Abstract
This is the first report of corolla‐borne secretory trichomes that substitute in role for a non‐functional disc in a species of the neotropical genus Lundia A. DC. (Bignoniaceae). The floral biology and flowering phenology of Lundia cordata were investigated at two remnants of tropical rainforest in northeastern Brazil. This species is a typically ornithophilous liana, with reddish, tubular, scentless flowers. The flowers are resupinate, protandrous and last for 2 d. There is a vestigial non‐functional perigynous disc and nectar is secreted by glandular trichomes distributed along the internal surface of the corolla. The nectar is stored at the base of the corolla tube, thus showing secondary nectar presentation. The nectariferous trichomes are multi‐cellular, uniseriate, with a basal foot cell rooting in the epidermis, one neck cell, and a glandular head with 13 cells on average. Three species of hummingbirds (Amazilia fimbriata, Eupetomena macroura and Phaethornis pretrei) serve as pollinators. Phaethornis ruber, Xylocopa bees, wasps and diurnal moths are considered nectar thieves.
Key words: Bignoniaceae, Lundia, pollination, nectariferous trichomes, hummingbirds, tropical rainforest, northeastern Brazil
INTRODUCTION
The pantropical family Bignoniaceae has about 800 species, approx. 110 genera and is centred in the neotropics (Gentry, 1982; Takhtajan, 1997). All species in this family are zoophilous (Gentry, 1974a, b), with bees being the major pollinators (Gentry, 1974a; Endress, 1994). Pollination by birds, bats, hawkmoths and butterflies also occurs (Weber and Vogel, 1986; Gentry, 1990; Endress, 1994; Lohmann, 2002), with one known occurrence of pollination by lemurs in Madagascar (Zjhra, 1998).
The flowers of the Bignoniaceae usually have a well‐developed nectariferous disc around the ovary; the nectar is released by stomatal sap‐holes (Galetto, 1995; Rivera, 2000). However, some taxa, such as Clytostoma, Cydista, Phryganocydia (Gentry, 1982; Rivera, 1996; Lohmann, 2002) and Lundia (Schumann, 1897; Gentry, 1982) lack a functional disc. In these cases, pollinator attraction can take place by deceit (mimetism of nectariferous flowers; Gentry, 1974a, b; Vogel, 1993; Lohmann, 2002).
The genus Lundia A. DC. comprises about 12 species (Gentry, 1982; Mabberley, 1987), and is distributed from southern Mexico to Brazil and Bolivia (Gentry, 1982). Absence of a floral disc is stated as being typical of this genus in all taxonomic descriptions (Bureau, 1868; Schumann, 1897; Kränzlin, 1921; Gentry, 1980, 1982). The occurrence of nuptial nectar was mentioned by Gentry (1980) and ascribed to trichomes around the base of the ovary. The few available studies into the floral biology of Lundia refer to nectarless flowers in L. obliqua Sond. (Amaral, 1992; Vogel, 1993). Deceptive floral attraction of L. obliqua by mimicry was presumed by Vogel (1993), who recorded this species flowering amongst Arrabidaea triplinervia (Mart. ex DC.) Baill. ex Bur., its putative model. As the floral UV patterns of the two species turned out to differ considerably (Amaral, 1992), doubt has been cast on the specificity of this relationship, and the mimetism of L. obliqua appears to be more generalized.
In this paper, we describe for the first time the presence of corolla‐borne nectar in a species of Lundia. We also report on the floral biology, flowering phenology and flower visitors of L. cordata A. DC.
MATERIALS AND METHODS
Study sites
Observations were made from May to July 1998 and in August 1999 at the Estação Ecológica do Tapacurá (EECT) (08°01′S, 35°11′W) and Brejo do Bituri (08°07′30′′S, 34°52′30′′W) situated, respectively, in the municipalities of São Lourenço da Mata and Brejo da Madre de Deus. Both are remnants of Atlantic forest in Pernambuco state, northeastern Brazil. The latter is a tropical semi‐deciduous forest, being 110–230 m a.s.l. and approx. 55 km from the coast. The former is a tropical montane forest, approx. 800 m a.s.l., surrounded by Caatinga vegetation, thus constituting an island of evergreen forest (locally called ‘Brejo de Altitude’), approx. 250 km from the coast. The local average annual rainfall in both sites is about 1300 mm, with a mean annual temperature of 24 °C [see Sales et al. (1998) and FIDEM (1987) for detailed descriptions of the study sites].
