Abstract
In several species of the Oleaceae, mannitol, already present at considerable levels, accumulates in response to stress. This family comprises both deciduous and evergreen species, and we investigated the role of mannitol in deciduous malacophyll and evergreen sclerophyll species growing under the same conditions in the field. The relationship between mannitol content and changes in rainfall or temperature was also studied. The mannitol content of leaves of Fraxinus ornus L., F. angustifolia Vahl., Olea europaea L. and Phillyrea media L. was determined by gas chromatography. Leaf samples were collected once a month for 1 year. In the two ash species, the seasonal pattern of mannitol content appeared the same: a gradual increase in spring, peaking in summer, followed by a gradual decrease. The mannitol content was similar in both species, ranging between 260 and 720 µmol g–1 d. wt. The seasonal pattern of mannitol content in Olea and Phillyrea was similar for both species, but unlike that of Fraxinus did not show a summer peak. Rainfall was negatively correlated with the seasonal increase of mannitol content in ash. Mannitol content increased gradually during drought, reaching a maximum value at the end of the dry season. Temperature did not have a direct influence on mannitol content. In Olea and Phillyrea, variations in mannitol content were poorly correlated with rainfall or temperature, indicating that mannitol does not have a primary role in the response of these species to the hot, dry summer conditions.
Key words: Fraxinusornus, Fraxinusangustifolia, Oleaeuropaea, Phillyreamedia, ash, drought, gas chromatography, mannitol, Oleaceae, olive, seasonal variation, stress
INTRODUCTION
Mannitol is one of the most widespread polyols in the plant kingdom, occurring in more than 100 species of vascular plants. Like other polyols, it may participate in a wide range of physiological processes in plants, including: as a carbon storage and translocation compound; as a regulator of the pool of the cellular reductants, NADPH and NADP (Stoop and Mooibroek, 1998); as a hydroxyl radical scavenger; as a cryoprotectant; and especially as an osmotically active compatible solute (Popp and Smirnoff, 1995).
Mannitol occurs in most members of the family Oleaceae, where it comprises a significant portion of the soluble carbohydrate pool (Stoop et al., 1996; Peltier et al., 1997). In several species of the Oleaceae, mannitol accumulates in response to abiotic stress. For example, in Fraxinus ornus and F. excelsior growing in a mesoxerophilic stand in the French intermediate Alps, the leaf mannitol content increased in response to summer drought conditions (Marigo and Peltier, 1996; Guicherd et al., 1997; Peltier et al., 1997). In experiments with potted trees, the mannitol content of leaves of young olive plants grown under salt stress increased with the external saline concentration and returned to control levels during the relief period (Tattini et al., 1996).
The aim of this work was to investigate whether mannitol plays a similar role in different species of Oleaceae growing under the same environmental conditions in the field. For this reason we followed the seasonal variation in leaf mannitol content of two deciduous malacophyll species, Fraxinus ornus and F. angustifolia, and two evergreen sclerophyll species, Olea europaea and Phillyrea media, growing in the same area in northern Sicily. We also examined the relationship between mannitol accumulation and two predominant climatic factors, the amount of rainfall and the mean daily temperatures recorded during the sampling period.
MATERIALS AND METHODS
Site description and plant material
The plant material was collected from adult trees of Fraxinus ornus L., F. angustifolia Vahl., Olea europaea L. and Phillyrea media L. growing on clay soil on two west‐facing slopes (37°55′43′′N, 14°07′10′′E and 37°55′17′′N, 14°07′47′′E), at 450 m a.s.l. in the Madonie Mountains, in north‐western Sicily. From the thermo‐pluviometric data available, the mesoclimatic conditions of the area may be ascribed to the ‘Lower Thermomediterranean Lower Subhumid’, according to the classification of Rivas‐Martínez, with a dry period of about 4·5 months (Brullo et al., 1996). Microclimatic conditions were followed by measuring maximum and minimum temperatures, relative humidity and rainfall at the study sites. Measurements were recorded weekly during the winter and daily during the summer. Missing measurements were obtained by statistically correlating the data collected at the sites with those of the closest weather stations of the Servizio Idrografico Regionale (Cefalù and Castelbuono), using G.I.S. software (IDRISI).
