Abstract
A broad survey of most of the major geyser basins within Yellowstone National Park (Wyoming, USA) was conducted to identify the flowering plants which tolerate high rhizosphere temperatures (≥40 °C) in geothermally heated environments. Under such conditions, five species of monocots and four species of dicots were repeatedly found. The predominant flowering plants in hot soils (>40 °C at 2–5 cm depth) were grasses, primarily Dichanthelium lanuginosum. Long‐term (weeks to months) rhizosphere temperatures of individual D. lanuginosum above 40 °C were recorded at several different locations, both in the summer and winter. The potential role of heat shock proteins (HSPs) in the apparent adaptation of these plants to chronically high rhizosphere temperatures was examined. Antibodies to cytoplasmic class I small heat shock proteins (sHSPs) and to HSP101 were used in Western immunoblot analyses of protein extracts from plants collected from geothermally heated soils. Relatively high levels of proteins reacting with anti‐sHSP antibodies were consistently detected in root extracts from plants experiencing rhizosphere temperatures above 40 °C, though these proteins were usually not highly expressed in leaf extracts from the same plants. Proteins reacting with antibodies to HSP101 were also present both in leaf and root extracts from plants collected from geothermal soils, but their levels of expression were not as closely related to the degree of heat exposure as those of sHSPs.
Key words: Geothermal, Yellowstone, heat stress, heat shock proteins, extreme environments, Dichanthelium lanuginosum
INTRODUCTION
One approach to understanding the cellular mechanisms of plant stress tolerance has been to focus on plants adapted to extreme environments, such as hot deserts (Kappen, 1981; Nobel, 1995; Ingram and Bartels, 1996). From such studies valuable information has been gained regarding the nature of plant adaptations to environmental stresses. Some examples include: photosynthetic adaptations to heat stress (Bjorkman et al., 1980; Seeman et al., 1986), adaptations to dehydration stress, e.g. CAM plants (Cushman and Bohnert, 1999) and resurrection plants (Chandler and Bartels, 1999), and growth adaptations to desert environments (Mulroy and Rundel, 1977; Drennan and Nobel, 1996).
Surface geothermal areas (sometimes referred to as solfataras) are extreme environments that may also serve as sources for plants adapted to stressful conditions. Due to the relatively rare nature and geographical isolation of large, undisturbed geothermal areas such as the Kronotsky Reserve in Kamchatka and Yellowstone National Park (YNP), USA, little is known about plants adapted to growth and reproduction in the harsh physical conditions common to surface geothermal environments.
Early research into such plants was carried out by Hesselbo (1913) and Lange (1973), who studied the flora around hot springs in Iceland and noted the predominance of bryophytes. More recently, Glime and Iwatsuki (1994) have studied the physical and chemical effects on plant community structure of two geothermal fields located on Hokkaido, Japan. They concluded that vascular plants were limited primarily by root zone (i.e. rhizosphere) temperature, with higher temperatures favouring mosses and lichens. Regarding New Zealand geothermal soils and associated plants, Given (1980) concluded that heat flow through the soil was probably the most significant factor in determining species composition, with bryophytes occupying the hotter zones. A recent paper by Burns (1997) examined the vegetative composition and structure along a stress gradient at another geothermal area in New Zealand. Again, soil temperature was the primary determinant of plant species composition.
Several studies involving both phylogenetic and physiological aspects of flowering plants growing in thermal areas of YNP have been conducted. For example, Sheppard (1971) performed a vegetative pattern survey of only four steaming ground sites but described several species of mosses and angiosperms that flourished where soil temperatures reached 50–65 °C at a depth of 10 cm. Another study (Miller, 1968) provided aerial mapping of thermal areas and mentioned a few of the species noted by Sheppard (1971). Two other investigations involved Mimulus guttatus (Scrophulariaceae), which sometimes grows along the banks of streams flowing from hot springs in YNP. Rice (1973) examined photosynthetic rate vs. temperature, and Delmer (1974) studied the thermostability of cytoplasmic proteins as well as the effects of temperature on growth of this species in tissue culture. In neither case, however, did M. guttatus display unusual heat resistance.
We have previously reported on the heat and acid tolerance of the most common flowering plant observed in several of YNP’s active geothermal areas (Stout et al., 1997). Here we present more information on this and other commonly occurring plants in YNP’s geyser basins, including data regarding the expression of heat shock proteins (HSPs) in these plants.
MATERIALS AND METHODS
Field conditions and plant sampling
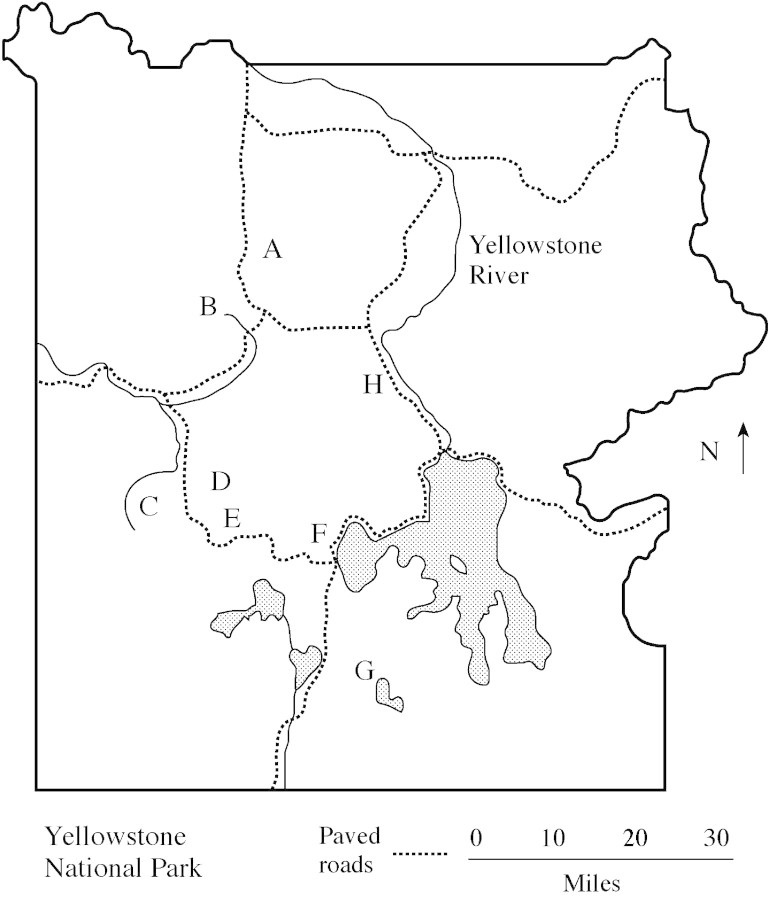
Figure 1 shows the major geothermal areas within YNP surveyed for heat‐tolerant plants. The places shown on this map include most of the major geyser basins within YNP. Each of these geyser basins was explored to identify several active surface‐geothermal sites. The precise location of each of the 30 study sites surveyed for this report is listed in Table 1. Such sites usually fell into one of the following three general categories: (1) areas adjacent to hot springs and their discharge streams; (2) inside or adjacent to fumaroles (holes in volcanic areas from which hot gases escape); and (3) steaming ground. For each geyser basin shown in Fig. 1, several active geothermal sites, typically ranging from approx. 0·5 to 10 m2, were surveyed for the presence of plants at high soil temperatures (above 40 °C). This temperature was chosen because the general consensus appears to be that plants growing at chronic temperatures at or above 40 °C would be considered to be heat tolerant (Brock, 1967; Levitt, 1980; McWilliams, 1980; Kappen, 1981). Soil temperature was measured in the field at depths of 2–5 cm, typically within the rhizosphere of the vascular plants found there. At the larger (>10 m2) steaming ground sites, randomly located quadrats (855 cm2) were sampled along 10 m transects. Plant species were determined by comparing field‐collected plants with type specimens in the Montana State University herbarium with assistance from herbarium staff.
