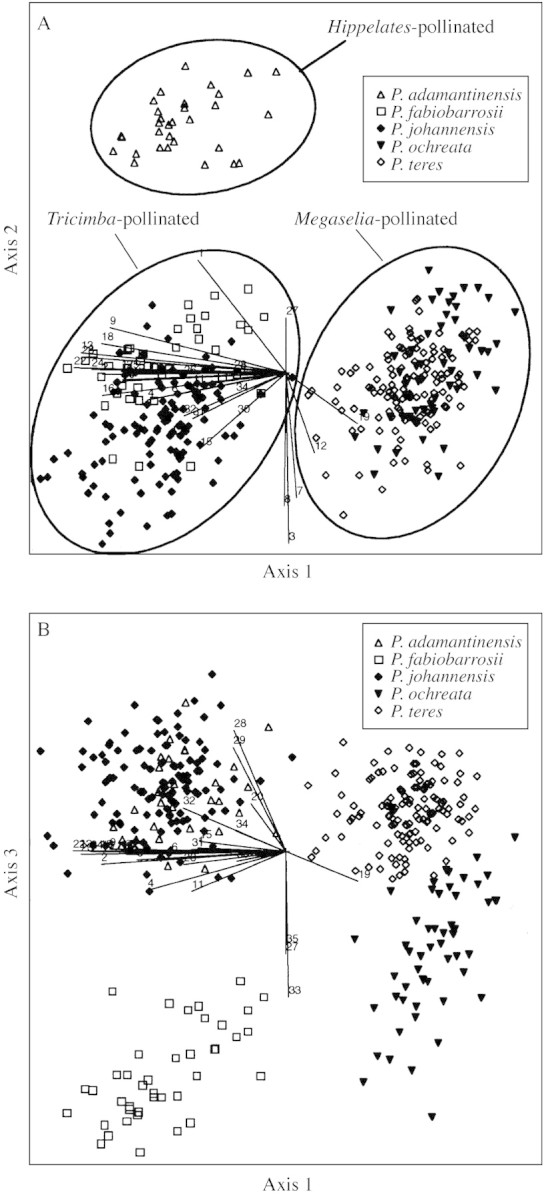
Fig. 3. Biplot representation of the scores on the first three axes of the principal component analysis of 35 morphological characters of Pleurothallis adamantinensis, P. fabiobarrosii, P. johannensis, P. ochreata and P. teres. See Table 2 for character codes. Percentage of variance accumulated on the first three axes = 70·54 (first axis = 44·89; second axis = 14·79; third axis = 10·86).
The same relationships between species were also observed in the cluster analyses. In analyses including all characters and those including floral characters only, there were five groups, each formed exclusively by all conspecific populations (Fig. 4A and B). Higher level groups were formed by the species pairs P. johannensis with P. fabiobarrosii, and P. ochreata with P. teres. In the analysis using vegetative characters only, the Grão Mogol population of P. ochreata did not group with the other conspecific populations. This population clustered with the populations of P. teres (Fig. 4C). Higher level groups were formed by the species pairs P. johannensis with P. teres, and P. ochreata with P. fabiobarrosii.
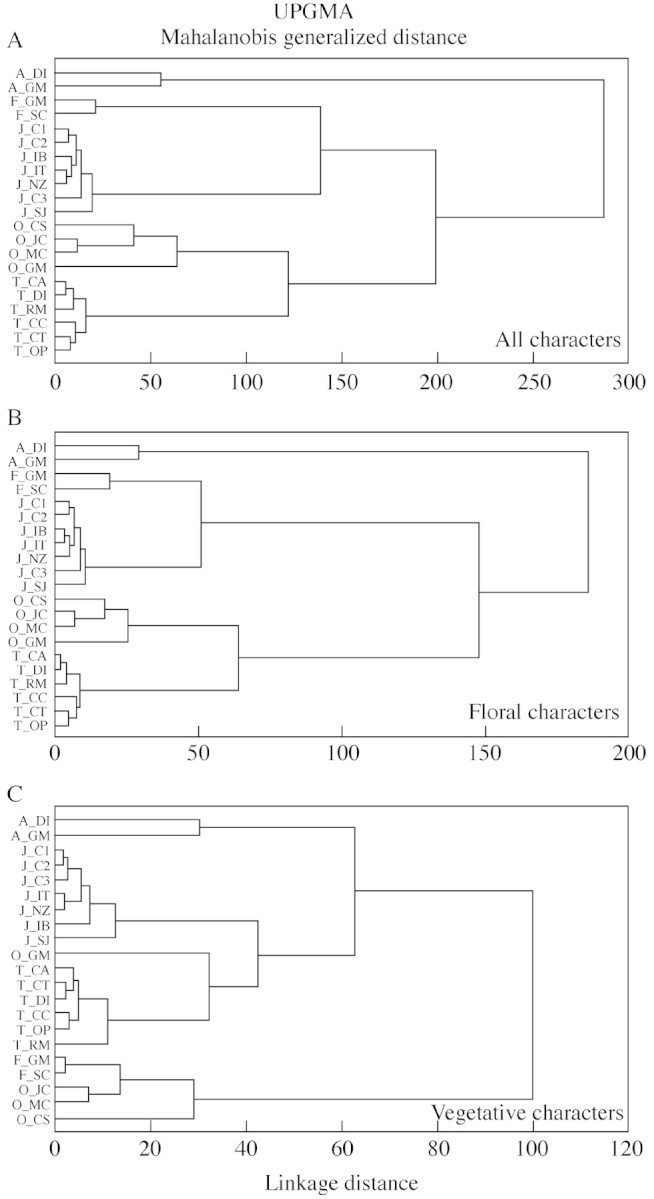
Fig. 4. Dendrograms showing the phenetic relationship among the populations of Pleurothallis adamantinensis, P. fabiobarrosii, P. johannensis, P. ochreata and P. teres based on 35 morphological characters (A), 26 floral characters (B) or nine vegetative characters (C) using Mahalanobis generalized distance calculated from the pooled residual covariances within groups matrix and UPGMA as the clustering algorithm. See Table 1 for population codes. Cophenetic correlation = 0·852 (A), 0·886 (B) and 0·773 (C).
