Abstract
Programmed cell death (PCD) in plants is considered an integral part of development. Evidence of DNA fragmentation, occurring at specific sites and times during embryo formation in maize (Zea mays L.), was obtained using terminal deoxyribonucleotidyl transferase‐mediated dUTP‐fluorescein nick end labelling (TUNEL) and by genomic DNA ladder detection. During the crucial period of elaboration of the primary shoot and root axis (14–20 d after pollination), TUNEL‐positive nuclei are present in the scutellum, coleoptile, root cap and principally in the suspensor. Additional evidence of a form of programmed cell death occurring in these tissues comes from the detection of a DNA ladder. Upon completion of the differentiation process, all embryonic cells are TUNEL‐negative, indicating that possible programmed cell death events during maize embryogenesis are confined to structures or organs that do not contribute to the adult plant body.
Key words: Zea mays L., maize, embryogenesis, programmed cell death, TUNEL procedure, DNA ladder
INTRODUCTION
Programmed cell death (PCD) is considered an integral part of development both in animals and plants. Such an inbuilt death programme is triggered in response to external or internal signals. Cell death may be envisaged as the necessary counterpart of cell division in determining the shape and morphology of tissues and organs during differentiation. Paradoxically, cell death is essential for the development and survival of an organism.
In animal development, PCD has been extensively studied and the features of the process have been analysed at the cytological, biochemical and molecular levels (Vaux and Korsmeyer, 1999). When it is associated with specific biochemical and morphological events, such as DNA strand breaks, activation of caspases, chromatin condensation and nuclear fragmentation into distinct bodies, cell death is defined as apoptosis. Plants also undergo programmed cell death in a variety of situations. Some of these are developmentally regulated, while others are the consequence of biotic and abiotic stresses (Greenberg, 1996; Havel and Durzan, 1996a; Mittler and Lam, 1996; Martienssen, 1997; Pennell and Lamb, 1997; Lam et al., 1999; Lam and Greenberg, 2000; Wu and Cheung, 2000). The parallel between animal and plant cell death is under discussion, and similarities between such events have recently been reported (Danon et al., 2000).
In maize, in particular, cell death events are known to occur throughout normal development both in the sporophyte and in the gametophyte (Buckner et al., 1998, 2000). Cell death takes place during the formation of unisexual male and female flowers in microsporogenesis and megasporogenesis (Calderon‐Urrea and Dellaporta, 1999), during xylem differentiation (Schindler et al., 1995), in roots during aerenchyma form ation (Gunawardena et al., 2001) and in leaf senes cence.
During maize seed formation both in the embryo and endosperm, cells are thought to die at predictable times and places, though details of these events are still scanty. The suspensor, through which nutrients are transferred from maternal tissue to the developing embryo, is thought to undergo programmed cell death once its functions are accomplished (Yeung and Meinke, 1993). Cell death has also been demonstrated to occur in developing endosperm (aleurone and central endosperm), the function of which is to store and supply nutrients to the germinating seed (Young et al., 1997; Young and Gallie, 2000).
To date, the genetic control of maize embryogenesis has been investigated by analysing mutants affected during embryo development. Less attention has been devoted to the study of cell death events that occur naturally during the process of embryo formation. In this report we present cytological and molecular evidence, based on the terminal deoxyribonucleotidyl transferase (TdT)‐mediated dUTP‐fluorescein nick end‐labelling (TUNEL) method (Gorczyca et al., 1993) and on DNA ladder detection, that programmed cell death probably occurs in specific sites and at specific moments during maize embryo formation.
MATERIALS AND METHODS
Plant material
Seeds analysed in these experiments were obtained from maize plants of the inbred line W64A grown in the field or under glasshouse conditions at the University of Milan, Italy.