Fig. 1. Location of geothermal areas (geyser basins) surveyed within Yellowstone National Park, USA. Thirty study sites encompassed active surface‐geothermal zones distributed among eight large areas: Amphitheater Springs (A); Norris Basin (B); Rush Lake/Firehole River (C); White Creek (D); Rabbit Creek (E); West Thumb area (F); Heart Lake (G); and Forest Springs/Mud Volcano area (H). The location of each individual study site is listed in Table 1.
Table 1.
Location of the 30 individual study sites
| Geothermal area | Geothermal area | ||
| Site number | Latitude/longitude | Site number | Latitude/longitude |
| (A) Amphitheater Springs | (D) White Creek | ||
| 1 | 44·8034°N/110·7255°W | 1 | 44·5325°N/110·7963°W |
| 2 | 44·8022°N/110·7251°W | 2 | 44·5309°N/110·7922°W |
| 3 | 44·8026°N/110·7257°W | (E) Rabbit Creek | |
| 4 | 44·8005°N/110·7274°W | 1 | 44·5216°N/110·8106°W |
| 5 | 44·7996°N/110·7284°W | 2 | 44·5209°N/110·8092°W |
| 6 | 44·7996°N/110·7274°W | 3 | 44·5199°N/110·8100°W |
| (B) Norris | 4 | 44·5158°N/110·8108°W | |
| 1 | 44·7370°N/110·7003°W | 5 | 44·5081°N/110·8087°W |
| 2 | 44·7363°N/110·7033°W | (F) West Thumb | |
| 3 | 44·7342°N/110·7070°W | 1 | 44·4174°N/110·5715°W |
| 4 | 44·7279°N/110·7104°W | 2 | 44·4333°N/110·5816°W |
| (C) Rush Lake/Firehole River | (G) Heart Lake | ||
| 1 | 44·5630°N/110·8350°W | 1 | 44·3105°N/110·5336°W |
| 2 | 44·5634°N/110·8315°W | 2 | 44·3062°N/110·5267°W |
| 3 | 44·5621°N/110·8239°W | 3 | 44·3023°N/110·5191°W |
| 4 | 44·5617°N/110·8278°W | (H) Forest Springs/Mud Volcano | |
| 5 | 44·5599°N/110·8324°W | 1 | 44·7060°N/110·4594°W |
| 6 | 44·5588°N/110·8433°W | 2 | 44·6245°N/110·4361°W |
Geothermal areas correspond with those shown in Fig. 1.
Plant materials collected in the field for protein analyses were immediately wrapped in aluminium foil and frozen in liquid nitrogen. These specimens were kept in liquid nitrogen until they were returned to the laboratory where they were stored at –80 °C until use.
Temperature measurements
Soil temperatures were determined using a digital thermocouple thermometer (Model 115; Barnant Company, Barrington, IL, USA) equipped with a 10 cm long penetration probe (603‐1000; Barnant). Leaf surface temperatures were measured using a surface microprobe (623‐1000; Barnant). Data‐loggers (Forestry Suppliers, Inc., Jackson, MS, USA) equipped with thermistor sensor cables were used to monitor and record long‐term rhizosphere (1–5 cm depth) and air temperature variations.
Protein extraction, gel electrophoresis and Western blot analyses
Protein extraction was performed essentially as described by Hernandez and Vierling (1993), with several modifications. For protein sample preparation, 0·1–0·5 g (f. wt) of plant tissue was ground in liquid nitrogen using a mortar and pestle. The resulting powder was immediately ground in a protein extraction buffer (2–3 ml buffer per 0·1 g f. wt of tissue). The protein extraction buffer consisted of 60 mm Tris–HCl (pH 8), 60 mm dithiotreitol (DTT), 2 % (w/v) sodium dodecyl sulfate (SDS), 15 % (w/v) sucrose, 5 mm ϵ‐amino‐η‐caproic acid, 1 mm benzamidine, 0·275 % diethyl dithiocarbamic acid and 4 % polyvinylpyrrolidone (insoluble, mol wt 240 000). After grinding, samples were heated to 95 °C for 5 min, and insoluble material pelleted by centrifugation for 5 min at 12 000 g (max) at room temperature. To the resulting supernatant was added five volumes of –20 °C acetone, with mixing. Samples were stored at –20 °C overnight. The following day, samples were centrifuged for 5 min at 2500 g (max) at room temperature to pellet protein precipitate. The pellet was typically resuspended in 1 : 1 (v : v) 2 × SDS polyacrylamide gel electrophoresis (SDS‐PAGE) sample buffer (20 % glycerol, 4 % SDS, 10 % DTT, 0·126 mm Tris–HCl, pH 6·8). Protein concentrations were estimated by precipitating a 10 µl aliquot of the resuspended protein pellet in 0·5 ml 0·1 m ammonium acetate in methanol overnight at room temperature. Protein precipitate was collected by centrifugation at 11 000 g (max) for 10 min. The pellet was resuspended in 0·3 ml 0·1 m ammonium acetate in methanol and immediately centrifuged at 11 000 g (max) for 10 min. The pellet was then resuspended in 0·5 ml ice‐cold acetone and centrifuged at 11 000g (max) for 10 min. This pellet was air‐dried at room temperature for 10–15 min and then dissolved in 0·1 ml 0·05 m NaOH. Protein concentration was quantitatively estimated by the bicinchoninic acid (BCA) method (Smith et al., 1985) using a kit from Pierce Chemical Company (Rockford, IL, USA). The procedures for SDS‐PAGE and Western immunoblots were as previously described (Stout et al., 1997).