Intraspecific morphological variability and genetic variability
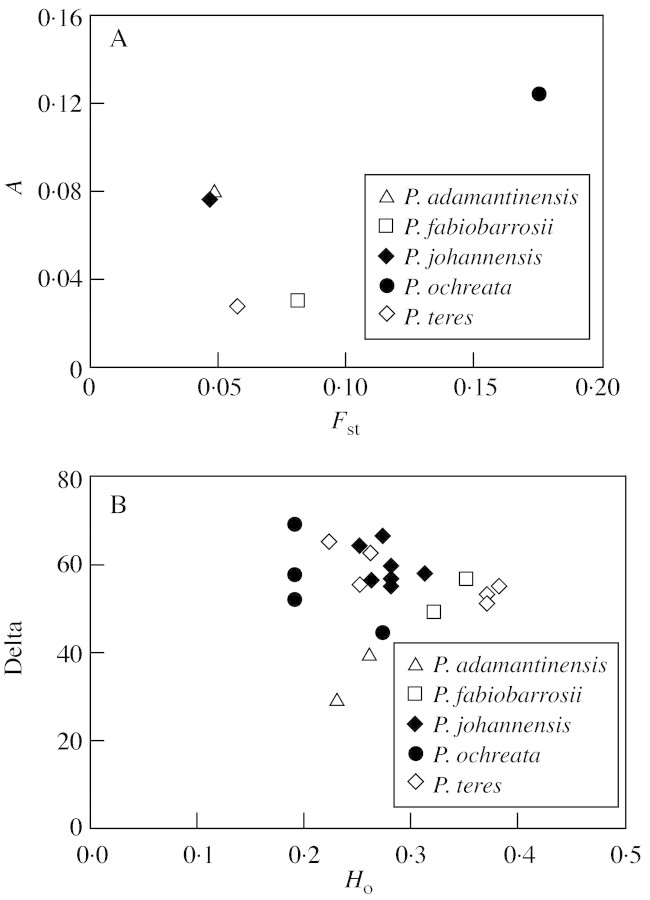
All A values were statistically significant (P < 0·002), indicating that there is morphological structuring among conspecific populations in all species. The highest A value was observed in P. ochreata (A = 0·1255), due mainly to the differentiation in leaf characters of the Grão Mogol population (Figs 2D, 4C and 5C). Intermediate values were observed in P. johannensis (A = 0·0774), due mainly to the differentiation of the São João Del Rei population, which has individuals with more sulcate leaves (Fig. 5E and F), and in P. adamantinensis (A = 0·0809), which has relatively high differentiation in leaf traits between the two populations (Figs 4C and 5A). Relatively low A values were found in P. teres (A = 0·0281) and P. fabiobarrosii (A = 0·0321) despite the apparent separation of the populations of the latter in the PCA (Fig. 5B and D). The correlation between species values of A and Fst was not significant (Fig. 6A; r = 0·6486; d.f. = 3; P > 0·05). A similar result was obtained for population values of delta and genetic variability (Ho; Fig. 6B; r = 0·1010; d.f. = 19; P > 0·05).
Fig. 5. Biplot representation of the scores on the first two (three for P. johannensis) axes of the principal component analysis of 35 morphological characters of Pleurothallis adamantinensis (A), P. fabiobarrosii (B), P. ochreata (C), P. teres (D) and P. johannensis (E and F). See Table 1 for population codes and Table 2 for character codes. Percentage of variance accumulated on the first two axes = 40·37 (A; first axis = 27·52; second axis = 12·85), 41·06 (B; first axis = 28·12; second axis = 12·94), 41·94 (C; first axis = 26·27; second axis = 15·67) and 33·84 (first axis = 21·03; second axis = 12·81). Percentage of variance accumulated on the first three axes of P. johannensis = 43·25 (first axis = 18·25; second axis = 13·80; third axis = 11·20).
