Abstract
Magnetic resonance imaging was used to localize the site of essential oil accumulation in fruit of Carum copticum L. (Apiaceae). A chemical shift method is described that utilized the spectral properties of the aromatic monoterpene thymol, the major component of the essential oil, to image thymol selectively. The presence of essential oil secretory structures in the fruit and an essential oil containing a high proportion of thymol were confirmed with optical microscopy and gas chromatography‐mass spectrometry, respectively. Selective imaging of whole C. copticum fruits showed that thymol accumulation was localized to the secretory structures (canals) situated in the fruit wall. The technique was considered non‐invasive as the seeds used in the imaging experiments remained intact and viable.
Key words: Carum copticum, ajowan, nuclear magnetic resonance, magnetic resonance imaging, chemical shift imaging, histochemistry, essential oil, monoterpenoids, thymol
INTRODUCTION
Techniques involving nuclear magnetic resonance (NMR) have been used increasingly over the past decade in the study of plants (De Graaf, 1998; Brown and Semelka, 1999). NMR imaging (MRI) is a non‐invasive analytical technique, making it attractive for application to biological specimens and, as sample preparation is unnecessary, artefacts are avoided (Young, 1988; Mitchell, 1999).
MRI has been used to study several aspects of plant physiology and metabolism, including phloem and xylem water flow (Kockenberger et al., 1997), disease and senescence processes (Goodman and Glidewell, 1997), accumulation of heavy metals and pesticide residues (Ratcliffe and Roscher, 1998), sucrose concentration and metabolism (Goodman et al., 1993; Tse et al., 1996), lipid and water distribution (Halloin et al., 1993), and chemical composition (Gussoni et al., 1994). In all of these publications, the non‐invasive nature of the technique has been emphasized.
This paper describes the application of chemical shift selective MRI to localization of essential oil accumulation in fruits of Carum copticum (ajowan). The method is an example of non‐invasive plant histochemistry, approaching the concept of histochemistry from a perspective quite different from conventional methods that involve cutting, fixing and staining. The application of MRI to the location of terpenoids in plants has been described in two previous reports, where it was used to image the aromatic monoterpene anethole accumulated in the fruit of Foeniculum vulgare (Sarafis et al., 1990), and to localize terpenoids in the female cones of two Podocarpaceae species (Glidewell et al., 2002).
Ajowan fruits are an important commercial product for the food/flavouring industry, and they accumulate up to 5 % essential oil in compartments referred to as canals or vittae (Bhargava and Haksar, 1959). Reports on the antimicrobial and antioxidant properties of thymol have been reviewed in a previous article (Gersbach et al., 2001), and similar properties have been attributed to extracts of ajowan seeds (Mehta et al., 1994; Srivastava et al., 1999).
The composition of the essential oil has been analysed extensively. Up to 55 % is the aromatic monoterpene thymol, and there are significant amounts of the thymol precursors p‐cymene and γ‐terpinene (Balbaa et al., 1973; Ashraf and Bhatty, 1975; Nagalakshmi et al., 2000). The relatively high essential oil content of the ajowan fruit, with a high proportion of thymol, make it a promising subject for MRI. The aromatic ring structure of the monoterpene thymol, with its aromatic protons and their particular spectral characteristics, is a feature that is conducive to detection by NMR methods. In ajowan, the main component of the fruit is lipid in the form of reserve oil (triglyceride). This is present in greater abundance than monoterpenes, but the triglyceride and essential oil are located in separate, well‐defined compartments, the seed endosperm and vittae, respectively. This, combined with the well separated NMR peaks of the two substances, suggests the choice of chemical shift selective imaging (CSSI). The principles of CSSI and its application to the analysis of two‐component systems, such as water and lipid in biological specimens, have been reported in several publications (Sun et al., 1994; Pope et al., 1995; Gerald et al., 1996; Schick et al., 1997; Schick, 1998; Weis et al., 1998).