Floral attributes and flowering phenology
Records were made of flower morphology, size and colour, length of anthesis, number of flowers per inflorescence and availability of nectar. Measurements of floral parts were based on ten flowers. Nectar‐producing sites were verified by longitudinal sectioning of previously bagged buds in pre‐anthesis at about 0430 h. Nectar volume and sugar concentration were also measured in previously bagged flowers using, respectively, micro‐syringes (Microliter®, 5 and 10 µl), and a hand‐held temperature compensated refractometer (Atago®, 0–50 %).
Data on flowering phenology were obtained to verify the blooming pattern according to Gentry (1974a, b) and Newstrom et al. (1994).
Voucher specimens of Lundia cordata were deposited in the Herbarium of the Universidade Federal de Pernambuco, Brazil (UFP), and in the Herbarium of the Institut für Botanik der Universität Wien, Vienna (WU).
Anatomy of the nectariferous trichome carpet
Anatomical observations of nectariferous hairs were made using flowers fixed in 70 % ethanol and 70 % FAA (formalin–acetic acid–ethanol). For light microscopy, corollas were embedded in Technovit 7100, cut with a Reichert‐Jung‐Microtome (7 µm), stained with toluidine blue and safranin, and mounted in Entellan. The density of nectar trichomes was determined using a microscopical drawing attachment. Two representative square millimetres along the corolla tube were counted. For scanning electron microscopy, the selected material was critical‐point dried, sputter‐coated with gold, and viewed and photographed under a JEOL T 300 scanning electron microscope.
Visitor records
Floral visitors were observed on the spot using binoculars from dawn (approx. 0400 h) to late afternoon (1730 h), on both the first and second day of anthesis. Visits were monitored over approx. 40 h. The frequency of visits and the behaviour of each visitor were noted. The site of pollen deposition on the body of the visitors was also recorded.
RESULTS
Floral attributes and flowering phenology
Lundia cordata is a liana with terminal, corymbose panicles (Fig. 1A), flowering from mid‐May to mid‐July in the Reserva Ecológica do Tapacurá and from mid‐June to August in the Brejo do Bituri. Each patch at the study sites had about 25–30 inflorescences and three to six anthetic flowers per inflorescence per day, i.e. 80–150 flowers per day.
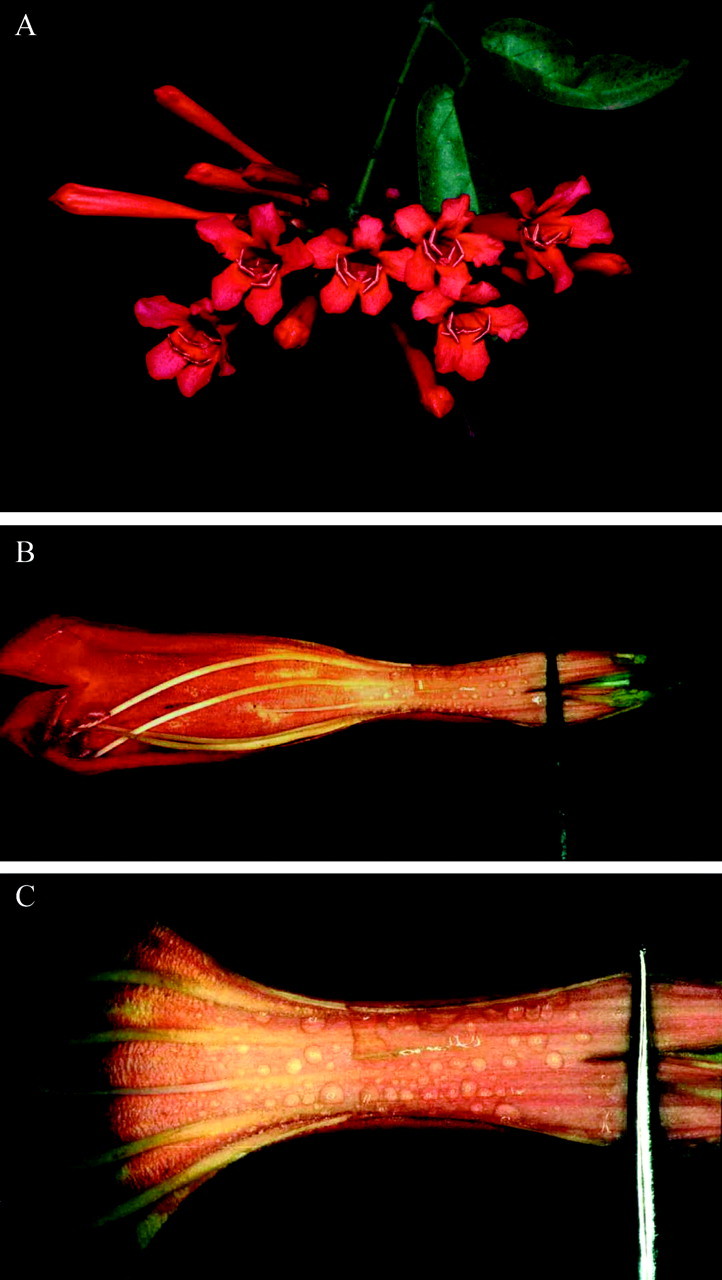
Fig. 1. A, Inflorescence of Lundia cordata. B and C, Details of the internal surface of the corolla with nectar droplets just before the anthesis.