Histological analysis
Immature seeds were dissected from cobs at fixed intervals, from 14 to 27 d after pollination (DAP). Samples were immediately fixed in freshly prepared 4 % p‐formaldehyde in phosphate‐buffered saline (PBS) (130 mm NaCl, 7 mm Na2HPO4, 3 mm NaH2PO4) for 12 h. The fixed material was placed in 70 % ethanol and stored at 4 °C until processed. Embedding procedures were performed as described previously (Procissi et al., 1997). Sections (8 µm thick) attached to poly‐lysine‐coated slides were deparaffinized with xylene for 10 min and then rehydrated by passing through a graded ethanol series for 3 min each. The different embryonic developmental stages were characterized by staining sections with safranin‐fast green and with DAPI (4,6‐diamidino‐2‐phenylindole; 1 mm in PBS for 10 min).
In situ detection of DNA fragmentation
To detect DNA fragmentation, the TUNEL procedure was applied, using the In Situ Cell Death Detection Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Tissue was made permeable by incubating sections with Proteinase K (2 µg ml–1) for 15 min at 37 °C. DNA fragmentation was detected directly after the TUNEL reaction by yellow‐green fluorescence of incorporated fluorescein‐12‐dUTP in the nuclei. Slides were examined using an Axioskop microscope (Carl Zeiss, Oberkochen, Germany) equipped with a filter set for fluorescein isothiocyanate (FITC) and photomicrographs were taken with Kodak Ektachrome 320T film. Alternat ively, incorporated fluorescein was detected by anti‐fluorescein antibody conjugated with alkaline phosphatase. After substrate reaction, stained sections were analysed under a light microscope and photomicrographs were taken using Kodak 64T film. A negative control was included in each experiment by omitting TdT from the reaction mixture. Under these conditions all nuclei should be unstained. As a positive control, permeabilized sections were incubated with DNase I (1 U ml–1) for 10 min at room temperature before processing. All nuclei in all seed tissues (embryo axis, scutellum, endosperm, pericarp) should be stained as a result of the induced DNA strand breaks. Experiments reported here were repeated at least three times; all results were in agreement across the experiments.
DNA extraction and ladder detection
Using W64A plants as a source material, genomic DNA was extracted from immature embryos following the CTAB protocol described in Ausubel et al. (1994). For the ladder detection, the ApoAlert™ LM‐PCR Ladder Assay Kit (Clontech Laboratories Inc., Palo Alto, CA, USA) was used, according to the manufacturer’s instructions. As the first step, dephosphorylated adaptors were ligated to the ends of the DNA fragments generated during cell death. A 24‐mer primer was then used in a PCR reaction in which fragments with adaptors on both ends were exponentially amplified. Ligation reactions were set up in 70 µl volume with different DNA quantities. In our conditions, results were obtained starting from 0·5 µg of genomic DNA and using 150 ng of ligated DNA in the PCR mixture with 20 and 25 cycles. Twenty microlitres from each reaction was run on a 1·2 % agarose/EtBr gel. A positive control reaction was performed using the calf thymus DNA supplied with the kit. A negative control was included, consisting of the PCR mixture without the adaptor‐ligated DNA, and a further control was obtained by omitting ligase in the first step.
RESULTS
In situ detection of DNA fragmentation in immature embryos
The TUNEL procedure was applied to longitudinal sections of developing maize seeds to verify the occurrence of cell death events during embryo formation. In particular, attention was focused on the developmental interval between 14 and 27 DAP, from leaf stage 1 (L1) up to leaf stage 4 (L4), according to the classification of Abbe and Stein (1954).
Cell death events were demonstrated at specific sites during the developmental stages considered. Specific TUNEL signals, i.e. yellow‐green fluorescence of fluorescein, which reveal nuclei undergoing DNA fragmentation, were detected mainly in the apical region of the embryo, in the scutellum layers adjacent to the coleoptile (Fig. 1) and in the suspensor (Fig. 2).