MATERIALS AND METHODS
Volatile analysis
Mature dry mericarps of Carum copticum (Apiaceae) were collected from stock plants grown at the University of Western Sydney, Australia. Volatiles were sampled by solvent extraction: 250 mg of the fruit was extracted in 1·0 ml methanol for 24 h at room temperature. The extract was analysed by gas chromatography‐mass spectrometry to confirm the presence of aromatic monoterpenes suitable for detection by NMR microscopy. The instrument was an HP‐5890 gas chromatograph fitted with a J&W DB‐1 30·0 m × 0·25 mm × 1·0 µm column, and interfaced with an HP‐5971 mass selective detector. The injector temperature was 240 °C, detector temperature 260 °C and the oven temperature programme was 80–150 °C at 2·5 °C min–1, then 150–250 °C at 5 °C min–1. Volatiles were identified by mass spectra and Kovats retention indices calculated from the retention times of C8–C14 hydrocarbon standards under identical operating conditions.
Bright‐field microscopy
Whole mericarps and transverse sections were examined and photographed using bright‐field microscopy. The microscope was an Olympus SZH zoom stereomicroscope equipped with a DF‐Plan ×1·0 objective and ×2·5 projection eyepiece. The zoom control was adjustable from 7·5 to ×60·0. The aperture was adjusted to the minimum setting. The microscope was also fitted with an Olympus C35AD‐4 camera, operated by an Olympus PM‐10AD5 automatic photomicrographic system attachment. Images were recorded on 100 ASA print film.
NMR spectroscopy
A high resolution 1H‐NMR spectrum of thymol was acquired with a Varian Unity Plus 300 MHz spectrometer, interfaced with a 7·05 Tesla/89 mm wide‐bore Oxford magnet system. The sample of pure thymol was obtained from Aldrich (Sydney, Australia), and was dissolved in CDCl3 containing 0·1 % CHCl3. All chemical shifts were reported relative to TMS (TMS = 0 ppm).
MRI
Experiments were carried out to acquire NMR images of aromatic monoterpenes present in secretory structures in the ajowan seeds using a chemical shift selective method. The NMR imaging experiments were performed on the same spectrometer system as for the acquisition of the high resolution 1H‐NMR spectrum, which was also equipped with a Varian micro‐imaging accessory. The MRI probe was a V‐120 supplied by Doty Scientific Instruments (Columbia, SC, USA), containing coils for the generation of magnetic field gradients for spatial delineation.
Images of 300 µm transverse slices across ajowan schizocarps and mericarps were acquired by means of a chemically selective fast‐spin‐echo sequence, having a chemical shift selective 90° Gaussian shaped excitation pulse of 8·0 ms duration and 400 Hz band‐width, and a slice selective 180° Gaussian shaped re‐focusing pulse of 2·0 ms duration and 800 Hz band‐width. Gradient strengths were 10·0 G cm–1 for the slice selection and 30·0 G cm–1 for the readout and phase encoding gradients. The spin‐echo time was 12·0 ms and the repetition time 1·0 s.
RESULTS AND DISCUSSION
Volatile analysis and optical microscopy
The GC‐MS analysis of the methanol extract showed that thymol was the major volatile component of Carum copticum (ajowan) fruit. The volatiles identified and their qualitative contribution to the essential oil were as follows: thymol 45·60 %, γ‐terpinene 23·79 %, p‐cymene 21·25 %, β‐pinene 3·44 %, α‐pinene 1·40 %, carvacrol 1·20 %, camphene 0·88 %, β‐myrcene 0·65 % and limonene 0·51 %. The remaining 1·28 % contained about 15 trace compounds (<0·10 % each) that were not identified.
Images acquired by light microscopy confirmed the fruit of C. copticum to be a schizocarp with two single‐seeded associated with essential oil accumulation in the Apiaceae (O’Brien and McCully, 1981; Sarafis et al., 1990). Two canals were located in the mesocarp on the flat inner side of the mericarp where the two mericarps are joined and four were located around the convex outer surface of the mericarp in the mesocarp (Fig. 1B).
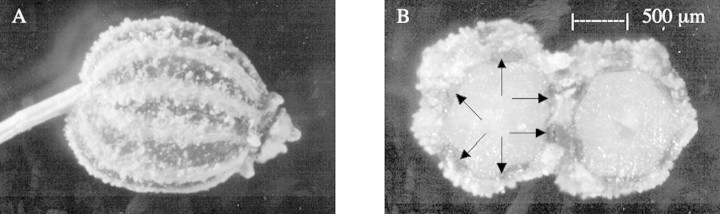
Fig. 1. A, Intact schizocarp of Carum copticum, ×150. B, Transverse section through schizocarp showing location of oil canals (arrows) in one mericarp. There were six canals in each mericarp, situated in the fruit wall.