Flowers are resupinate, hermaphrodite, tubular, scentless and reddy‐orange to vermilion on the first day of anthesis, turning pink on the second day. The effective length of the corolla tube (sensu Wolf et al., 1976) is 45·6 ± 1·7 mm (n = 11). Four stamens and one staminode are inserted in the corolla tube. They lean onto the topographically lower wall section of the corolla and are slightly exerted, as is the style (Fig. 1A and B). The anthers are villous and the ovary is densely hispid. The cupular calyx is comparatively small (5 mm long, 3 mm wide), truncate and has no extranuptial nectaries. Anthesis starts at about 0530 h. The occurrence of protandry was detected, the male phase taking place during the first day of anthesis (when the bilamellate stigma is closed). The female phase occurs on the second day of anthesis, when the two lobes of the stigma are spread and the anthers have wilted. The sugar concentration of the nectar was 10·2 ± 0·3 % (n = 9) and 10·6 ± 0·5 % (n = 10) in flowers of the first and second day of anthesis, respectively (range 8–14 %). The sugar concentration of nectar during the bud phase had a similar range. The mean volume of nectar per flower (at 1000 h) was about 55·8 ± 6·1 µl (n = 9) on the first day, and 48·0 ± 10·1 µl (n = 10) on the second day.
Anatomy of the vestigial nectary and nectariferous trichomes
A disc‐like structure present at the base of the ovary (Fig. 2A) is non‐secretory and no stomata (sap holes) were found on it. Non‐glandular hairs cover this structure and the ovary surface.
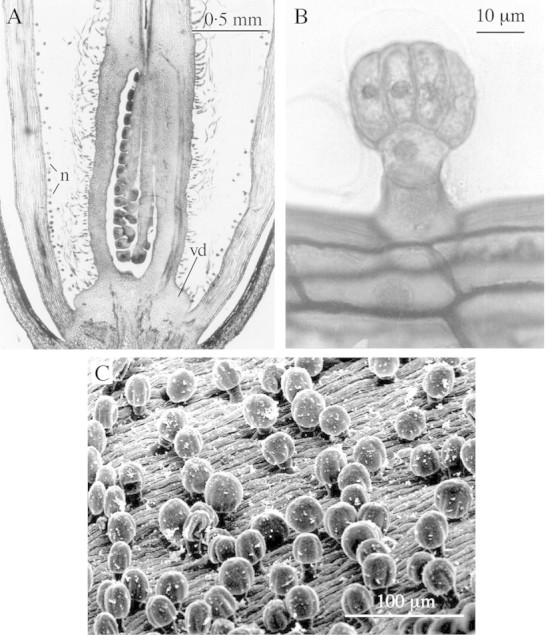
Fig. 2. A, Longitudinal section of the basal portion of a Lundia cordata flower. vd, Vestigial disc; n, nectariferous trichome carpet. B, Detail of a nectariferous trichome from the internal surface of the corolla. C, Scanning electron micrograph of the nectariferous trichome carpet.
Nectar is secreted by glandular trichomes distributed over the internal surface of the corolla. The trichome carpet extends from about the point of insertion of the filaments down to the base of the corolla tube (Figs 1B, C and 2A). Nectar secretion starts in the bud stage, 1 d before anthesis. At the beginning of the secretory phase, the nectar appears in drops (Fig. 1B and C), which then fuse and flow towards the base of the corolla tube, where the fluid accumulates.