HSP antisera
Rabbit antisera used for the detection of HSPs on immunoblots were as follows. For small HSPs (sHSPs), antiserum against class I low molecular weight HSPs from Oryza sativa (Jinn et al., 1993) was used. For HSP101, antiserum against Triticum sp. (Wells et al., 1998) HSP101 was used. The cross‐reactivity of these antibodies to proteins present in crude protein extracts from Dichanthelium lanuginosum and the other plant species collected in YNP was tested as follows. Plant specimens (0·5–2 g f. wt) were incubated in a medium containing 1 % sucrose and 5 mm potassium phosphate buffer, pH 6·0 (Jinn et al., 1989), for 2–4 h, either at room temperature (22–25 °C) or at 40 °C (heat‐treated). Protein extracts were obtained from these plants and immunoblots were performed as described above.
RESULTS
Geothermal plant surveys
In an effort to identify heat‐tolerant (rhizosphere temperature ≥40 °C) flowering plant species in geothermal soils of Yellowstone National Park, a broad survey was conducted of most of Yellowstone’s major geyser basins (Fig. 1). Over 500 individual flowering plants were logged at 30 different active geothermal sites among the eight geyser basins surveyed. Collectively, these individual plants represented only four species of dicots and five species of monocots, as outlined in Table 2.
Table 2.
Vascular plants, with observed rhizosphere temperatures >40 °C, found in 30 geothermally heated survey sites within Yellowstone National Park
| No. ofsurvey sites at | Rhizosphere temperature (°C) | ||
| Family/ | which the species | ||
| species | was found | Maximum | Mean ± s.e. (n) |
| Dicotyledons | |||
| Asteraceae | |||
| Gnaphalium chilense (P) | 13 | 47 | 43·7 ± 2·33 (36) |
| Heterotheca depressa (P) | 4 | 45 | 42·1 ± 1·93 (22) |
| Euphorbiaceae | |||
| Euphorbia glyptosperma (A) | 6 | 51 | 44·0 ± 3·63 (19) |
| Polygonaceae | |||
| Rumex acetosella (P, E) | 5 | 43 | 41·3 ± 1·70 (10) |
| Monocotyledons | |||
| Juncaceae | |||
| Juncus tweedyi (P) | 9 | 51 | 43·8 ± 3·76 (33) |
| Poaceae | |||
| Agrostis rossiae (A) | 4 | 45 | 42·0 ± 3 (5) |
| Agrostis scabra (P) | 20 | 51 | 44·0 ± 3·02 (32) |
| Dichanthelium lanuginosum (P) | 30 | 57 | 45·1 ± 4·22 (330) |
| Panicum capillare (A, E) | 11 | 55 | 44·3 ± 3·24 (60) |
A, Annual; E, exotic; P, perennial.
n, Total number of distinct individuals measured.
Species of the family Poaceae were both the most prevalent and the most heat‐tolerant plants we observed in Yellowstone’s geothermal areas (Table 2). Perennial Dichanthelium lanuginosum (Elliott) Gould var. sericeum (Schmoll) Spellenberg (Panicum occidentale Scribn.; Panicum thermale Bolander), hot springs panicgrass (Schmoll, 1939; Spellenberg, 1975), was found at all the geothermal areas we surveyed. An annual grass, Panicum capillare L., was also one of the more heat‐tolerant and frequently observed angiosperms on geothermally heated ground. In contrast to P. capillare, no D. lanuginosum plants were observed outside of active solfataras. Another abundant heat‐tolerant grass found in YNP’s geothermal areas was Agrostis scabra Willd. Although all three grass species were frequently rooted in moss mats when growing on the hotter steaming ground sites or in steam vents, A. scabra was almost always found in such mats at higher soil temperatures. Finally, with regard to grasses, our survey revealed consistently high soil temperatures for Agrostis rossiae Vasey, a rare annual found only at a few thermal sites in YNP and usually sympatric with A. scabra.
Of the remaining five species listed in Table 2, Gnaphalium chilense Sprengl., Heterotheca depressa (Rydb.) Dorn and Rumex acetosella L. were also noted by Sheppard (1971) to occur at soil temperatures over 40 °C (at a depth of 10 cm, however). Although Sheppard (1971) did not record temperatures for Euphorbia glyptosperma Engelm., he did note that the species was growing near his study sites. Similarly, Juncus tweedyi Rydb. has been observed by Yellowstone botanists to occur at thermal sites but as far as we know no temperatures have been recorded prior to our survey.
Long‐term rhizosphere temperatures
To broaden previously reported information regarding the chronic temperatures experienced by D. lanuginosum in geothermally heated soils (Stout et al., 1997), temperature dataloggers were used to record the rhizosphere temperatures of individuals of this species in three types of geothermal environments and in geothermally heated soils over the winter. Long‐term D. lanuginosum rhizosphere temperatures were simultaneously monitored in: (1) plants adjacent to a hot spring; (2) plants on relatively dry, steaming soil; and (3) plants in an area bordering these active geothermal sites. Having collected such data every summer from 1996 to 2001, we present rhizosphere temperatures typical of July and August in Fig. 2. Roots of D. lanuginosum adjacent to hot springs (Fig. 2A) and on steaming soil (Fig. 2B, upper tracing) were often exposed to chronic temperatures between 35 and 50 °C. Rhizosphere temperatures of plants in cooler soils adjacent to active geothermal areas (Fig. 2B, lower tracing) were 10–20 °C lower during the same period. The decrease in temperatures that occurred between 5 and 6 August was due to rainfall from a thunderstorm.