The information collected by light microscopy and GC‐MS confirmed that ajowan fruits accumulated significant quantities of essential oil containing a high combined proportion of aromatic monoterpenes (thymol, carvacrol and p‐cymene), and the presence of secretory structures where accumulation of the terpenoids is believed to be localized.
NMR and MRI
The high resolution NMR spectrum of thymol contained signals from the aromatic protons and the methyl and hydroxyl groups. The spectrum was used as a reference to identify signals arising from thymol in the subsequent spectrum of whole ajowan fruit. The peaks were assigned so as to correspond to the numbered groups in the structure of thymol, included with the spectrum (Fig. 2A).
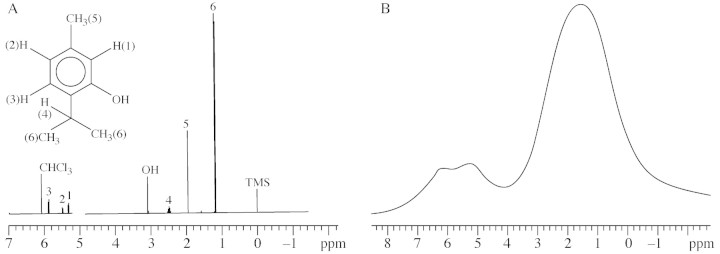
Fig. 2. A, 1H‐NMR spectrum of pure thymol. Assignment of peaks corresponds to the adjacent structure of thymol. CHCl3 at δ = 6·1 ppm is the residual peak of chloroform in the CDCl3 solvent. All chemical shifts are reported relative to TMS at δ = 0 ppm. B, 1H‐NMR spectrum of whole dried ajowan fruit. The signal at δ = 6·2 ppm arises from the aromatic protons of thymol; the olefinic protons in reserve oil (triglyceride) produce the peak at δ = 5·2 ppm; the broad resonance at δ = 1·5 ppm contains signals from the methyl and methylene groups of reserve oil, and the hydroxyl and methyl groups of thymol.
The 1H‐NMR spectrum of the whole ajowan fruit revealed three main signals (Fig. 2B). The signal at δ = 1·5 ppm contained several unresolved resonances resulting from the methyl and methylene groups of reserve oil, with a contribution from the methyl and hydroxyl groups of thymol; resonance from the aromatic protons of thymol appeared at δ = 6·2 ppm, while the peak at δ = 5·2 ppm has been confirmed by 2‐dimensional correlated spectroscopy (2D‐COSY) spectroscopy to derive from olefinic protons adjacent to methylene groups in the reserve oil (Aue et al., 1976; Sarafis et al., 1990).
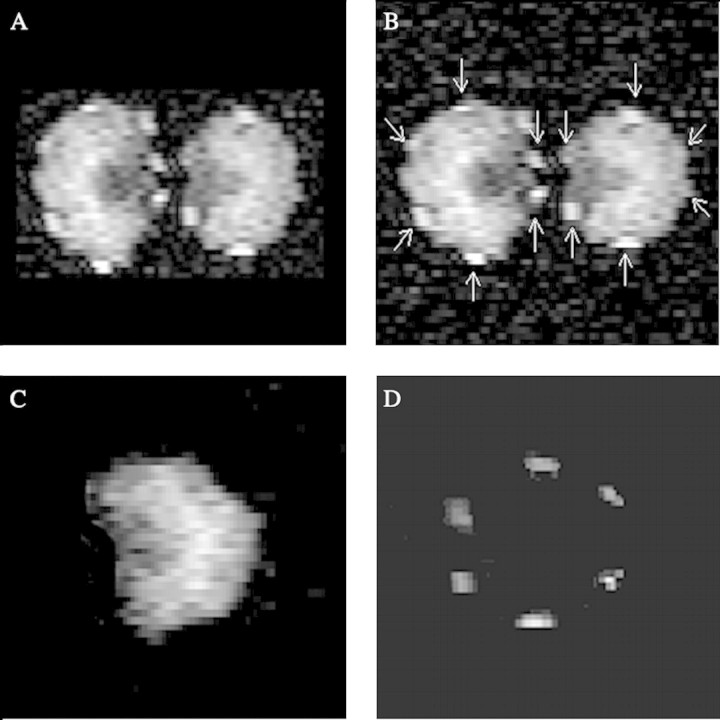
The images in Fig. 3A and B are non‐selective, and represent total lipid distribution: reserve oil in the endosperm and thymol in the vittae. The location of the vittae is evident in these images (highlighted in Fig. 3B), which can be compared with the optical microscopic image of a transverse slice through the schizocarp in Fig. 1B, where the canals are also indicated.