Leaf samples were collected once a month, from March 1998 to March 1999, from three trees of Fraxinus ornus, F. angustifolia and Phillyrea media, and two trees of Olea europaea. At midday, five south‐facing leaves were collected from each tree and immediately frozen in liquid nitrogen. In the laboratory, leaves were lyophilized, powdered with liquid nitrogen in a mortar and stored at –20 °C.
Mannitol determination
Aliquots of the powdered leaves (300 mg) were extracted twice in 10 ml 80 % ethanol (v/v) and once in 10 ml H2O, overnight at room temperature. Combined extracts were partitioned against 10 ml of CHCl3, and a 10 ml aliquot of the aqueous phase was passed through an ion exchange column (Sephadex QAE‐A‐25; Sigma, Steinheim, Germany) equilibrated with ammonium buffer, pH 9·5, and brought to neutrality with water (Redgwell, 1980; Loescher et al., 1982). An aliquot (10 ml) of the eluate was dried at 60 °C using a rotary vacuum evaporator (Rotovapor; Büchi, Milan, Italy). The dry residue was dissolved in 5 ml 99 % pyridine, to which 250 µl hexamethyldisilazane and 100 µl trimethylchlorosilane were added for derivatization.
Analyses were carried out using a temperature‐programmed Hewlett‐Packard 6890 gas chromatograph with an HP 5973 mass spectrometry detector, on an HP 5MS column (30 m × 250 µm × 0·25 µm coating thickness). Peaks were identified by comparison with standards and with the NIST database. For quantitative analysis, arabinose was added to the samples before derivatization as an internal standard, since it was never found in the plant extracts. In preliminary experiments, the accuracy of the method was tested by adding mannitol as an internal standard to replicate samples before extraction; recovery was 98 %.
Mean values including standard deviations were calculated and the significance (P < 0·05) of differences between sets of data was tested using a Student’s t‐test. Pearson’s correlation coefficient was calculated to measure the degree of correlation between data, and the significance (P < 0·05) was tested using a one‐tailed test.
RESULTS
Climatic conditions
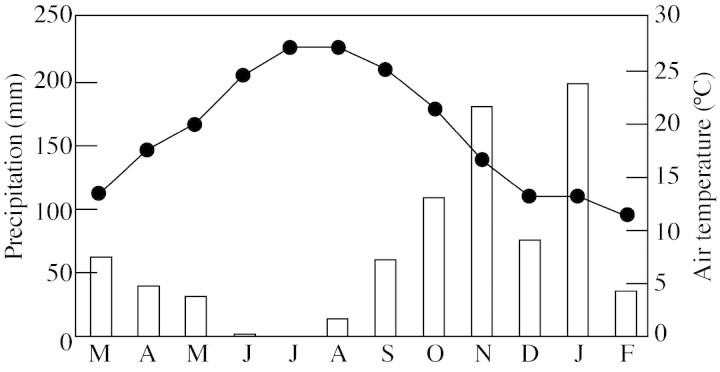
The total annual rainfall in the period March 1998–February 1999 was 800 mm. The mean annual temperature was 19·3 °C and mean summer temperatures were between 25 and 27 °C (Fig. 1). Peak daily temperatures of 38 °C were recorded at the study sites in July and August, and the maximum mean temperatures occurred in the months of July, August and September (27, 32 and 25 °C, respectively). In the same months rainfall was 0, 12·8 and 60·6 mm, respectively, with three rainy days in August and five in September. Maximum rainfall occurred in January, with 198 mm falling in 9 d.
Fig. 1. Mean monthly precipitation (bars) and mean monthly air temperature (circles) at the study site in the Madonie Mountains during the period 1 Mar. 1998 to 28 Feb. 1999.