The nectariferous trichomes (Fig. 2C) are multicellular and uniseriate, with a basal foot cell rooting in the epidermis, one neck cell and a glandular head with 13 cells on average (9–17, n = 10) (Fig. 2B and C), the four central cells forming a tetrad, surrounded by the peripheral cells. Each trichome is approx. 55 µm high and the mean head diameter is approx. 40 µm. The distance between hairs is irregular. Their density increased from approx. 375 hairs per mm2 in the area 5 mm below the point of insertion of the filaments to approx. 585 hairs per mm2 in the basal section of the tube.
Floral visitors and pollinators
At the study sites, flowers of L. cordata were legitimately visited only by hummingbirds: Amazilia fimbriata, Eupetomena macroura (in EECT) and Phaethornis pretrei (at both sites). Phaethornis ruber was also seen visiting flowers of L. cordata. However, unlike the other species, P. ruber visited illegitimately, since it took nectar by puncturing the base of the corolla tube (either a primary or secondary thief; sensu Inouye, 1980). Bees (Xylocopa spp.), wasps and diurnal moths were also observed robbing nectar through holes at the corolla base, acting as primary (bees) or secondary thieves (wasps and moths).
Considering the behaviour of each hummingbird species, P. pretrei can be regarded as a trapliner at the populations occurring in Brejo do Bituri, visiting flowers at regular intervals of 30–35 min. In Tapacurá, this species was seen just once, making it an occasional visitor. Despite being a mostly territorial species when visiting other ornithophilous species at Tapacurá (A. V. Lopes et al., unpubl. res.), Eupetomena macroura behaved as a trapliner when visiting flowers of L. cordata. This species was observed from 0600 to 1700 h. Amazilia fimbriata also behaved as a trapliner, but mainly visited flowers before 1300 h. The site of pollen deposition on the body of legitimate visitors was the chin, according to the sternotribic placement of the anthers.
DISCUSSION
Flowers of L. cordata have typical ornithophilous attributes (sensu Faegri and Pijl, 1979): the corolla is orange to reddish, nectar production is abundant (with a low concentration of sugar) and there are no nectar guides or scent.
The flowering phenology of L. cordata is of the annual type according to Newstrom et al. (1994), and seems to be in agreement with the Cornucopia type proposed by Gentry (1974a, b), in that it flowers for several weeks and produces a large number of flowers each day. Gentry (1974a, b) also suggested that genera of the Bignoniaceae lacking a disc are normally associated with the Multiple‐bang flowering pattern. This is probably due to the lack of nectar production and the mimetic behaviour of these species ‘which are dependent for pollination on exploratory visits by nectar‐seeking bees’ (Gentry, 1974a, b). However, the phenological pattern of L. cordata and also the presence of floral (nuptial) nectar does not fit with the description of the Multiple‐bang syndrome.
There is no other study of flowering phenology of Lundia, thus generalizations are not possible across the whole genus. The flowering phenology of the mimetic species should also be followed to gain a better understanding of the phenological patterns in the genus.
The occurrence of strict protandry in L. cordata excludes spontaneous self‐pollination. Moreover, as noted by Gentry (1990), nearly all neotropical Bignoniaceae are self‐incompatible. Studies on reproductive systems of some species have demonstrated that the late‐acting, self‐incompatibility system seems to be quite widespread in the family (see Gibbs and Bianchi, 1999, and references therein). With respect to Lundia spp., just one species, L. obliqua, has been investigated with regard to its reproductive system (Amaral, 1992); late‐acting self‐incompatibility was shown to occur. In fact, L. cordata showed low (<8 %) fruit set under natural conditions, thus suggesting the occurrence of self‐incompatibility. Field investigations are necessary to confirm this supposition. Low fruit set in Lundia has also been noted by others (Amaral, 1992).
Gentry (1980) was the first to note, without naming a particular species, that floral nectar occurs in Lundia, and that it may be produced by ‘. . . a dense ring of glandular trichomes around the base of the ovary’. Gentry was probably referring to the pilosity, which, in L. cordata, covers a perigynous ring‐wall that we interpret here as a rudimentary disc. We found that this structure and the hairs covering it were non‐glandular and non‐secretory. The true source of nectar, in L. cordata at least, are the glandular hairs of the corolla tube. More scattered glandular hairs occur on the inner corolla tube and filament‐bases in many other Bignoniaceae (Rivera, 1996, 1998). In Campsis radicans (L.) Seem. these trichomes even secrete small amounts of nectareous fluid, despite the flower having a well‐developed nectariferous disc (S. Vogel, unpubl. res.). Such a condition may have preceded the reduction of the disc. Trichomatic glands are widespread in the Bignoniaceae, fulfilling a variety of functions. They often cover the interior of the calyx and also form extranuptial nectaries on the outside of the calyx of many species.