Fig. 6. Relationship between A values and Fst (A) and between delta values and Ho (B) of 21 populations of Pleurothallis adamantinensis, P. fabiobarrosii, P. johannensis, P. ochreata and P. teres.
The Mantel test showed no significant correlation between Mahalanobis generalized distance and geographic distance or between Mahalanobis generalized distance and Nei’s genetic distance in P. johannensis (P > 0·15 in all cases) and P. teres (P > 0·17 in all cases).
Phylogenetic analysis
The branch‐and‐bound search found eight equally most parsimonious trees of 220 steps in length, a consistency index (CI) of 0·84 and a retention index (RI) of 0·79. One of the trees is shown in Fig. 7. A strongly supported (100 % bootstrap), closely related clade with all five Pleurothallis spp. studied here was indicated. The samples from the three different populations of P. ochreata formed a sister clade (90 % support) to the remaining species with strong bootstrap support (100 %). The clade with the remaining species received only 56 % bootstrap support and was internally unresolved except for the two samples of P. teres, which were sister to each other (75 % support). The relationships outside the Pleurothallis spp. group in this study were all strongly supported.
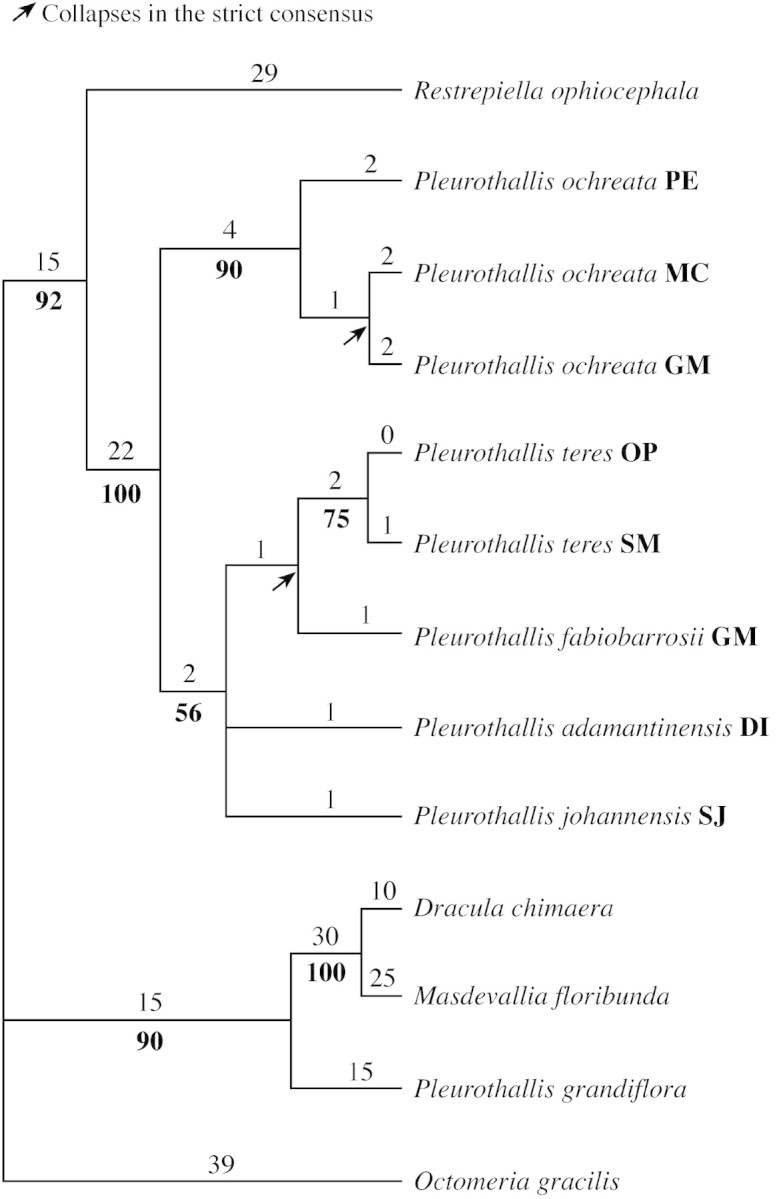
Fig. 7. One of the eight equally most parsimonious trees based on ITS sequencing data (L = 220; CI = 0·84; RI = 0·79). Fitch branch lengths are shown above the branches and bootstrap percentages below. Arrows indicate branches that collapse in the strict consensus of all trees.