Mannitol content
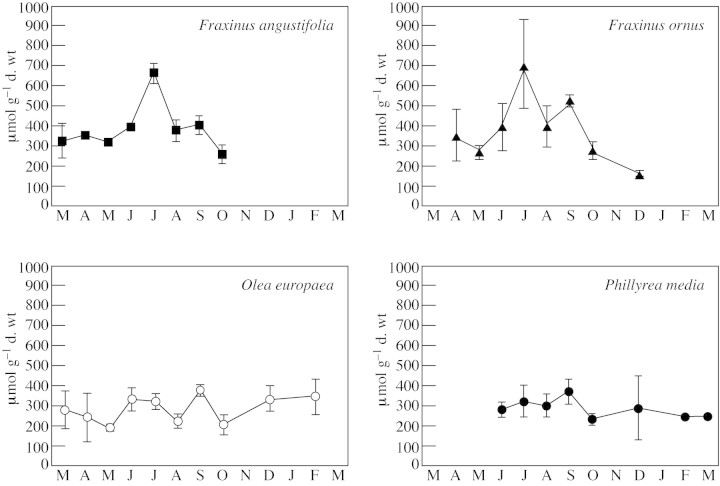
Leaves of F. angustifolia appeared in March, and had an average mannitol content of 323 µmol g–1 d. wt. There was a significant increase in mannitol content in June of around 20 %, while the maximum content (669 µmol g–1 d. wt) was attained in July, an increase of almost 70 % over that in June. The mannitol content fell significantly in August, to 381 µmol g–1 d. wt. The mannitol content in October (260 µmol g–1 d. wt) was almost half that recorded in September, and was significantly lower than that in July (Fig. 2).
Fig. 2. Seasonal variation in mannitol content of leaves of F. ornus, F. angustifolia, O. europaea and P. media. Bars indicate standard deviations.
The leaves of F. ornus appeared in April, and had an average mannitol content of 355 µmol g–1 d. wt. In June, the average concentration increased by 45 % and in July it reached the maximum level measured (717 µmol g–1 d. wt). The difference between mannitol content recorded in June and July was not significant due to the high standard deviation which was the result of the extraordinarily high mannitol content of the leaves of one individual (973 µmol g–1 d. wt). However, the summer peak was significant when compared with the mannitol content in spring. The mannitol content in August was 405 µmol g–1 d. wt and the content in October was significantly lower than that in September (287 µmol g–1 d. wt). The leaves were still on the tree in December, when their mannitol content was 174 µmol g–1 d. wt (Fig. 2).
The leaves of Olea sampled in March had an average mannitol content of 277 µmol g–1 d. wt; this content oscillated slightly during the year, without significant variations. The lowest value (181 µmol g–1 d. wt) was measured in May, while the highest content was found in September (379 µmol g–1 d. wt). Values in December and July were similar (Fig. 2).
The first sampling of leaves of Phillyrea was carried out in June, when leaves had a mean mannitol content of 287 µmol g–1 d. wt. Annual variations in this species were also not significant. Maximum and minimum mean mannitol contents were measured in September (381 µmol g–1 d. wt) and October (242 µmol g–1 d. wt) (Fig. 2).
Comparing the mannitol content of the leaves of the two ash species, the seasonal pattern appeared the same: a gradual increase beginning in spring, a summer peak in July, followed by a gradual decrease. The mannitol content was similar for both species, except in September, when the mannitol content of F. ornus was significantly higher than that of F. angustifolia.
The seasonal pattern of average mannitol content was comparable for Olea and Phillyrea, and it differed from that observed in Fraxinus in the absence of a summer peak. This difference between sclerophylls and malacophylls was particularly evident in July, when the mannitol content of the two evergreens was about half that of the ash trees, and in September when the mannitol content of F. ornus was significantly higher than that of the other species.