Fig. 3. NMR images of transverse slice across ajowan schizocarp (A and B) and mericarp (C and D). Spatial resolution is 25 µm × 25 µm and slice thickness is 300 µm. A, Non‐selective image of overall lipid distribution, comprising reserve oil and thymol; B, same image as in A showing location of oil canals; C, chemical shift selective image (δ = 5·2 ppm) of reserve oil (triglyceride) distribution; D, chemical shift selective image (δ = 6·2 ppm) of thymol distribution. The image corresponds with the location of the canals as shown in Fig. 1B, and confirms that thymol is localized in the canals.
Peak resolution in the spectrum of ajowan fruit was adequate for selective imaging of thymol. The obvious choice for selective imaging of thymol was by excitation at δ = 6·2 ppm, the peak resulting from resonance of the aromatic protons of thymol. Two chemically selective NMR images were acquired, one indicating the distribution of reserve oil or triglyceride in a single seed (Fig. 3C) and the other the distribution of thymol, corresponding to the six oil canals located in the mericarp (Fig. 3D). The latter probably included a contribution from p‐cymene, which made a significant contribution to the essential oil and, having aromatic protons, will result in resonance in the range of δ = 6·2 ppm. Averaging of 512 transients was required for each pixel to produce the image of thymol.
The selective image of thymol (Fig. 3D) verified that thymol accumulation and, consequently, a significant proportion of essential oil accumulation, was confined to the canals in the mesocarp of the ajowan fruit. The image corresponds with the location of the canals in the optical image (Fig. 1B): thymol in the fruit was identified by the volatile analysis and by the comparison of the high resolution NMR spectrum of thymol with the spectrum of the whole fruit. Because of the selectivity of the method used, it can be stated that the image in Fig. 3D results from thymol, and also that it indicates its spatial distribution in the fruit.
The use of CSSI for localizing chemical constituents in plant material was completely non‐invasive, and this is its greatest advantage over conventional methods. Fruits used in the imaging experiments remained intact throughout, and the seeds contained in the fruits retained their viability. The seeds germinated and developed normally subsequent to the experiments. Although the images are referred to as slices, or sections, through the plant material, there was no mechanical sectioning or cutting involved in the conventional sense; the ‘slice’ is accomplished by means of magnetic field gradients.
CSSI is yet to be routinely used in histochemical studies involving plant material or essential oil, but, in addition to the method described here, CSSI has been successfully applied to histochemistry of plant materials, such as anethole in fennel seeds (Sarafis et al., 1990). Therefore, it should be feasible to image other compounds selectively, and in different types of plant material (Stark and Bradley, 1999). In addition to ajowan and fennel, the fruits of other Apiaceae species have oil canals, for example, those of cumin contain significant amounts of the aromatic monoterpene cuminaldehyde, making it a prospective subject for chemically selective NMR imaging.
Imaging of essential oil components in leaves or flowers should also be possible, although this would require some modification of the technique due to compositional differences. In leaves, for example, which commonly have structures that accumulate essential oil, the major component, by a significant margin, is water, and the problem arises of resonances from molecules in comparatively very low concentration being obscured by that of water.
NMR microscopy should be considered as a technique that is yet to be even moderately exploited in its application to plant histochemistry, and one that can be further developed and improved for the non‐invasive imaging and selective identification and localization of compounds in plant material.