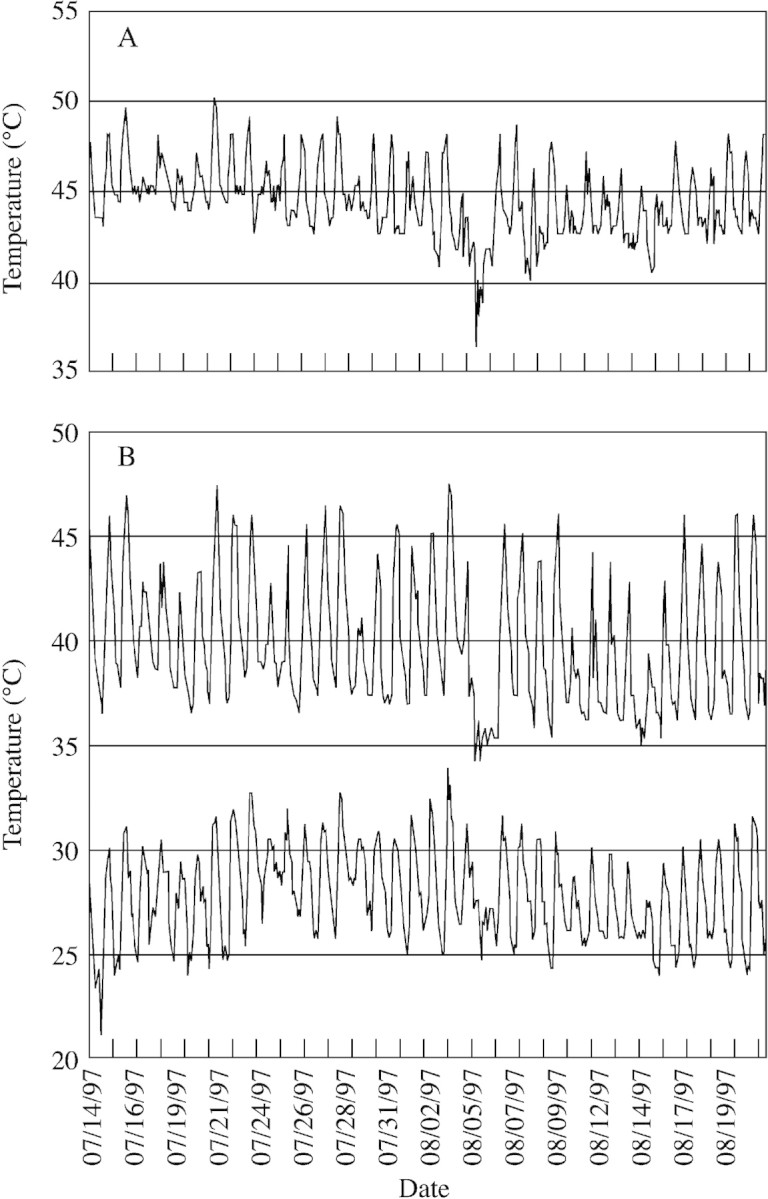
Fig. 2. Simultaneous datalogger readouts of Dichanthelium lanuginosum rhizosphere temperatures on three types of geothermally heated soils in the Rabbit Creek thermal area (Fig. 1, site 5) of YNP. A, Near (5–10 cm) the edge of a hot springs (85 to 90 °C). B, On relatively dry, steaming soil (approx. 20 m from hot springs) (upper tracing) and on geothermally inactive, exposed ground approx. 50 m from the active geothermal sites above (lower tracing).
Can geothermal heat also maintain warm temperatures for both roots and leaves of D. lanuginosum during the cold winters in YNP, where mean ambient temperatures typically range from –5 to –10 °C (Gates, 1961)? During the winters of 1996–97 and 1997–98, dataloggers were used to record rhizosphere and leaf temperatures of individual D. lanuginosum adjacent to thermal streams and on steaming ground sites. Representative results are presented in Fig. 3. Geothermal heat sustained high rhizosphere temperatures (Fig. 3A and B, upper tracing) as well as warm leaf temperatures (Fig. 3B, lower tracing) of some D. lanuginosum individuals during the winter months.
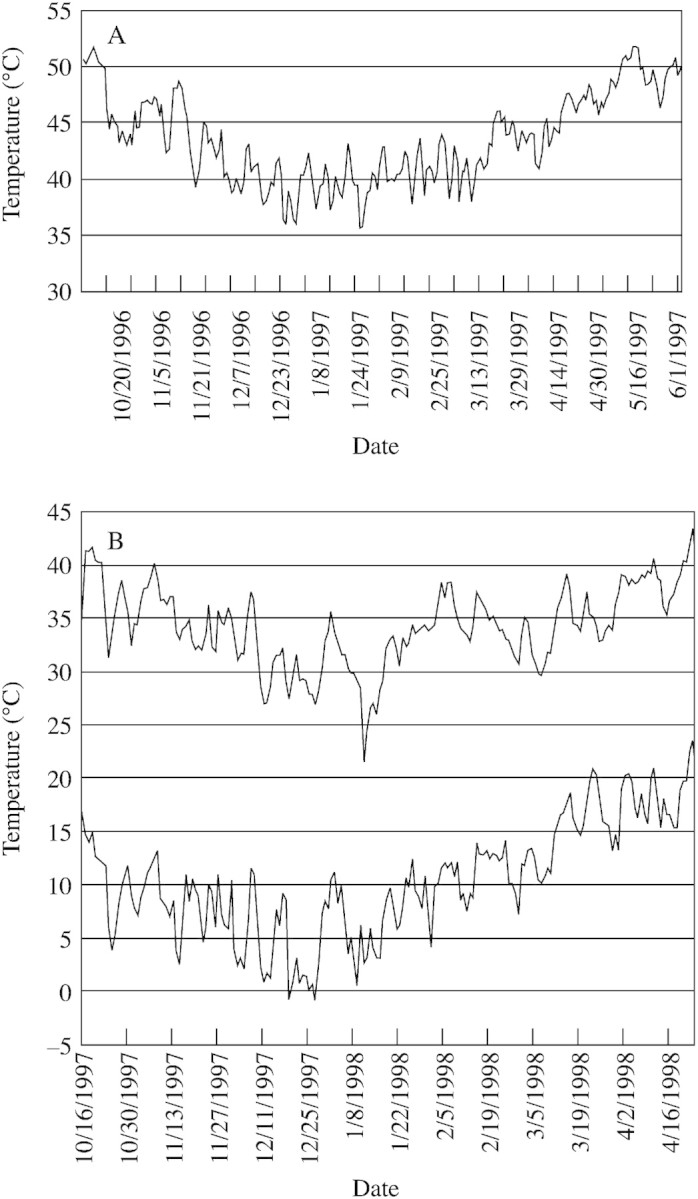
Fig. 3. Temperature datalogger readouts from the winters of 1996–97 and 1997–98, showing rhizosphere and leaf temperatures of clusters of Dichanthelium lanuginosum plants. A, Rhizosphere temperatures of plants adjacent to (5–10 cm) a hot springs outflow stream located in the Rabbit Creek thermal area (Fig. 1, site 5) of YNP. B, Plants on a steaming ground site in the Amphitheater Springs thermal area (Fig. 1, site 1) of YNP, showing simultaneous rhizosphere (upper tracing) and leaf (lower tracing) temperatures.