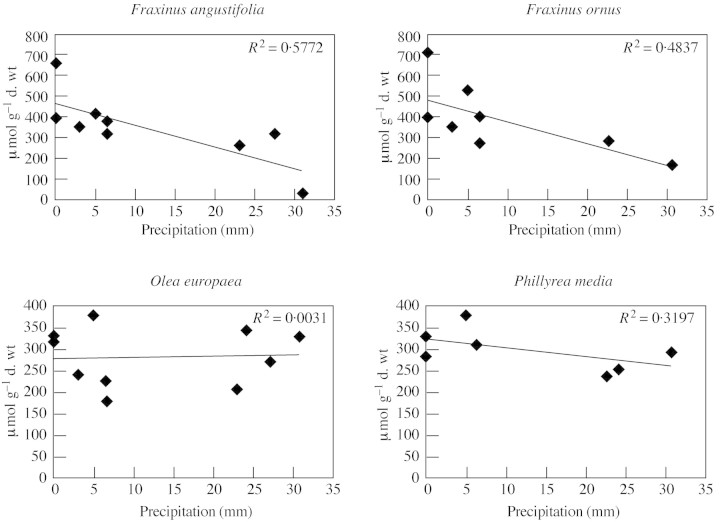
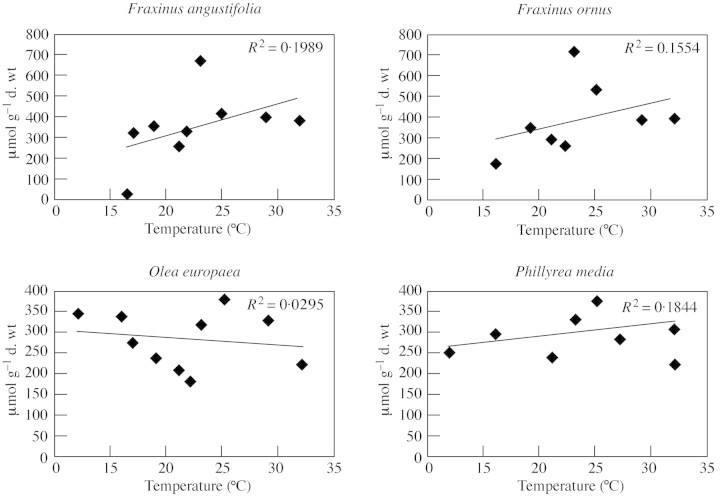
We examined the correlation between the leaf mannitol content of the four species and mean temperature or total rainfall during the period preceding leaf sampling. In the case of F. angustifolia and F. ornus, the correlation between the amount of rainfall during the 7 d preceding sampling and mannitol content was negative (r = –0·76 and –0·70, respectively; P < 0·05) (Fig. 3). In contrast, no significant correlation was found between variations in mannitol content and temperature (Fig. 4). In the case of Olea and Phillyrea, variations in mannitol content were not significantly correlated either to rainfall or to temperature (Fig. 3 and 4).
Fig. 3. Relationship between leaf mannitol content and rainfall in the week preceding leaf sampling.
Fig. 4. Relationship between leaf mannitol content and the mean temperature recorded on the day of leaf sampling.
Besides mannitol, which was always the main soluble carbohydrate in leaf extracts, gas chromatography analyses revealed the occurrence of mannose, glucose, myo‐inositol, sucrose and other unidentified soluble carbohydrates in low concentrations.
DISCUSSION
Mannitol was the main constituent of the pool of soluble carbohydrates in the leaves of the four species of Oleaceae examined, and it was generally present at higher levels than those reported in the literature for the same or related species (Flora and Madore, 1993; Tattini et al., 1996; Peltier et al., 1997). During summer, the mannitol content of the leaves of F. ornus and F. angustifolia was about twice that found during a summer drought in leaves of F. ornus and F. excelsior growing in France; in this case another compatible solute, malate, showed an increase parallel to, but higher than, that of mannitol (Marigo and Peltier, 1996; Guicherd et al., 1997; Peltier et al., 1997). In the ash trees growing in Sicily, malate did not seem to have a predominant role in the response to water stress, either in the case of F. angustifolia which did not show any significant seasonal variation, or in the case of F. ornus, which had a rather low malate content despite showing a summer peak in the concentration of this organic acid (Oddo et al., 2002).