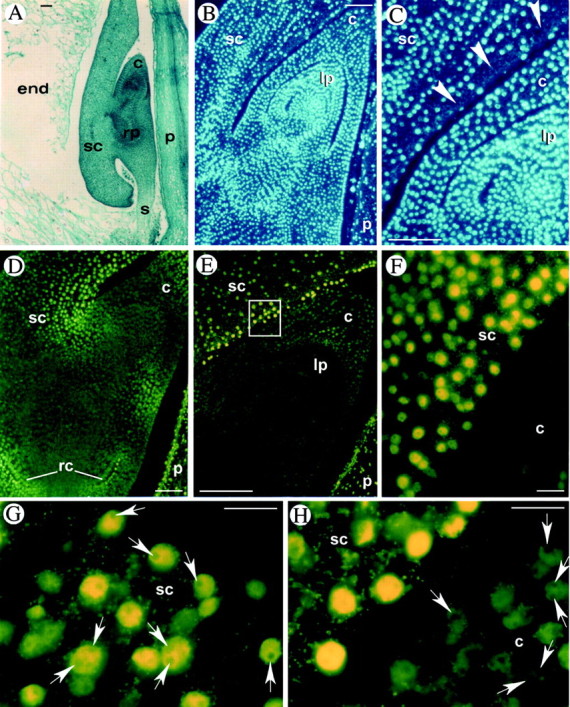
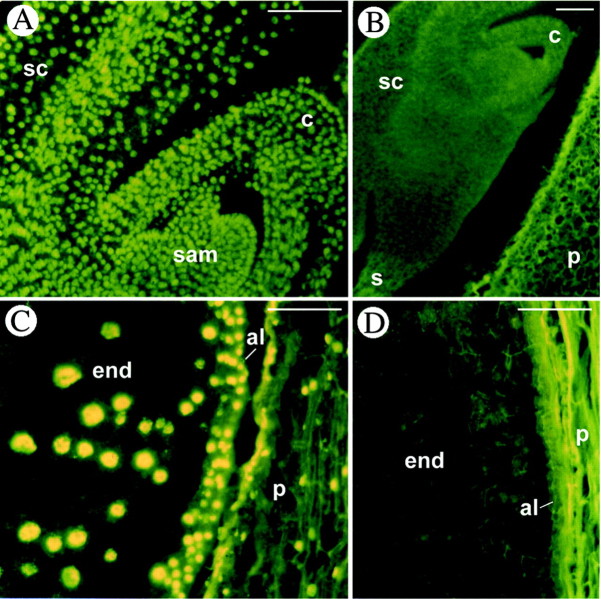
Fig. 1. Programmed cell death in maize embryos at early developmental stages (stages L1–L2). A, Safranin‐fast green staining of a longitudinal section of an embryo at 14 DAP. B and C, DAPI staining of longitudinal sections at 17 DAP showing evidence of nuclear loss (arrowheads) in scutellum layers surrounding the shoot. D–H, In situ detection of DNA fragmentation by the TUNEL procedure (yellow fluorescence on nuclei). TUNEL‐positive nuclei are evident in the scutellum layers surrounding the coleoptile (D–F), in the coleoptile and in the root cap (D). F (enlargement of E) shows the difference between TUNEL‐positive (yellow) and TUNEL‐negative (dark green) nuclei. G, One or two nucleoli (arrows) are present in TUNEL‐positive nuclei of the scutellum at 14 DAP. H, Nucleoli are absent in TUNEL‐positive nuclei and present (arrows) in TUNEL‐negative nuclei at 16 DAP. c, Coleoptile; end, endosperm; lp, leaf primordium; p, pericarp; rc, root cap; rp, root primordium; s, suspensor; sc, scutellum. Bars = 100 µm for A–E and 20 µm for F–H.