HSP expression in plants from geothermal soils
Given the high temperatures to which they are exposed, plants in geothermally heated soils might express relatively high levels of HSPs. This would be expected if plant evolution in this thermally stressful environment relied on this cellular mechanism for maintaining native protein structure at denaturing temperatures. To address this question, the expression of sHSPs and of HSP101 in plants collected from thermal soils in YNP was examined since these two categories of HSPs have been strongly implicated in thermotolerance in plants (Waters et al., 1996; Queitsch et al., 2000).
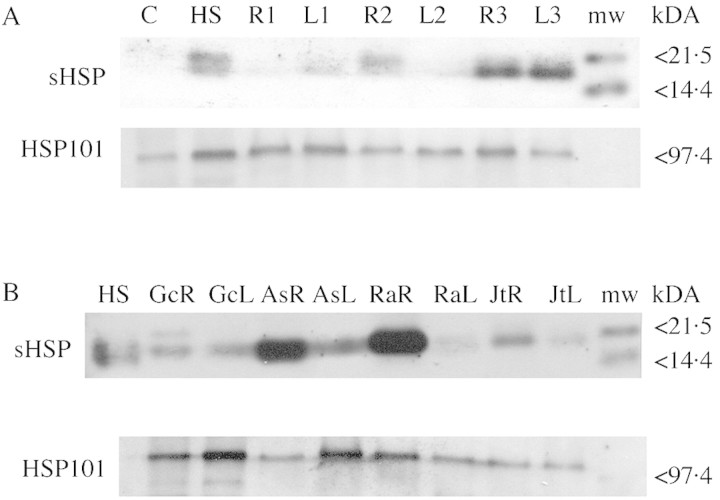
Roots of individual D. lanuginosum plants typically express proteins reacting with antibodies to sHSPs at soil temperatures above approx. 40 °C (Fig. 4A). In most cases, such proteins were not simultaneously expressed in leaves of these plants. Leaf surface temperatures of D. lanuginosum typically did not exceed 35 °C even in geothermally heated soils (e.g. see Fig. 3B, lower tracing). Only in cases of unusually hot soils did we observe anti‐sHSP antibodies reacting to proteins from D. lanuginosum leaf extracts (e.g. Fig. 4A). Root and leaf extracts from some of the other plant species listed in Table 2 were also reacted with anti‐sHSP antibodies. Individual Gnaphalium chilense, Agrostis scabra, Rumex acetosella and Juncus tweedyi from geo thermal soils expressed presumptive sHSPs at rhizosphere temperatures above 40 °C (Fig. 4B) with expression being much higher in roots than in leaves, which were typically 15–20 °C cooler than the roots.
Fig. 4. Expression of sHSP and HSP101 in plants collected from geothermally heated soils in Yellowstone National Park. Total protein extracts from whole 3‐ to 4‐week‐old Dichanthelium lanuginosum seedlings (laboratory grown) that had been maintained at 22–25 °C (C) or heated at 40 °C for 2 h (HS) and from field‐collected plants were electrophoretically separated on SDS‐polyacrylamide gels (5 µg protein per lane), transferred to nitrocellulose paper and reacted to antibodies against sHSP and HSP101. A, Extracts from roots and leaves of individual D. lanuginosum plants collected from geothermal soils with rhizosphere temperatures of 34 °C (R1, L1), 42 °C (R2, L2) and 48 °C (R3, L3). B, Extracts from roots (R) and leaves (L) of individual plants with rhizosphere temperatures ranging from 40 to 43 °C; Gc, Gnaphalium chilense; As, Agrostis scabra; Ra, Rumex acetosella; Jt, Juncus tweedyi. Blots are representative of results obtained from at least three replicate experiments using extracts from different individuals collected from two different geothermal study sites (Amphitheater Springs, site 4; Rabbit Creek, site 1).
The expression of proteins reacting with antibodies to HSP101 in both root and leaf extracts from D. lanuginosum and other plant species listed in Table 2 is also shown in Fig. 4. In contrast to sHSP‐reactive proteins, levels of proteins immunoreactive with the anti‐HSP101 antibodies were less well correlated with the degrees of heat exposure in D. lanuginosum (Fig. 4A). In general, however, relatively higher levels of these proteins were found in root extracts from plants collected from hot soils (rhizosphere temperatures above 40 °C) compared with leaf extracts from the same plants, with the exception of Agrostis scabra (Fig. 4B).
DISCUSSION
The primary aim of this study was to identify heat‐tolerant (chronic rhizosphere temperatures ≥40 °C) flowering plant species found on geothermally heated soils over a broad area of YNP. Despite the great physical (e.g. soil pH, mineralization, moisture content) diversity of surface geothermal areas in YNP (Brock, 1967), we found a relatively small diversity of heat‐tolerant flowering plants (Table 2) present in most of YNP’s major geyser basins (Fig. 1). Since the chief common feature of these diverse geyser basins was geothermally heated soils, our results are consistent with previous investigations of geothermal plant communities (Shepard, 1971; Given, 1980; Glime and Iwatsuki, 1994; Burns, 1997) in that all of these studies concluded that soil temperature is the most significant physical factor influencing plant species composition in geothermally heated environments.
From our broad survey of diverse geothermal areas in YNP, D. lanuginosum var. sericeum (Spellenberg, 1975) was the most frequently observed heat‐tolerant vascular plant. Commonly known as hot springs panicgrass in western North America, this plant has previously been noted to be associated with active thermal areas in YNP, as Panicum thermale (Brock and Brock, 1968; Miller, 1968; Sheppard, 1971) and as Panicum ferventicola (Schmoll, 1939). As Panicum thermale, it has also been reported to be associated with other surface geothermal areas in western North America (Brewer, 1868; Gillette et al., 1961; Malloch, 1978), especially on warm, acid ground (Hitchcock and Chase, 1910). Spellenberg (1975) divided D. lanuginosum into three varieties in western North America: (1) the widespread var. fasciculatum (populations on non‐thermal soils, but also on thermal soils in Lassen Volcanic National Park); (2) var. sericeum (populations associated with surface geothermal areas in the Rocky Mountains); and (3) a small population of var. thermale on warm soils at The Geysers, Sonoma County, California. This population has been the focus of recent studies (Pavlik, 2001; Pavlik and Enberg, 2001) examining the demographic and ecophysiological responses of these rare plants to soil temperature and soil water. Genetic analysis, e.g. using inter simple sequence repeat markers (Wolfe et al., 1998), of these three varieties may give clues to the cellular bases for the apparent adaptation to thermal soils of at least two of them.