DISCUSSION
Species’ delimitation and phylogenetic relationships
The results of the ordination and cluster analyses agree with the species’ delimitation proposed by Borba et al. (2000) and also supported by allozyme (Borba et al., 2001c), chemical (Borba et al., 2001b) and anatomical data (Scatena and Nunes, 1996). Borba et al. (2000) and Borba and Semir (2001) pointed out the floral similarity of P. johannensis with P. fabiobarrosii and showed that these species differ mainly in vegetative characters and minor lip characters. These conclusions are confirmed by the ordination analysis, in which the two species are separated mainly by vegetative characters. Conversely, the results of the cluster analysis showed that the two species can also be recognized based only on their floral characters, especially lip width, lip isthmus length, lip calli and apex angles of flower parts.
Based on the low genetic similarity between P. johannensis and P. fabiobarrosii [Nei’s (1978) unbiased genetic identity = 0·565] and between P. ochreata and P. teres (genetic identity = 0·647), Borba et al. (2001c) suggested that floral similarity in these two species pairs may be due to convergence to attract similar pollinators (see Figs 2–3 in Borba and Semir, 2001). Conversely, divergence in different pollination mechanisms may have occurred in the closely related P. johannensis and P. teres, species with high genetic similarity (0·774, rising to 0·80 if the highly differentiated Santa Maria Madalena population of P. teres is excluded from the analysis). Those authors also suggested that in this species group vegetative characters may be more informative in assessing phylogenetic relationships than floral ones. The species genetically most similar to P. ochreata is P. fabiobarrosii, and P. teres is most similar to P. johannensis. The occurrence of these same relationships in the cluster analysis using only vegetative characters and also the strong association of the three pollination guilds with the three groups found both on the first three axes of the ordination (mainly formed by floral characters) and in the cluster analysis using only floral characters strengthen the hypotheses presented by Borba et al. (2001c).
Recently, the understanding of phylogenetic relationships in Orchidaceae has been strongly challenged. Convergence and radiation in floral traits related to pollination mechanisms have been demonstrated in several orchid groups (e.g. Johnson et al., 1998; van den Berg et al., 2000; Williams et al., 2001), some suggested much earlier (e.g. van der Pijl and Dodson, 1966, p. 94). The role of floral and vegetative characters in assessing phylogenetic relationships has been re‐evaluated (e.g. Chase and Palmer, 1989, 1992). Despite the recent debate about the existence of pollination syndromes and specialized pollination systems (see Herrera, 1996; Ollerton, 1996; Waser et al., 1996; Johnson and Steiner, 2000), our data indicate that specialized pollination systems are correlated with floral character suites, which are, to some extent, predictable and largely compatible with the basic idea of pollination syndromes. Similar results have been found in other plant groups (e.g. Sakai et al., 1999) and are possibly widespread in angiosperms.
The phylogenetic analysis using ITS sequences indicates that P. teres, P. fabiobarrosii, P. adamantinensis and P. johannensis are closely related species, considering the number of nucleotide substitutions among the species and comparing them with the number of substitutions between the two populations of P. teres and among the three populations of P. ochreata. These levels of variation are similar to those of species complexes in other Orchidaceae–Epidendroideae groups (Ryan et al., 2000; van den Berg et al., 2000). Taking into account the occurrence of only six substitutions between P. ochreata and the other four species, we suggest that these are closely related groups. However, P. ochreata is not necessarily the closest species to the remainder because other species of this group have not been sampled. It is important to note that only P. ochreata has nodding inflorescences, the remaining species having erect inflorescences. Furthermore, P. ochreata is the only species occurring in northeast Brazil, the rest being found exclusively in southeast Brazil.
The low levels of divergence in ITS sequences do not conclusively show convergence in this group, but they do indicate that either convergence or radiation in floral traits must have occurred at least once. Based on our allozyme data (Borba et al., 2001c) and the geographical distribution of the species, we suggest that it is more plausible that convergence must have occurred between P. ochreata and P. teres. To clarify these relationships, other species belonging to this group must be included in the analysis, as must other genes.
It should be noted that Pleurothallis has been shown to be polyphyletic, requiring division into smaller genera to reflect phylogenetic relationships (Pridgeon et al., 2001; Pridgeon and Chase, 2001). The species studied here belong to Acianthera, but only some of the transfers have been made to date.
Differentiation in P. ochreata
Pleurothallis ochreata is a species with a wide distribution in northeastern Brazil, especially in Bahia, but it is also found in Pernambuco and Paraíba. The Grão Mogol population, in the north of Minas Gerais, is the only population known in southeastern Brazil, representing the southern extreme of distribution for this species (see Fig. 1). Although the ‘campos rupestres’ of Grão Mogol belong to the same mountain chain as the core area of P. ochreata distribution (the Espinhaço chain), they are geographically isolated, and high endemism has been found in this region (Giulietti and Pirani, 1988).