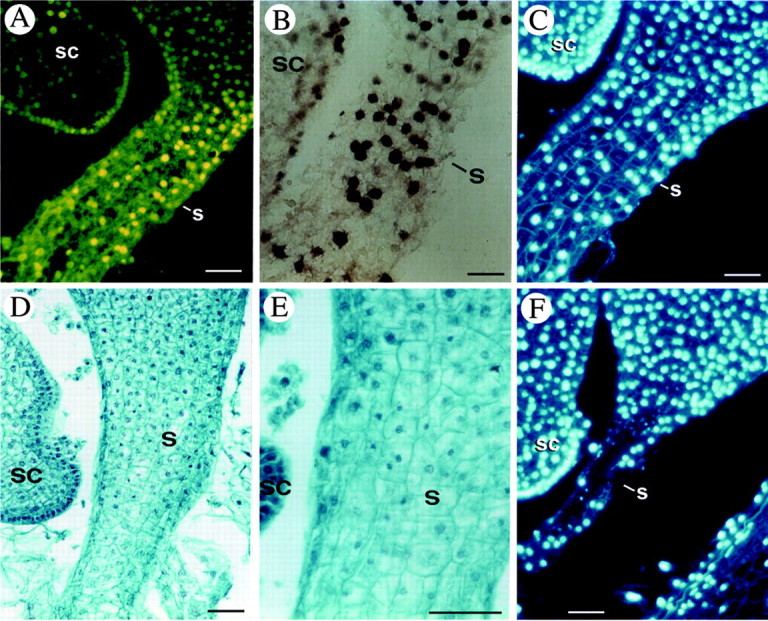
Fig. 2. Programmed cell death in the suspensor of the maize embryo. A and B, TUNEL‐positive nuclei in the suspensor at 14 DAP visualized directly (A) by fluorescein fluorescence and indirectly (B) by the secondary anti‐fluorescein‐AP conjugate. C and F, DAPI staining of the suspensor nuclei in longitudinal sections at 14 DAP (C) and at 16 DAP (F). D and E, Safranin‐fast green staining of a longitudinal section at 15 DAP. sc, Scutellum; s, suspensor. Bars = 50 µm.
In detail, at 14 DAP (stage L1; Fig. 1A), TUNEL‐positive cells were particularly evident in the cell layers of the scutellum surrounding the shoot primordium (Fig. 1D–F). Fluorescent nuclei were also visible in the coleoptile and in the root cap cells (Fig. 1D), while nuclei of the cells of the embryo axis were all TUNEL negative. Particularly striking is the difference between positive fluorescent nuclei of the scutellum layers surrounding the shoot and the negative nuclei of the nearby leaf primordia (Fig. 1E and F). Close analysis of the positive nuclei reveals a difference that appeared on subsequent days of development: at 14 DAP in the positive nuclei, nucleoli (the negative dots inside the nucleus) were still visible (Fig. 1G), whereas later on, at 16 DAP, fluorescence increased and nucleoli were no longer identifiable. Figure 1H, in particular, reveals the difference between the nuclei of the scutellum where nucleoli are no longer visible and the nuclei of the nearby adjacent leaf primordia where one or two nucleoli are evident. In DAPI‐stained sections (Fig. 1B and C), at 17 DAP, the scutellum cell layers surrounding the coleoptile appeared devoid of a few nuclei, thus corroborating the TUNEL data in favour of a presumed death of these cells.
As for the suspensor (Fig. 2), DNA fragmentation was demonstrated at 14 DAP by TUNEL assay, both directly (yellow‐green fluorescence of incorporated fluorescein) (Fig. 2A) and indirectly (by a colorimetric reaction) (Fig. 2B). DAPI staining at subsequent times (14 and 16 DAP) showed a progressive disappearance of nuclei starting from the upper part of the suspensor (Fig. 2C and F). Histological analysis confirmed this observation since a morphological difference in the appearance of nuclei could be observed at 15 DAP (Fig. 2D and E): in the upper portion of the suspensor, nuclei showed chromatin condensation, whereas in the lower part they were still rounded and swollen. On the basis of these observations, DNA fragmentation precedes chromatin degradation and the degeneration process proceeds from the top towards the bottom of the suspensor.
At later developmental stages (27 DAP, stage L4; Fig. 3), the whole of the embryo was TUNEL‐negative, except for only a few fluorescent nuclei at the level of the scutellar node. In the two maternal tissues, the pericarp and nucellus, brightly fluorescent nuclei (visible only in Fig. 1D and E) were evident at all developmental stages analysed.