The trichomatic nectariferous carpet of L. cordata is considered substitutive in the sense of Vogel (1997), because it has evolved secondarily in a subgroup that has lost a nectar‐secreting organ of another, non‐homologous type.
Secondary nectar presentation, or indirect availability of the nectar, was observed in flowers of L. cordata, because the nectar is produced by trichomes distributed along the internal surface of the corolla and is accumulated at the base of the corolla tube. This strategy facilitates nectar removal by one or a few tongue licks.
Corolla‐borne nectar has not previously been described in Lundia. Our results call for new investigations of other Lundia species, but also of other disc‐less species of the Bignoniaceae: mostly Clytostoma, Cydista and Phrygano cydia. Interestingly, of all the species in these genera, L. cordata is probably the only one that is bird‐pollinated: the others do not have ornithophilous characteristics and are probably bee‐pollinated (L. Lohmann, pers. comm.).
Bignoniaceae are most closely related to Scrophu lariaceae (Takhtajan, 1997), some species of which also lack a disc but have nectariferous trichomes instead. The four disc‐less genera of Bignoniaceae (Clytostoma, Cydista, Phryganocydia and Lundia) belong to the tribe Bignonieae, all other genera of which have a nectariferous disc (Gentry, 1974a). A first phylogenetic analysis of Bignonieae based on the chloroplast gene ndhF (L. Lohmann, unpubl. res.) reveals that Lundia is derived within this tribe (and here rather distant from the other disc‐less taxa). In addition, the tribe Bignonieae as a whole has also been shown to be derived within the family Bignoniaceae (Spangler and Olmstead, 1999). Since the basal (and most of the advanced) tribes have nectariferous discs, as do the majority of species within Bignonieae, the absence of nectariferous discs in Lundia is judged an apomorphy for the genus.
The nectar‐sterile perigynous ring still found in flowers of L. cordata and L. obliqua also indicates the former presence of a disc, suggesting loss of a nectary. While many species of the genus appear to have adopted a deceptive syndrome, the re‐appearance of nectar‐production in L. cordata suggests that a reversal from deceit to reward has taken place in some taxa of Lundia.
ACKNOWLEDGEMENTS
We thank Lúcia G. Lohmann (Missouri Botanical Garden) for fruitful discussions on Bignoniaceae, for permission to use unpublished data, for critically reviewing the manuscript and for providing the taxonomic identification of the Lundia species; Dr Marlies Sazima (Unicamp, Brazil) for valuable discussions and suggestions; Paulo Martins (UFRPE, Brazil) for permission to work at the Estação Ecológica do Tapacurá (EECT); Dr Ralf Buchner and Mag. Susanne Sontag (University of Vienna) for their help with LM and SEM preparations; Dr Arthur Davis (University of Saskatchevan, Canada) for kindly improving our English; Edinaldo Mendes da Silva (EECT) for pleasant company and help during the field work; and the CAPES, CNPq, FACEPE and FBPN‐MacArthur Foundation for essential financial support. This paper is part of the doctoral thesis of the first author, presented at Universidade Estadual de Campinas (São Paulo, Brazil).