Young plants of Olea europaea grown under controlled conditions had a mannitol content of about 60 µmol g–1 f. wt (Flora and Madore, 1993). In another series of experiments, the mannitol content of leaves of young olive plants under salinity stress increased from 160 to about 220 µmol g–1 d. wt (Tattini et al., 1996). In both cases, glucose was the main constituent of the pool of soluble carbohydrates, and was present at much higher concentrations than mannitol. In contrast, our results are in agreement with those of Priestley (1977) and Drossopoulos and Niavis (1988), who found that mannitol represented about 70 % of the soluble carbohydrates in olive leaves. The occurrence of mannitol in Phillyrea latifolia was reported by Zimmermann et al. (1975), but quantitative data have not been reported.
With regard to mannitol accumulation, it was possible to discern a difference in behaviour between deciduous malacophyll species and evergreen sclerophyll species of the same family: the two Fraxinus species showed a significant increase in mannitol content during the summer, while this seasonal accumulation did not occur in Olea and Phillyrea. It is possible that rainfall played a determinant role in inducing the seasonal increase of mannitol content in F. angustifolia and F. ornus, as there was a negative correlation between the leaf mannitol content of F. angustifolia and F. ornus and the amount of rainfall during the 7 d preceding leaf sampling. The mannitol content increased gradually during the drought, reaching a maximum value at the end of the dry season. These observations agree with those of Guicherd et al. (1997), who found that the mannitol content in young plants of F. excelsior increased during water stress and decreased rapidly upon watering. In contrast, temperature did not appear to have a direct effect on the mannitol content of the two species of Fraxinus.
Interestingly, these two species of ash are traditionally cultivated in Sicily for the production of manna, the solidified phloem sap obtained after incision of the bark. The composition of manna is quite variable; the mannitol content ranges between 20 and 60 %, while other minor components include water, glucose, levulose, coumarins and organic acids (Lentini et al., 1983; Oddo et al., 2000). It is well known that the dry season is a necessary requisite for manna production, even though it is not completely clear to what extent dry conditions promote production or simply facilitate collection of the dried sap. Milburn et al. (1980) suggested that drought promotes the rate and duration of exudation of the unusually concentrated sap. Our results show that dry conditions actually influence the mannitol content of the leaves of the ash species examined.
The poor correlation between mannitol content in Olea and Phillyrea and rainfall or temperature suggests that mannitol does not play a fundamental role in the response to drought or high temperature in these two species. However, it is probable that the slightly higher concentrations of mannitol present in summer and winter may confer physiological advantages typical of this organic solute, such as protection of membrane structure, or may function as an energetically favourable storage compound (Loescher, 1987; Stoop et al., 1996).
Plant responses to environmental conditions and stresses are extremely plastic and are regulated by many factors. To gain a complete picture of the response of these four species to stress conditions other parameters, such as photosynthetic rate, stomatal conductance and leaf water potential, must be measured. However, the results reported here answer our initial working hypotheses; even though all the species under investigation posses the genetic information and enzymatic equipment for mannitol biosynthesis, this osmolyte does not have the same role in deciduous malacophylls and evergreen sclerophylls, at least with regard to the response to drought conditions. Furthermore, when species show a summer peak in mannitol content this is correlated to drought conditions and not to high temperatures.
ACKNOWLEDGEMENTS
This work was partially supported by the Ente Parco delle Madonie and by a Palermo University grant (ex 60 %).