Borba et al. (2001b) studied the alkaloid profile of these Pleurothallis spp. and found that the Grão Mogol population of P. ochreata is differentiated from the remaining conspecific populations by an inversion of the relative abundance of 1‐hydroxymethylpyrrolizidine isomers. However, Borba et al. (2001c) found no clear genetic differentiation in this population compared with the same populations sampled by Borba et al. (2001b) in their study of alkaloids. These populations also attract the same pollinator species (Borba and Semir, 2001) and are interfertile (Borba et al., 2001a).
We have observed the occurrence of phenotypic plasticity in leaf traits in P. ochreata from Bahia and Pernambuco: individuals growing in open areas usually have fleshier, shorter leaves, but when cultivated in the glasshouse they produce larger, more flattened leaves, similar to those of individuals growing in shaded sites or as epiphytic individuals, and similar to the leaves of the holotype of the species. This characteristic led Pabst (1956) to give specific status to the rupicolous populations as P. bahiensis Pabst, but this was subsequently synonymized by the same author (Pabst and Dungs, 1975). Plasticity was not observed in leaves of individuals of the Grão Mogol population (individuals of these populations have been cultivated in our glasshouse for nearly 4 years).
Due to the differences in both leaf and chemical (Borba et al., 2001b) characters, we propose subspecific status for the Grão Mogol population of P. ochreata:
Pleurothallis ochreata subsp. cylindrifolia Borba and Semir subsp. nov. TYPE: BRAZIL. Minas Gerais: Grão Mogol, Serra do Barão, 16°33′S, 42°54′W, xii.1997, E. L. Borba 505 (holotype here designated: UEC!; isotype: BHCB!, HUEFS!, SP!, SPF!). Fig. 2D.
A subspecie typica characteribus floralibus maxime affinis sed ab ea foliis angustioribus teretis sulcatisque differt.
Leaves erect, cylindrical, sulcate, fleshy, approx. 65 (45–78) mm length, approx. 7 (6–9) mm lateral diameter, approx. 6 (5–7) mm dorsiventral diameter, approx. 4 (3–5) mm thickness, approx. 5 (4–7) mm distance between margins.
Specimens examined. Pleurothallis ochreatasubsp. cylindrifolia. Minas Gerais: Grão Mogol, Serra do Barão, E. L. Borba 1320, xii.1997 (UEC). Grão Mogol, Serra do Barão, E. L. Borba 1321, xii.1997 (UEC). Grão Mogol, Serra do Barão, E. L. Borba 1322, xii.1997 (UEC). Grão Mogol, Serra do Barão, E. L. Borba 1323, xii.1997 (UEC). Grão Mogol, Serra do Barão, E. L. Borba 1324, xii.1997 (UEC). Pleurothallis ochreatasubsp.ochreata. Bahia: Abaíra, W. Ganev 1668, 16.xii.1992 (HUEFS). Brejões, A. L. Peixoto 1627 & O. L. Peixoto, 15.xii.1981 (UEC). Jacobina, Romero et al. 5657, iv.1999 (UEC). Lençóis, L. R.Noblick 1740, 3.iv.1980 (HUEFS). Lençóis, L. Coradin et al. 6526, s.d. (SP). Lençóis, Serra da Chapadinha, S. J. Mayo 43, 5.vii.1994 (HUEFS). Monte Santo, L. P. Queiroz4606, 24.viii.1996 (HUEFS). Morro do Chapéu, Gerrit Davidse & W. G. D’Arcy 11928, 3.iv.1976 (SP). Morro do Chapéu, L. P. Queiroz 1288, 19.xi.1986 (HUEFS). Morro do Chapéu, L. P. Queiroz 4279, 14.iii.1995 (HUEFS). Morro do Chapéu, Serra Pé do Morro, R. M. Harley 3180, 29.vi.1996 (HUEFS). Mucugê, Rio Cumbuca, R. M. Harley 18172, 15.ii.1977 (UEC). Mucugê, N. L. Menezes et al. s.n., 20.vii.1981 (SP 195336). Palmeiras, Pai Inácio, A. Pereira 1750, 24.iv.1995 (HUEFS). Piatã, Serra de Santana, L. P. Queiroz 51520, 10.ii.1992 (HUEFS). Rio de Contas, Pico das Almas, R. M. Harley 27349 & D. J. N. Hind, 22.xii.1988 (SP). Umburanas, Serra do Curral, L. P. Queiroz 5165, 9.iv.1999 (HUEFS). Pernambuco: Camocim de São Félix, J. Semir s.n., v.1998 (UEC).