The other vascular plant species that we observed in hot soils in YNP’s geyser basins exhibit only a few common characteristics. Most of these plant species are commonly considered weedy, exotic or both, with a marked tendency to either polyploidy or reproduction by selfing. Most of these species flourish in a wide range of soil conditions and geographic areas, e.g. P. capillare is widespread throughout North America (Zuloaga and Morrone, 1996). One exception, however, is Agrostis rossiae. This annual grass is endemic to YNP and was found only on thermal soils (rhizosphere temperatures >40 °C). Because of the rare nature of A. rossiae, little is known about this species. The habit of grasses growing in moss mats on the hotter soils that we observed was noted by Sheppard (1971), who suggested that moss mats might provide grasses with moisture resulting from steam condensation and insulation from potentially lethal hot soil.
We have previously shown that rhizosphere temperatures of D. lanuginosum adjacent to hot springs outflow streams may exceed 50 °C around midday in July and August and remain above 30–40 °C overnight (Stout et al., 1997). Here we provide additional information regarding long‐term rhizosphere and leaf temperatures of D. lanuginosum in several different geothermally heated environments. The simultaneous temperature datalogger readouts presented in Fig. 2 clearly demonstrate that geothermal heating, whether from hot springs or from steam percolation through soils, maintained rhizosphere temperatures above 35 °C, and up to 50 °C during midsummer in YNP. This is typically 10–15 °C above those of plants on exposed (solar‐heated) ground adjacent to the active geothermal soils (Fig. 2B, lower tracing). Geothermal heat maintained warm rhizosphere temperatures during the winter months in YNP (Fig. 3) and may also have contributed to warming the leaves (Fig. 3B, lower tracing). Since active geothermal areas remain snow‐free during the winter, solar heating may also have contributed to the leaf warming. The observation that some winter rhizosphere temperatures (Fig. 3) were comparable with summer rhizosphere temperatures (Fig. 2) is remarkable, since this indicates that the roots of these plants may have been experiencing heat stress even during frigid Yellowstone winters.
The cellular mechanisms that are primarily responsible for the apparent adaptation to geothermally heated soils of D. lanuginosum, and of the other heat‐tolerant vascular plant species observed in Yellowstone’s geyser basins, are unknown. HSPs are likely candidates responsible for the ability of these plants to tolerate the stressful rhizosphere temperatures. The relative significance of HSPs, compared with the expression of thermally stable proteins, e.g. in the adaptation strategies of organisms to thermally stressful environments, is currently unclear (Coleman et al., 1995; Knight and Ackerly, 2001). sHSPs have been implicated as components of macromolecular protein chaperone mechanisms underlying plant thermotolerance (Hernandez and Vierling, 1993; Park et al., 1996; Waters et al., 1996; Knight and Ackerly, 2001). Recent evidence (Queitsch et al., 2000) supports the idea that HSP101 expression is essential for thermotolerance in plants, perhaps by functioning to re‐activate denatured proteins (Glover and Lindquist, 1998) to promote the translation of some cellular mRNAs (Wells et al., 1998), or both.
We thus chose to examine expression of a class of sHSPs and HSP101 in plants collected from geothermally heated soils in YNP. There are several reports of the expression of HSPs in crop plants under field conditions (Burke et al., 1985; Kimpel and Key, 1985; Hernandez and Vierling, 1993), but, to the best of our knowledge, there are only a few published reports of the expression of HSPs in native plants collected from their natural habitats, including geothermal environments (Neumann et al., 1989). Both diurnal variation in HSP72/73 (Colombo et al., 1995) and seasonal variation in HSP70 (Wisniewski et al., 1996) have been observed in woody plants. In two studies involving leaf sHSPs, Dr Charles Knight (Stanford University) has found that leaves from plants collected in the field have higher levels of these HSPs than those from comparable glasshouse‐grown plants, and that leaves from the chaparral plant Ceanothus cuneatus have higher levels of sHSP when collected from plants on south‐facing slopes compared with plants on cooler north‐facing slopes (pers. comm.). In our earlier report (Stout et al., 1997), we provided precursory evidence for sHSP expression in whole‐plant protein extracts from D. lanuginosum, with rhizosphere temperatures above 45 °C, collected from geothermally heated ground.
This study confirms that heat‐tolerant flowering plants collected from geothermally heated soils in YNP express proteins reacting with antibodies to both sHSPs and HSP101. Proteins reacting with antibodies to sHSPs were present in root extracts from most of the D. lanuginosum plants that we collected from geothermally heated soils. The exceptions were plants experiencing rhizosphere temperatures below approx. 35 °C (Fig. 4A). The immunodetectable levels of sHSPs in D. lanuginosum increased in a heat‐correlated manner, from rhizosphere temperatures of approx. 40 °C, up to 48 °C. Typically, we detected little or no expression of proteins reacting with sHSP antibodies in extracts from leaves of D. lanuginosum plants collected from geothermally heated soils. In this case, the exceptions were leaves from plants growing in the hottest soils (rhizosphere temperatures above 45 °C). The apparent expression levels of HSP101 in D. lanuginosum from geothermally heated soils are less well correlated with the degree of heat exposure than are sHSP expression levels (Fig. 4A). Detectable levels of proteins reacting with HSP101 antibodies were found in both root and leaf extracts, over a wide range of rhizosphere temperatures. In general, however, there appeared to be higher levels of presumptive HSP101 in extracts from D. lanuginosum plants growing in the hotter soils, but the degree of increased expression in response to increased heat was much less dramatic than that of sHSPs.
Proteins reacting to both sHSP and HSP101 antibodies were also present in extracts from roots of other flowering plant species collected from geothermally heated soils in YNP. As with D. lanuginosum, relatively high expression levels of apparent sHSPs were observed in extracts from plants experiencing rhizosphere temperatures above 40 °C at the time they were collected (Fig. 4B). Also similar to D. lanuginosum, the apparent expression of HSP101 was less well correlated with the degree of heat exposure than that of sHSPs. However, the relative cross‐reactivity of the anti‐HSP antibodies to target proteins was a factor. Most of the plant species examined were comparable when pre‐screened for antibody reactivity using specimens heat‐treated in the laboratory (see Materials and Methods). Protein extracts from heat‐treated G. chilense, however, were weakly reactive to the sHSP antibodies and unreactive with the HSP101 antibodies. Since we presume that expression levels of these HSPs would be comparable among plant species heat‐shocked in the laboratory, we conclude that such proteins in our specimens of G. chilense lack reactive epitopes that are present on HSP101 proteins from the other plant species we tested.