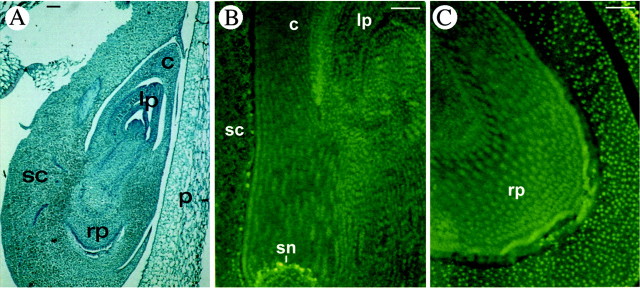
Fig. 3. Programmed cell death in maize embryos at a late developmental stage (stage L4). A, Safranin‐fast green staining of a longitudinal section of an embryo at 27 DAP. B and C, TUNEL procedure on the upper portion of the embryo (B) and on the lower portion (C). The only fluorescent nuclei visible are those corresponding to the scutellar node. c, Coleoptile; lp, leaf primordiuma; p, pericarp; rp, root primordium; sc, scutellum; sn, scutellar node. Bars = 100 µm.
Appropriate control treatments were conducted for every set of slides (Fig. 4). When the DNase I treatment preceded the TUNEL procedure, all nuclei in all regions of the embryo (Fig. 4A) and of the endosperm (Fig. 4C) were fluorescent (positive control). On the other hand, no fluorescence was observed in the same tissues when TdT enzyme was omitted from the reaction (negative control) (Fig. 4B and D).
Fig. 4. Controls of the TUNEL procedure. A and C, Positive controls. All nuclei of the different regions of the embryo (A) and endosperm (C) are TUNEL‐positive. B and D, Negative controls. All nuclei in the embryo (B) and in the endosperm (D) are TUNEL‐negative. al, Aleurone; c, coleoptile; end, endosperm; p, pericarp; sam, shoot apical meristem; sc, scutellum; s, suspensor. Bars = 100 µm.
Ladder detection in immature embryos
As demonstrated above, apparently only a small percentage of nuclei in our biological system undergo cell death (approx. 5 %, according to a rough count). Therefore, DNA fragments produced by endonuclease activity during the degradation process were not detectable following electrophoresis of genomic DNA directly on ethidium bromide‐stained agarose gels (data not shown).
Purified genomic DNA from embryos at 17 DAP was assayed by the LM‐PCR procedure (as described in Materials and Methods). Profiles obtained using 1 µg of DNA in the ligation reaction and following 20 and 25 PCR cycles are shown in Fig. 5 (lanes 1 and 2, respectively). The positive control is shown in lane 3. Comparing the embryo profiles and the positive control shows that despite the high sensitivity of the kit, the efficiency of the reaction was low and the profiles became less clear as the cycle number is increased from 20 to 25. The low efficiency might be due to the poor availability of 5′ phosphorylated ends in the genomic DNA, an observation supported by the fact that the amount of DNA added to the ligation mix was a limiting factor. However, specificity of the amplified bands can be corroborated by the results obtained in the sample in lane 4, where ligation of adaptors was omitted and no amplification products were detected. In the negative control, consisting of the amplification mix without genomic DNA, no amplification products were obtained (data not shown).