The absence of differences in floral characters, attraction of the same pollinator species (Borba and Semir, 2001), interfertility (Borba et al., 2001a) and genetic similarity, in which the Grão Mogol populations is nested within the other conspecific populations in both allozyme (Borba et al., 2001c) and ITS analyses, support the argument for subspecific rather than specific status for this population. This conclusion is reinforced by the geographical distribution because P. ochreata subsp. cylindrifolia is restricted to the southern extreme of the range of the species. Despite the difference in flowering time between the Grão Mogol population in the field and other conspecific populations, all of them flower together when grown in the glasshouse (Borba and Semir, 2001). This difference in flowering time in the field may result from differences in the rainy season at these localities (Borba and Semir, 2001). Even though it is relatively common for allozymic studies to confirm subspecies or geographic ‘races’ previously detected using either morphological or chemical data (e.g. Desrochers and Bohm, 1995), Hillis (1987) pointed out that ‘historically distinct evolutionary lineages may not show any particular level of divergence at either morphological or molecular level’, a point also stressed by Avise (1994).
Intraspecific morphological and genetic variability
Besides the absence of correlation between genetic and morphological variability in the populations, the absence of correlation in the way this variability is distributed among the conspecific populations is striking. For example, although P. ochreata showed both the highest A value (indicating high morphological differentiation) and Fst (indicating high genetic differentiation among populations; Borba et al., 2001c), these occur for different reasons: the high value of A is mainly due to differences in leaf characters in the Grão Mogol population, and the high value of Fst is due to differences in other populations (the highest values of genetic identity were found between the Grão Mogol population and the others; Borba et al., 2001c). Pleurothallis johannensis had a moderate A value, attributable to individuals with more deeply sulcate leaves in the São João Del Rei populations; however, this species had a low Fst, with high similarity between this population and the remaining ones (Borba et al., 2001c). Similar results were obtained with P. adamantinensis. Equally, although P. teres had low values of A and Fst, no correlation between morphological and genetic similarity among the populations was observed, resulting in a different topology for the dendrograms (see Fig. 2 in Borba et al., 2001a). These results are not completely unexpected because lack of congruence in partitioning of the variability among conspecific populations has been observed even when comparing different molecular markers (Avise, 1994).
It is important to note that in both morphological and genetic analyses, significant differences were found among conspecific populations. These populations grow on rock formations, as isolated islands of ‘campo rupestre’ vegetation. Because of the discontinuity of the mountain ranges and outcrops where these species occur, they have disjunct distributions. This characteristic has been suggested as the factor responsible for the great diversity and high endemism of these areas, among the highest in all Brazilian vegetation types (Joly, 1970; Giulietti and Pirani, 1988).
Absence of correlation between morphological and genetic variability has frequently been found in various plant groups and in animals (Turner, 1974; Mitton, 1978; Handford, 1980; Jain et al., 1980; Gilles, 1984; Elisens et al., 1992; Johannesson et al., 1993; Avise, 1994; Andersson, 1995; Karakousis and Kyriakopoulou‐Sklavounou, 1995). Giles (1984) stressed that inferences about rates of evolution and variation must not be taken to apply to the whole organism, but apply only to particular sets of characters. Recently, the importance of using different molecular markers to determine evolutionary significant units for conservation has been discussed, and several studies have used different methods resulting in different indicators (Avise, 1994; Newton et al., 1999). Our results confirm these statements and show that morphological, genetic and geographical indicators should be taken into account for conservation management or germplasm collection in this group and other plant groups of ‘campo rupestre’ vegetation (e.g. Jesus et al., 2001).
ACKNOWLEDGEMENTS
We thank Alec Pridgeon for providing some sequences for outgroups, Paulo I. K. L. Prado and Vera N. Solferini for discussion on some topics, Alec Pridgeon, Martin Ingrouille and Julie H. A. Dutilh for improvements to the manuscript, Angela B. Martins for the Latin diagnosis and for providing specimens of P. ochreata, Karla S. Yotoko for help in the laboratory, Juliana M. Felix, Veridiana N. Vaccarelli, Márcio Lucca and Graciela S. Oliver for help on field trips, and Geraldo W. Fernandes for giving us permission to collect on his ranch. E.L.B. received a fellowship from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 97/08800‐1). This study was funded by a grant from FAPESP to J.S. (97/08795‐8), and molecular work was funded by the Royal Botanic Gardens, Kew.