Since hydrothermally modified soils sometimes contain high levels of toxic metals (Phelps and Buseck, 1980; Stauffer and Thompson, 1984) and since high concentrations of some toxic metals such as arsenite and cadmium are known to induce HSP expression in both plants and animals (Feder and Hofmann, 1999), it is possible that elevated levels of HSPs in some of the plants growing on hydrothermally modified ground in YNP may be due to toxic metals in the soil. The soils from which plants were collected for the HSP expression experiments shown in Fig. 4 were chemically analysed, including tests for arsenic and lead. The soil samples from these two geothermal study sites (Amphitheater Springs, site 4, and Rabbit Creek, site 1, as shown in Table 1) displayed no indication of unusually high levels of heavy metals (data not shown).
Collectively, our results tend to support the idea that cytoplasmic class I sHSPs may contribute more than HSP101 proteins to the ability of some plants to acclimate to, and perhaps adapt to, geothermally heated environments in YNP. This conclusion is based on our observation that apparent HSP101 expression levels did not increase to nearly the same degree as those of sHSPs in response to increasing rhizosphere temperatures. This presumes that there is a quantitative relationship between the amount of a class of HSP expressed and its relative role in contributing to thermotolerance. Our conclusion is tempered somewhat by the fact that quantitative estimates of apparent sHSPs and HSP101 expression in these plants in relation to soil temperature has been complicated by two factors. One was the individual variation among plants collected from the field, and the second was some differences in relative antibody cross‐reactivity among different plant species. Nevertheless, this is one of the few reports confirming HSP expression in native plants in response to high temperature exposure imposed by natural environmental conditions.
ACKNOWLEDGEMENTS
We thank T. Kerstetter for excellent technical assistance, C. Seibert and J. Rumely (MSU Herbarium) for assistance in plant identification, and Professors Daniel Gallie (UC Riverside) and Chu‐Yung Lin (National Taiwan Uni versity) for their kind gifts of antibodies. This work was supported by USDA NRICGP grant 95‐37106‐2447, by the Montana State University MONTS program, and by NASA grant NAG 5‐8807 to the Montana State University Thermal Biology Institute. This is journal article 2002‐13 from the Montana Agricultural Experiment Station.
Supplementary Material
Received: 11 December 2001; Returned for revision: 20 February 2002; Accepted: 2 May 2002
References
- BjorkmanO, Badger MR, Armond PA.1980. Response and adaptation of photosynthesis to high‐temperatures. In: Turner NC, Kramer PJ, eds. Adaptation of plants to water and high‐temperature stress New York: John Wiley & Sons, 233–249. [Google Scholar]
- BrewerWH.1868. Notice of plants found growing around hot springs in California. Proceedings of the California Academy of Natural Sciences 3: 120–121. [Google Scholar]
- BrockTD.1967. Life at high temperatures. Science 158: 1012–1019. [DOI] [PubMed] [Google Scholar]
- BrockTD, Brock ML.1968. Life in a hot‐water basin. Natural History 77: 47–53. [Google Scholar]
- BurkeJJ, Hatfield JL, Klein R, Mullet JE.1985. Accumulation of heat shock proteins under field conditions. Plant Physiology 78: 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BurnsB.1997. Vegetation change along a geothermal stress gradient at Te Kopia steamfield. Journal of the Royal Society of New Zealand 27: 279–294. [Google Scholar]
- ChandlerJ, Bartels D.1999. Plant desiccation. In: Lerner HR, ed. Plant responses to environmental stress New York: Marcel Dekker Inc., 575–590. [Google Scholar]
- ColemanJS, Heckathorn SA, Hallberg RL.1995. Heat‐shock proteins and thermotolerance: linking molecular and ecological perspectives. Trends in Ecology and Evolution 10: 305–306. [DOI] [PubMed] [Google Scholar]
- ColomboSJ, Timmer VR, Colclough ML, Blumwald E.1995. Diurnal variation in heat tolerance and heat shock protein expression in black spruce (Picea mariana). Canadian Journal of Forest Research 25: 369–375. [Google Scholar]
- CushmanJC, Bohnert HJ.1999. Crassulacean acid metabolism: molecular genetics. Annual Review of Plant Physiology and Plant Molecular Biology 50: 305–332. [DOI] [PubMed] [Google Scholar]
- DelmerDP.1974. Studies on the nature of adaptations of the monkey flower, Mimulus gutattus, to a thermophilic environment. Canadian Journal of Botany 52: 1509–1514. [Google Scholar]
- DrennanPM, Nobel PS.1996. Temperature influences on root growth for Encelia farinosa (Asteraceae), Pleuraphis rigida (Poaceae), and Agave deserti (Agavaceae) under current and doubled CO2 concentrations. American Journal of Botany 83: 133–139. [Google Scholar]
- FederME, Hofmann GE.1999. Heat‐shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annual Review of Physiology 61: 243–82. [DOI] [PubMed] [Google Scholar]
- GatesDM.1961. Winter thermal radiation studies in Yellowstone Park. Science 134: 32–35. [DOI] [PubMed] [Google Scholar]
- GilletteGW, Howell JT, Leschke H.1961. The flora of Lassen Volcanic National Park, California. Wasmann Journal of Biology 19: 1–43. [Google Scholar]
- GivenDR.1980. Vegetation on heated soils at Karapiti, central North Island, New Zealand, and its relation to ground temperature. New Zealand Journal of Botany 18: 1–13. [Google Scholar]
- GlimeJM, Iwatsuki Z.1994. Geothermal communities of Ponponyama, Hokkaido, Japan. Journal of the Hattori Botanical Laboratory 75: 133–147. [Google Scholar]
- GloverJR, Lindquist S.1998. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94: 73–82. [DOI] [PubMed] [Google Scholar]
- HernandezLD, Vierling E.1993. Expression of low molecular weight heat‐shock proteins under field conditions. Plant Physiology 101: 1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HesselboA.1913. The bryophyta of Iceland. In: Rosenvinge LK, Warming E, eds. The Botany of Iceland, part II 4. Kopenhagen, London: John Wheldon and Co. [Google Scholar]
- HitchcockAS, Chase A.1910. The North American Species of Panicum.Contents of the United States National Herbarium 15: 231–232. [Google Scholar]