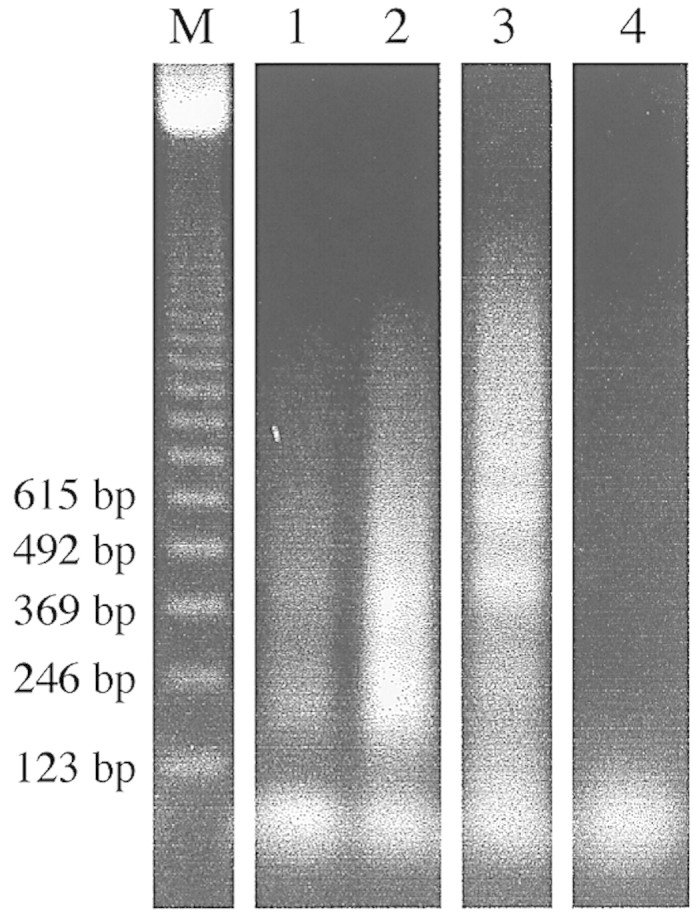
Fig. 5. Purified genomic DNA assayed by LM‐PCR. Profiles obtained from 17 DAP embryos after 20 (lane 1) and 25 (lane 2) PCR cycles and calf thymus DNA after 25 cycles (lane 3) are visible loading 20 µl of the reaction on 1·2 % agarose/EtBr gel. Lane 4, Negative control. Sizes of markers (lane M) are indicated.
DISCUSSION
Our investigation focused on cell death in the maize seed, and, in particular, on its occurrence during embryo development. It is generally accepted that in normal animal and plant morphogenesis PCD eliminates cells at specific places and/or at specific times to contribute towards the formation of correctly shaped structures and organs. It also removes cells that are present in excess, or tissues needed at just one stage of development but not required later.
The cytological and molecular data clearly indicate that DNA fragmentation, characteristic of programmed cell death, occurs during maize embryo formation. PCD events are observed during the crucial period of elaboration of the primary shoot and root axis, when regionalization of the embryo proper into the scutellum and embryo axis occurs. TUNEL‐positive nuclei are visible in the cell layers of the scutellum surrounding the shoot primordium and in the coleoptile, but never in leaf primordia. This observation is relevant in the context of the assumption that the scutellum and coleoptile differ from leaves both in terms of origin and function. Scanlon and Freeling (1998) proposed that these two organs do not derive from the shoot apical meristem, as leaves do, but instead differentiate before the formation of the tunica corpus‐shaped apex. Recently, Bommert and Werr (2001) affirmed that the protodermal cell fate is maintained in the scutellum and coleoptile, two organs set apart from the shoot/root axis in a single regionalization event. It is known that TUNEL positivity may be ascribed to programmed cell death, a high mitotic activity or to necrosis. The observation of Elster et al. (2000) that initial scutellar growth is mainly the result of cellular expansion rather than division supports the hypothesis that the fluorescence observed in the scutellum is the consequence of programmed cell death events in the context of morphogenetic processes. As to its function, the scutellum is utilized during seed germination as a food source for the developing embryo organ, whereas the coleoptile encloses and protects the embryonic shoot. The presence in the scutellum of cells destined to die may therefore be associated with the developmental processes or with the transient function of this organ. In the coleoptile, cells committed to programmed cell death have already been demonstrated by an immunological approach and are considered to be future xylem cells (Schindler et al., 1995).
Further evidence of programmed cell death in these tissues comes from the observation of nucleoli in the TUNEL‐positive cells of the scutellum. As demonstrated for suspensor and endosperm cells of Vicia faba (Wredle et al., 2001), dispersion of the nucleoli (one of the parameters of nuclear degradation), is probably occurring since nucleoli keep their round and condensed shapes for some time before they become no longer visible within the nuclei. For better characterization of their fate, our observations need to be integrated with ultrastructural investigations.
TUNEL‐positive nuclei are also evident in the suspensor, a structure that functions temporarily early in embryogenesis, and then later degenerates (Yeung and Meinke, 1993). Recently, Danon et al. (2000) pointed out that until now, no specific PCD hallmarks for the elimination of the suspensor have been reported. Our evidence indicates that PCD does take place in the course of the degeneration of the suspensor, according to a controlled spatial and temporal pattern.