Supplementary Material
Received: 19 December 2001; Returned for revision: 5 March 2002; Accepted: 30 April 2002
References
- AmaralMEC 1992. Ecologia floral de dez espécies da tribo Bignonieae (Bignoniaceae) em uma floresta semidecídua no município de Campinas, SP. PhD thesis, Universidade Estadual de Campinas, Campinas. [Google Scholar]
- BureauE.1868. Revision des genres Tynanthus et Lundia Adansonia (Baillon), Recueil périodique d’observations botaniques 8: 270–294. [Google Scholar]
- EndressPK.1994. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press. [Google Scholar]
- FaegriK, van der Pijl L.1979. The principles of pollination ecology. London: Pergamon Press. [Google Scholar]
- FIDEM.1987. Região Metropolitana do Recife – reservas ecológicas – série desenvolvimento urbano e meio ambiente. Recife: Alcântara Promoções e Publicidade S/A. [Google Scholar]
- GalettoL.1995. Nectary structure and nectar characteristics in some Bignoniaceae. Plant Systematics and Evolution 196: 99–121. [Google Scholar]
- GentryAH.1974a Coevolutionary patterns in Central American Bignoniaceae. Annals of the Missouri Botanical Garden 61: 728–759. [Google Scholar]
- GentryAH.1974b Flowering phenology diversity in tropical Bignoniaceae. Biotropica 6: 64–68. [Google Scholar]
- GentryAH.1980. Bignoniaceae part I (Crescentieae and Tourrettieae) In: Flora Neotropica Monograph 25. New York: New York Botanical Garden, 1–130. [Google Scholar]
- GentryAH.1982. Bignoniaceae In: Febres ZL de, Steyermark JA, eds. Flora de Venezuela 8 Caracas: Ediciones Fundación Educación Ambiental, 1–433. [Google Scholar]
- GentryAH.1990. Evolutionary patterns in neotropical Bignoniaceae. In: Gottsberger G, Prance GT, eds. Reproductive biology and evolution of tropical woody angiosperms. Memoirs of the New York Botanical Garden 55: 118–129. [Google Scholar]
- GibbsPE, Bianchi MB.1999. Does late‐acting self‐incompatibility (LSI) show family clustering? Two more species of Bignoniaceae with LSI: Dolichandra cynanchoides and Tabebuia nodosa Annals of Botany 84: 449–457. [Google Scholar]
- InouyeDW.1980. The terminology of floral larceny. Ecology 61: 1251–1253. [Google Scholar]
- KränzlinF.1921. Bignoniaceae novae III. In: Fedde F, ed. Repertorium specierum novarum regni vegetabilis 17: 119–121. [Google Scholar]
- LohmannLG.2002. Bignoniaceae. In: Henderson A, Mori S, eds. Flowering plant families of tropical America. New York: New York Botanical Garden Press (in press). [Google Scholar]
- MabberleyDJ.1997. The plant book. Cambridge: Cambridge University Press. [Google Scholar]
- NewstromLE, Frankie GW, Baker HG.1994. A new classification for plant phenology based on flowering patterns in lowland tropical rain forest trees at La Selva, Costa Rica. Biotropica 26: 141–159. [Google Scholar]
- RiveraGL.1996. Nectarios y tricomas florales en cuatro especies de Tecomeae (Bignoniaceae). Darwiniana 34: 19–26. [Google Scholar]
- RiveraGL.1998. Tricomas y emergencias florales en especies de Bignoniaceae de Argentina. Kurtziana 26: 99–115. [Google Scholar]
- RiveraGL.2000. Nuptial nectary structure of Bignoniaceae of Argentina. Darwiniana 38: 227–239. [Google Scholar]
- SalesMF, Mayo SJ, Rodal MJN.1998. Plantas vasculares das Florestas Serranas de Pernambuco – Um checklist da flora ameaçada dos Brejos de Altitude (Pernambuco – Brasil). Recife: Imprensa Universitária‐UFRPE. [Google Scholar]
- SchumannK.1897. Bignoniaceae. In: Engler A, Prantl K, eds. Die natürlichen Pflanzenfamilien. IV, 3b. Leipzig: Verlag von Wilhelm Engelmann, 189–252. [Google Scholar]
- SpanglerRJ, Olmstead RG.1999. Phylogenetic analysis of Bignoniaceae based on the cpDNA gene sequences rbcL and ndhF Annals of the Missouri Botanical Garden 86: 33–46. [Google Scholar]
- TakhtajanA.1997. Diversity and classification of flowering plants. New York: Columbia University Press. [Google Scholar]
- VogelS.1993. Betrug bei Pflanzen: Die Täuschblumen. Stuttgart: Akademie der wissenschaften und der Literatur. Mainz: Franz Steiner Verlag. [Google Scholar]
- VogelS.1997. Remarkable nectaries: structure, ecology, organophyletic perspectives I. Substitutive nectaries. Flora 192: 305–333. [Google Scholar]
- WeberA, Vogel S.1986. The pollination syndrome of Deplanchea tetraphylla (Bignoniaceae). Plant Systematics and Evolution 154: 237–250. [Google Scholar]
- WolfLL, Stiles FG, Hainsworth FR.1976. Ecological organization of a tropical, highland hummingbird community. Journal of Animal Ecology 32: 349–379. [Google Scholar]
- ZjhraM.1998. Phylogenetics, biogeography, and pollination ecology of endemic Malagasy Coleeae (Bignoniaceae). PhD Thesis, University of Wisconsin, Madison. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