Supplementary Material
Received: 13 February 2002; Returned for revision: 23 April 2002; Accepted: 9 May 2002
References
- BrulloS, Scelsi F, Siracusa G, Spampinato G.1996. Caratteristiche bioclimatiche della Sicilia. Giornale Botanico Italiano 130: 177–185. [Google Scholar]
- DrossopoulosJB, Niavis CA.1988. Seasonal changes of the metabolites in the leaves, bark and xylem tissues of olive tree. II. Carbohydrates. Annals of Botany 62: 321–327. [Google Scholar]
- FloraLL, Madore MA.1993. Stachyose and mannitol transport in olive (Olea europaea L.). Planta 189: 484–490. [Google Scholar]
- GuicherdP, Peltier JP, Gout E, Bligny R, Marigo G.1997. Osmotic adjustment in Fraxinus excelsior L.: malate and mannitol accumulation in leaves under drought conditions. Trees 11: 155–161. [Google Scholar]
- LentiniF, Mazzola P, Not R.1983. I frassini da manna. Natura e Montagna 30: 21–33. [Google Scholar]
- LoescherWH.1987. Physiology and metabolism of sugar alcohols in higher plants. Physiologia Plantarum 70: 553–557. [Google Scholar]
- LoescherWH, Marlow GC, Kennedy RA.1982. Sorbitol metabolism and sink‐source interconversions in developing apple leaves. Plant Physiology 70: 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MarigoG, Peltier JP.1996. Analysis of the diurnal change in osmotic potential in leaves of Fraxinus excelsior L. Journal of Experimental Botany 47: 763–769. [Google Scholar]
- MilburnJA, Tyree MT, Lo Gullo MA, Salleo S.1980. The physiology of sap transport in the genus Fraxinus Proceedings of the European Society of Plant Physiology 510–511. [Google Scholar]
- OddoE, Sajeva M, Bellini E.2002. Seasonal pattern of mannitol and malate accumulation in leaves of two manna ash species (Fraxinus ornus L. and F. angustifolia Vahl) growing in Sicily. Plant Biosystems 136: 29–34. [Google Scholar]
- OddoE, Saiano F, Bellini E, Alonzo G.2000. Analisi del contenuto in mannitolo di manna proveniente da due specie di frassino coltivate in Sicilia. Quaderni di Botanica Ambientale e Applicata 8: 61–63. [Google Scholar]
- PeltierJP, Marigo D, Marigo G.1997. Involvement of malate and mannitol in the diurnal regulation of the water status in members of Oleaceae. Trees 12: 27–34. [Google Scholar]
- PoppM, Smirnoff N.1995. Polyol accumulation and metabolism during water deficit. In: Smirnoff N, ed. Environment and plant metabolism: flexibility and acclimation. Oxford: BIOS Scientific Publishers, 199–215. [Google Scholar]
- PriestleyCA.1977. The annual turnover of resources in young olive trees. Journal of Horticultural Science 52: 105–112. [Google Scholar]
- RedgwellRJ.1980. Fractionation of plant extracts using ion‐exchange Sephadex. Analytical Biochemistry 107: 44–50. [DOI] [PubMed] [Google Scholar]
- StoopJMH, Mooibroek H.1998. Cloning and characterization of NADP‐mannitol dehydrogenase cDNA from the button mushroom, Agaricus bisporus, and its expression in response to NaCl stress. Applied and Environmental Microbiology 64: 4689–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StoopJMH, Williamson JD, Pharr DM.1996. Mannitol metabolism in plants: a method for coping with stress. Trends in Plant Science 1: 139–144. [Google Scholar]
- TattiniM, Gucci R, Romani A, Baldi A, Everard JD.1996. Changes in non‐structural carbohydrates in olive (Olea europaea) leaves during root zone salinity stress. Physiologia Plantarum 98: 117–124. [Google Scholar]
- ZimmermannMH, Ziegler H.1975. List of sugars and sugar alcohols in sieve‐tube exudates. In: Zimmermann MH, Milburn JA, eds. Encyclopedia of plant physiology, new series, vol. 1. New York: Springer Verlag, 480–503. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.