Later in development, when morphogenetic processes have been completed and the embryo only enlarges, fluorescent cells are no longer visible, except for a few positive nuclei corresponding to the scutellar node, in connection with the development of vascular tissue. Therefore, during embryogenesis, TUNEL‐positive cells are confined to structures or organs (the scutellum, suspensor and coleoptile, as well as the pericarp and nucellus) that do not contribute to the adult plant body, while the embryo axis is devoid of such cells.
Our results should be interpreted with caution. In recent years, data have been accumulating on the application of the TUNEL assay to different plants under physiological or induced conditions to reveal the occurrence of programmed cell death (Havel and Durzan, 1996b; Wang et al., 1996a, b, 1999; Groover and Jones, 1999; Marubashi et al., 1999; Takahashi et al., 1999). It is generally accepted that a positive TUNEL assay is not a sufficient criterion for the identification of PCD. We can therefore conclude that our TUNEL‐positive results are indicative of a relatively ordered process of chromatin degradation, in which the DNA has been broken down by an endonuclease.
However, the detection of a genomic DNA ladder, despite the difficulty of detecting such ladders in this heterogeneous tissue in which a limited number of cells undergo cell death, corroborates the hypothesis that programmed cell death does really occur during the complex process of maize embryo formation.
ACKNOWLEDGEMENT
This work was supported by a grant from the Ministero dell’Università e della Ricerca Scientifica (M.U.R.S.T.‐COFIN 98) to S.D.
Supplementary Material
Received: 22 March 2002; Returned for revision: 2 May 2002; Accepted: 8 May 2002
References
- AbbeEC, Stein OL.1954. The growth of shoot apex in maize: embryogeny. American Journal of Botany 41: 285–293. [Google Scholar]
- AusubelFM, Brent R, Kingston RE, Moore DD, Sidman JG, Smith J.1994. Current protocols in molecular biology Vol 1. New York: Green Publishing Associates and John Wiley & Sons, Inc. [Google Scholar]
- BommertP, Werr W.2001. Gene expression patterns in the maize caryopsis: clues to decisions in embryo and endosperm development. Gene 271: 131–142. [DOI] [PubMed] [Google Scholar]
- BucknerB, Johal GS, Janick‐Buckner D.2000. Cell death in maize. Physiologia Plantarum 108: 231–239. [Google Scholar]
- BucknerB, Janick‐Buckner D, Gray J, Johal GS.1998. Cell death mechanisms in maize. Trends in Plant Science 3: 218–223. [Google Scholar]
- Calderon‐UrreaA, Dellaporta SL.1999. Cell death and cell protection genes determine the fate of pistils in maize. Development 126: 435–441. [DOI] [PubMed] [Google Scholar]
- DanonA, Delorme V, Mailhac N, Gallois P.2000. Plant programmed cell death: a common way to die. Plant Physiology and Biochemistry 38: 647–655. [Google Scholar]
- ElsterR, Bommert P, Sheridan WF, Werr W.2000. Analysis of four embryo‐specific mutants in Zea mays reveals that incomplete radial organization of the proembryo interferes with subsequent development. Development Genes Evolution 210: 300–310. [DOI] [PubMed] [Google Scholar]
- GorczycaW, Gong J, Darzynkiewicz Z.1993. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assay. Cancer Research 53: 1945–1951. [PubMed] [Google Scholar]
- GreenbergJT.1996. Programmed cell death: a way of life for plants. Proceedings of the National Academy of Sciences of the USA 93: 12094–12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrooverA, Jones AM.1999. Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiology 119: 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GunawardenaAHLAN, Pearce DM, Jackson MB, Hawes CR, Evans DE 2001. Characterisation of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 212: 205–214. [DOI] [PubMed] [Google Scholar]