Supplementary Material
Received: 18 April 2001; Returned for revision: 2 September 2001; Accepted: 18 April 2002
References
- AnderssonE.1995. Age‐related differentiation among populations of Dactylorhiza traunsteineri (Orchidaceae) in eastern Sweden. Nordic Journal of Botany 15: 127–137. [Google Scholar]
- AviseJC.1994. Molecular markers, natural history and evolution. New York: Chapman and Hall. [Google Scholar]
- BorbaEL, Semir J.2001. Pollinator specificity and convergence in fly‐pollinated Pleurothallis (Orchidaceae) species: a multiple population approach. Annals of Botany 88: 75–88. [Google Scholar]
- BorbaEL, Semir J, Shepherd GJ.2001a Self‐incompatibility, inbreeding depression, and crossing potential in five Brazilian Pleurothallis (Orchidaceae) species. Annals of Botany 88: 89–99. [Google Scholar]
- BorbaEL, Trigo JR, Semir J.2001b Variation of diastereoisomeric pyrrolizidine alkaloids in Pleurothallis (Orchidaceae). Biochemical Systematics and Ecology 29: 45–52. [DOI] [PubMed] [Google Scholar]
- BorbaEL, Felix JM, Semir J, Solferini VN.2000. Pleurothallis fabiobarrosii, a new Brazilian species: morphological and genetic data, and notes on the taxonomy of Brazilian rupicolous Pleurothallis Lindleyana 15: 2–9. [Google Scholar]
- BorbaEL, Felix JM, Solferini VN, Semir J.2001c Fly‐pollinated Pleurothallis (Orchidaceae) species have high genetic variability: evidence from isozyme markers. American Journal of Botany 88: 419–428. [PubMed] [Google Scholar]
- ChaseMW, Palmer JD.1989. Chloroplast DNA systematics of the lilioid monocots: feasibility, resources, and an example from the Orchidaceae. American Journal of Botany 76: 1720–1730. [Google Scholar]
- ChaseMW, Palmer JD.1992. Floral morphology and chromosome number in subtribe Oncidiinae (Orchidaceae): evolutionary insights from a phylogenetic analysis of chloroplast DNA restriction site variation. In: Soltis PS, Soltis DE, Doyle JJ, eds. Molecular systematics of plants New York: Chapman and Hall, 324–339. [Google Scholar]
- DesrochersAM, Bohm BA.1995. Biosystematic study of Lasthenia californica (Asteraceae). Systematic Botany 20: 65–84. [Google Scholar]
- DodsonCH.1962. The importance of pollination in the evolution of the orchids of tropical America. American Orchid Society Bulletin 31: 525–534, 641,–649, 731–735. [Google Scholar]
- DoyleJJ, Doyle JL.1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin19: 11–15. [Google Scholar]
- ElisensWJ, Boyd RD, Wolfe AD.1992. Genetic and morphological divergence among varieties of Aphanostephus skirrhobasis (Asteraceae‐Astereae) and related species with different chromo some numbers. Systematic Botany 17: 380–394. [Google Scholar]
- FaegriK, van der Pijl L.1979. The principles of pollination ecology, 3rd edn. Oxford: Pergamon Press. [Google Scholar]
- FelsensteinJ.1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- FitchWM.1971. Towards defining the course of evolution: minimum change for a specific tree topology. Systematic Zoology 20: 406–416. [Google Scholar]
- GillesBE.1984. A comparison between quantitative and biochemical variation in the wild barley Hordeum murinum Evolution 38: 34–41. [DOI] [PubMed] [Google Scholar]
- GiuliettiAM, Pirani JR.1988. Patterns of geographic distribution of some plant species from the Espinhaço Range, Minas Gerais and Bahia, Brazil. In: Heyer WR, Vanzolini PE, eds. Proceedings of a workshop on Neotropical distribution patterns Rio de Janeiro: Academia Brasileira de Ciências, 39–69. [Google Scholar]
- HandfordP.1980. Heterozygosity at enzyme loci and morphological variation. Nature 286: 261–262. [DOI] [PubMed] [Google Scholar]
- HerreraCM.1996. Floral traits and plant adaptation to insect pollinators: a devil’s advocate approach. In: Lloyd DG, Barrett SCH, eds. Floral biology: studies on floral evolution in animal‐pollinated plants. London: Chapman and Hall. [Google Scholar]
- HillisDM.1987. Molecular versus morphological approaches to system atics. Annual Review of Ecology and Systematics 18: 23–42. [Google Scholar]
- JainSK, Wu L, Vaidya KR.1980. Levels of morphological and allozyme variation in Indian amaranths: a striking contrast. Journal of Heredity 71: 283–285. [Google Scholar]
- JesusFF, Solferini VN, Semir J, Prado PI.2001. Local genetic differentiation in Proteopsis argentea (Asteraceae), a perennial herb endemic in Brazil. Plant Systematics and Evolution 226: 59–68. [Google Scholar]
- JohannessonK, Johannesson B, Rolán‐Alvarez E.1993. Morphological differentiation and genetic cohesiveness over a microenvironmental gradient in the marine snail Littorina saxatilis Evolution 47: 1770–1787. [DOI] [PubMed] [Google Scholar]