- IngramJ, Bartels D.1996. The molecular basis of dehydration tolerance in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 377–403. [DOI] [PubMed] [Google Scholar]
- JinnTL, Yeh YC, Chen YM, Lin CY.1989. Stabilization of soluble proteins in vitro by heat shock proteins‐enriched ammonium sulfate fraction from soybean seedlings. Plant and Cell Physiology 30: 463–469. [Google Scholar]
- JinnTL, Wu SH, Yeh CH, Hsieh MH, Yeh YC, Chen YM, Lin CY.1993. Immunological kinship of class I low molecular weight heat shock proteins and thermostabilization of soluble proteins in vitro among plants. Plant and Cell Physiology 34: 1055–1062. [Google Scholar]
- KappenL.1981. Ecological significance of resistance to high temperature. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, eds. Encyclopedia of plant physiology, new series. Vol. 12A. Physiological plant ecology I. Berlin: Springer‐Verlag, 439–474. [Google Scholar]
- KimpelJA, Key JL.1985. Presence of heat shock mRNAs in field grown soybeans. Plant Physiology 79: 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KnightCA, Ackerly DD.2001. Correlated evolution of chloroplast heat shock protein expression in closely related plant species. American Journal of Botany 88:411–418. [PubMed] [Google Scholar]
- LangeB.1973. The Sphagnum flora of hot springs in Iceland. Lindbergia 2: 81–93. [Google Scholar]
- LevittJ.1980. Responses of plants to environmental stress New York: Academic Press, 402–433. [Google Scholar]
- McWilliamsJR.1980. Adaptation to high‐temperature stress. In: Turner NC, Kramer PJ, eds. Adaptation of plants to water and high‐temperature stress New York: John Wiley & Sons, 444–447. [Google Scholar]
- MallochBS.1978. Mapping rare plants at The Geysers. Fremontia 5: 30–32. [Google Scholar]
- MillerLD.1968. Steaming and warm ground in Yellowstone National Park: their location, geophysics, vegetation, and mapping with aerial multispectral imagery. PhD Thesis, University of Michigan, Ann Arbor. [Google Scholar]
- MulroyTW, Rundel PW.1977. Annual plants: adaptations to desert environments. Bioscience 27: 109–113. [Google Scholar]
- NeumannD, Nover L, Parthier B, Rieger R, Scharf K‐D, Wollgiehn R, Nieden UZ.1989. Heat shock and other stress response systems of plants. Biologisches Zentralblatt 108: 1–156. [PubMed] [Google Scholar]
- NobelPS.1995. Ecophysiology of roots of desert plants, with special emphasis on agaves and cacti. In: Waisel Y, Eshel A, Kafkafi U, eds. Plant roots: the hidden half New York: Marcel Dekker, 823–844. [Google Scholar]
- ParkSK, Shivaji R, Krans JV, Luthe DS.1996. Heat‐shock response in heat‐tolerant and non‐tolerant variants of Agrostis palustris Huds. Plant Physiology 111: 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PavlikBM.2001. Developing an ecosystem perspective from experimental monitoring programs. II. Ecophysiological responses of a rare geothermal grass to soil water. Environmental Management 28: 243–253. [DOI] [PubMed] [Google Scholar]
- PavlikBM, Enberg A.2001. Developing an ecosystem perspective from experimental monitoring programs: I. Demographic responses of a rare geothermal grass to soil temperature. Environmental Manage ment 28: 225–242. [DOI] [PubMed] [Google Scholar]
- PhelpsD, Buseck PR.1980. Distribution of soil mercury and the development of soil mercury anomalies in the Yellowstone geothermal area, Wyoming. Economic Geology 75: 730–741. [Google Scholar]
- QueitschC, Hong S‐W, Vierling E, Lindquist S.2000. Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis Plant Cell 12: 479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RiceBL.1973. Biology of Mimulus guttatus in thermal areas in Yellowstone National Park, Wyoming. PhD Thesis, Utah State University, Logan. [Google Scholar]
- SchmollHM.1939. A realignment of the Panicum thermale group. Madrono 5: 90–96. [Google Scholar]
- SeemanJR, Dowton WJS, Berry JA.1986. Temperature and leaf osmotic potential as factors in the acclimation of photosynthesis to high temperature in desert plants. Plant Physiology 80: 926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SheppardJS.1971. The influence of geothermal temperature gradients upon vegetative patterns in Yellowstone National Park. PhD Thesis, Colorado State University, Fort Collins. [Google Scholar]
- SmithPK, Krohn RI, Hermanso GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson, BJ, Klenk DC.1985. Measurement of protein using bicinchoninic acid. Analytical Biochemistry 150: 76–85. [DOI] [PubMed] [Google Scholar]
- SpellenbergR.1975. Synthetic hybridization and taxonomy of western north American Dichanthelium, group lanuginosa (Poaceae). Madrono 23: 134–153. [Google Scholar]
- StaufferRE, Thompson JM.1984. Arsenic and antimony in geothermal waters of Yellowstone National Park, Wyoming, USA. Geochimica et Cosmochimica Acta 48: 2547–2561. [Google Scholar]
- StoutRG, Summers ML, Kerstetter T, McDermott TR.1997. Heat‐ and acid‐tolerance of a grass commonly found in geothermal areas of Yellowstone National Park. Plant Science 130: 1–9. [Google Scholar]
- WatersER, Lee GJ, Vierling E.1996. Evolution, structure and function of the small heat shock proteins in plants. Journal of Experimental Botany 47: 325–338. [Google Scholar]
- WellsDR, Tanguay RL, Le H, Gallie DR.1998. HSP101 functions as a specific translational regulatory protein whose activity is regulated by nutrient status. Genes and Development 12: 3236–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WisniewskiM, Close TJ, Artlip T, Arora R.1996. Seasonal patterns of dehydrins and 70‐kDa heat‐shock proteins in bark tissues of woody plants. Physiologia Plantarum 96: 496–505. [Google Scholar]
- WolfeAD, Xiang QY, Kephart, SR.1998. Assessing hybridization in natural populations of Penstemon (Scrophulariaceae) using hypervariable inter simple sequence repeat markers. Molecular Ecology 7: 1107–1125. [DOI] [PubMed] [Google Scholar]
- ZuloagaFO, Morrone O.1996. Revision de las especies Americanas de Panicum subgenero Panicum seccion Panicum (Poaceae: Panicoideae: Paniceae). Annals of the Missouri Botanical Garden 83: 200–280. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.