- HavelL, Durzan DJ.1996a Apoptosis in plants. Botanica Acta 109: 268–277. [Google Scholar]
- HavelL, Durzan DJ.1996b Apoptosis during diploid parthenogenesis and early somatic embryogenesis of Norway spruce. International Journal of Plant Science 157: 8–16. [Google Scholar]
- LamE, Greenberg J.2000. Cell death: the ‘Yin’ path in the balancing of the life cycle. Plant Molecular Biology 44: vii–viii. [DOI] [PubMed] [Google Scholar]
- LamE, Pontier D, del Pozo O.1999. Die and let live – programmed cell death in plants. Current Opinion in Plant Biology 2: 502–507. [DOI] [PubMed] [Google Scholar]
- MartienssenR.1997. Fatal induction in plants. Current Biology 7: R534–R537. [DOI] [PubMed] [Google Scholar]
- MarubashiW, Yamada T, Niwa M.1999. Apoptosis detected in hybrids between Nicotiana glutinosa and N. repanda expressing lethality. Planta 210: 168–171. [DOI] [PubMed] [Google Scholar]
- MittlerR, Lam E.1996. Sacrifice in the face of foes: pathogen‐induced programmed cell death in plants. Trends in Microbiology 4: 10–15. [DOI] [PubMed] [Google Scholar]
- PennellRI, Lamb C.1997. Programmed cell death in plants. The Plant Cell 9: 1157–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ProcissiA, Dolfini S, Ronchi A, Tonelli C.1997. Light‐dependent spatial and temporal expression of pigment regulatory genes in developing maize seeds. The Plant Cell 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ScanlonJM, Freeling M.1998. The narrow sheath leaf domain deletion: a genetic tool used to reveal developmental homologies among modified maize organs. Plant Journal 13: 547–561. [Google Scholar]
- SchindlerT, Bergfeld R, Schopfer P.1995. Arabinogalactan proteins in maize coleoptiles: developmental relationship to cell death during xylem differentiation but not to extension growth. Plant Journal 7: 25–36. [DOI] [PubMed] [Google Scholar]
- TakahashiA, Kawasaki T, Henmi K, Shii K, Kodama O, Satoh H, Shimamoto K.1999. Lesion mimic mutants of rice with alterations in early signaling events of defense. Plant Journal 17: 535–545. [DOI] [PubMed] [Google Scholar]
- VauxDL, Korsmeyer SJ.1999. Cell death in development. Cell 96: 245–254. [DOI] [PubMed] [Google Scholar]
- WangH, Li J, Bostock RM, Gilchrist DG.1996a Apoptosis: a functional paradigm for programmed plant cell death induced by a host‐selective phytotoxin and invoked during development. The Plant Cell 8: 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WangM, Oppedijk BJ, Lu X, Van Duijn B, Schilperoort RA.1996b Apoptosis in barley aleurone during germination and its inhibition by abscisic acid. Plant Molecular Biology 31: 1125–1134. [DOI] [PubMed] [Google Scholar]
- WangM, Hoekstra S, van Bergen S, Lamers GEM, Oppedijk BJ, van der Heijden MW, de Priester W, Schilperoort RA.1999. Apoptosis in developing anthers and the role of ABA in this process during androgenesis in Hordeum vulgare L. Plant Molecular Biology 39: 489–501. [DOI] [PubMed] [Google Scholar]
- WredleU, Walles B, Hakman I.2001. DNA fragmentation and nuclear degradation during programmed cell death in the suspensor and endosperm of Vicia faba International Journal of Plant Sciences 162: 1053–1063. [Google Scholar]
- WuH, Cheung AY.2000. Programmed cell death in plant reproduction. Plant Molecular Biology 44: 267–281. [DOI] [PubMed] [Google Scholar]
- YeungEC, Meinke DW.1993. Embryogenesis in angiosperms: development of the suspensor. The Plant Cell 5: 1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YoungTE, Gallie DR.2000. Regulation of programmed cell death in maize endosperm by abscisic acid. Plant Molecular Biology 42: 397–414 [DOI] [PubMed] [Google Scholar]
- YoungTE, Gallie DR, DeMason DA.1997. Ethylene‐mediated programmed cell death during maize endosperm development of wild‐type and shrunken2 genotypes. Plant Physiology 115: 737–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.