- JohnsonSD, Steiner KE.2000. Generalization versus specialization in plant pollination systems. Trends in Ecology and Evolution 15: 140–143. [DOI] [PubMed] [Google Scholar]
- JohnsonSD, Linder HP, Steiner KE.1998. Phylogeny and radiation of pollination systems in Disa (Orchidaceae). American Journal of Botany 85: 402–411. [PubMed] [Google Scholar]
- JolyAB.1970. Conheça a vegetação brasileira. São Paulo: EDUSP. [Google Scholar]
- KarakousisY, Kyriakopoulou‐Sklavounou P.1995. Genetic and morphological differentiation among populations of the green toad Bufo viridis from northern Greece. Biochemical Systematics and Ecology 23: 39–45. [Google Scholar]
- KöeppenW.1948. Climatologia com un estudio de los climas de la Tierra. Mexico: Fondo de Cultura Economica [transl. by Peres PRH]. [Google Scholar]
- McCuneB, Mefford MJ.1999. PCOrd – Multivariate analysis of ecological data, Version 4·10. Gleneder Beach: MjM Software. [Google Scholar]
- MittonJB.1978. Relationship between heterozygosity for enzyme loci and variation of morphological characters in natural populations. Nature 273: 661–662. [DOI] [PubMed] [Google Scholar]
- NeiM.1978. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NewtonAC, Allnutt TR, Gillies ACM, Lowe AJ, Ennos RA.1999. Molecular phylogeography, intraspecific variation and the conservation of tree species. Trends in Ecology and Evolution 14: 140–145. [DOI] [PubMed] [Google Scholar]
- OllertonJ.1996. Reconciling ecological processes with phylogenetic patterns: the apparent paradox of plant‐pollinator systems. Journal of Ecology 84: 767–769. [Google Scholar]
- PabstGFJ.1956. Additamenta ad orchidologiam brasiliensem – III. Rodriguésia 30/31: 27–37. [Google Scholar]
- PabstGFJ, Dungs F.1975. Orchidaceae Brasiliensis, Vol. 1. Hildesheim: Brücke‐Verlag. [Google Scholar]
- PridgeonAM, Chase MW.2001. A phylogenetic reclassification of Pleurothallidinae (Orchidaceae). Lindleyana 16: 235–271. [Google Scholar]
- PridgeonAM, Solano R, Chase MW.2001. Phylogenetic relationships in Pleurothallidinae (Orchidaceae): combined evidence from nuclear and plastid DNA sequences. American Journal of Botany 88: 2286–2308. [PubMed] [Google Scholar]
- RyanA, Whitten WM, Johnson MAT, Chase MW.2000. A phylo genetic assessment of Lycaste and Anguloa (Orchidaceae: Maxil larieae). Lindleyana 15: 33–45. [Google Scholar]
- SakaiS, Kato M, Inoue T.1999. Three pollination guilds and variation in floral characteristics of Bornean gingers (Zingiberaceae and Costaceae). American Journal of Botany 86: 646–658. [PubMed] [Google Scholar]
- ScatenaVL, Nunes AC.1996. Anatomia de Pleurothallis rupestris Lindl. (Orchidaceae) dos campos rupestres do Brasil. Boletim de Botânica, Universidade de São Paulo 15: 35–43. [Google Scholar]
- StatSoft Inc.1995. STATISTICA for Windows (computer program manual). Tulsa: StatSoft, Inc. [Google Scholar]
- SunY, Skinner DZ, Liang GH, Hulbert SH.1994. Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theoretical and Applied Genetics 89: 26–32. [DOI] [PubMed] [Google Scholar]
- SwoffordDL.1998. PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4. Sunderland: Sinauer Associates. [Google Scholar]
- TurnerBJ.1974. Genetic divergence of Death Valley pupfish species: biochemical versus morphological evidence. Evolution 28: 281–294. [DOI] [PubMed] [Google Scholar]
- van den BergC, Higgins WE, Dressler RL, Whitten WM, Soto‐Arenas MA, Culham A, Chase MW.2000. A phylogenetic analysis of Laeliinae (Orchidaceae) based on sequence data from internal transcribed spacers (ITS) of nuclear ribosomal DNA. Lindleyana 15: 96–114. [Google Scholar]
- van der PijlL, Dodson CH.1966. Orchid flowers: their pollination and evolution. Coral Gables: University of Miami Press. [Google Scholar]
- WaserNM, Chittka L, Price MV, Williams NM, Ollerton J.1996. Generalization in pollination systems, and why it matters. Ecology 77: 1043–1060. [Google Scholar]
- WhittenWM, Williams NH, Chase MW.2000. Subtribal and generic relationships of Maxillarieae (Orchidaceae) with emphasis on Stanhopeinae: combined molecular evidence. American Journal of Botany 87: 1842–1856. [PubMed] [Google Scholar]
- WilliamsNH, Chase MW, Fulcher T, Whitten WM.2001. Molecular systematics of the Oncidiinae based on evidence from four DNA sequence regions: expanded circumscriptions of Cyrtochilum, Erycina, Otoglossum, and Trichocentrum and a new genus (Orchid aceae). Lindleyana 16: 113–139. [Google Scholar]
- WrightS.1978. Evolution and the genetics of populations, vol. 4. Variability within and among natural populations. Chicago: University of Chicago Press. [Google Scholar]
- ZarJH.1999. Biostatistical analysis. 4th edn. London: Prentice‐Hall. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.