Supplementary Material
Received: 4 February 2002; Returned for revision: 2 April 2002; Accepted: 10 May 2002
References
- AshrafM, Bhatty MK.1975. Studies on the essential oils of the Pakistani species of the family Umbelliferae. Pakistani Journal of Scientific and Industrial Research 18: 232–235. [Google Scholar]
- AueWP, Bartholdi E, Ernst RR.1976. Two‐dimensional spectroscopy: application to nuclear magnetic resonance. Journal of Chemistry and Physics 64: 2229–2246. [Google Scholar]
- BalbaaSI, Hilal SH, Haggag MY.1973. The volatile oil from the herb and fruits of Carum copticum at different stages of growth. Planta Medica 23: 311–320. [DOI] [PubMed] [Google Scholar]
- BhargavaPP, Haksar CN.1959. Examination of essential oil from ajowan seeds. Perfumery and Essential Oil Record 50: 204–209. [Google Scholar]
- BrownMA, Semelka RC.1999. MRI: basic principles and applications. 2nd edn. Wiley‐Liss: New York. [Google Scholar]
- DeGraafRA.1998. In‐vivo NMR spectroscopy: principle and techniques. Chichester, New York: Wiley. [Google Scholar]
- GeraldRE, Krasavin AO, Botto RE.1996. Selective‐echo method for chemical‐shift imaging of two‐component systems. Journal of Magnetic Resonance 123: 201–206. [Google Scholar]
- GersbachPV, Wyllie SG, Sarafis V.2001. A new histochemical method for localization of the site of monoterpene phenol accumulation in plant secretory structures. Annals of Botany 88: 521–525. [Google Scholar]
- GlidewellSM, Möller M, Duncan G, Mill RR, Masson D, Williamson B.2002. NMR imaging as a tool for non‐invasive taxonomy: comparison of female cones of two Podocarpaceae. New Phytologist 154: 197–207. [Google Scholar]
- GoodmanBA, Glidewell SM.1997. Use of magnetic resonance techniques in the study of disease and senescence processes in plants. Phyton: Annales Rei Botanicae 37: 81–94. [Google Scholar]
- GoodmanBA, Williamson B, Chudek JA.1993. Identification of the distribution of sugars in grapes using chemical shift selective NMR microscopy. Magnetic Resonance Imaging 11: 1039–1041. [DOI] [PubMed] [Google Scholar]
- GussoniM, Greco F, Pegna M, Bianchi G, Zetta L.1994. Solid state and microscopy NMR study of the chemical constituents of Afzelia cuanzensis seeds. Magnetic Resonance Imaging 12: 477–486. [DOI] [PubMed] [Google Scholar]
- HalloinJM, Cooper TG, Potchen EJ, Thompson TE.1993. Proton magnetic resonance imaging of lipid in pecan embryos. Journal of the American Oil Chemists Society 70: 1259–1262. [Google Scholar]
- KockenbergerW, Pope JM, Xia Y, Jeffrey KR, Komor E, Callaghan PT.1997. A non‐invasive measurement of phloem and xylem water flow in castor bean seedlings by nuclear magnetic resonance microimaging. Planta 201: 53–63. [Google Scholar]
- MehtaRL, Zayas JF, Yang SS.1994. Ajowan as a source of natural lipid antioxidant. Journal of Agricultural and Food Chemistry 42: 1420–1422. [Google Scholar]
- MitchellDG.1999. MRI principles. Philadelphia: Saunders. [Google Scholar]
- NagalakshmiS, Shankaracharya NB, Naik JP, Rao LJM.2000. Studies on chemical and technological aspects of ajowan (Trachyspermum ammi (L.) syn. Carum copticum Hiern) seeds. Journal of Food Science and Technology 37: 277–281. [Google Scholar]
- O’BrienTP, McCully B.1981. The study of plant structure. Melbourne, Victoria: Termarcarphi Pty Ltd. [Google Scholar]
- PopeJM, Jonas D, Walker RR.1995. Choice of soft pulse shapes for signal excitation in chemical shift selective imaging. Magnetic Resonance Imaging 13: 763–766. [DOI] [PubMed] [Google Scholar]
- RatcliffeRG, Roscher A.1998. Prospects for in vivo NMR methods in xenobiotic research in plants. Biodegradation 9: 411–422. [Google Scholar]
- SarafisV, Rumpel H, Pope J, Kuhn W.1990. Non‐invasive histo chemistry of plant materials by magnetic resonance microscopy. Protoplasma 159: 70 – 73. [Google Scholar]
- SchickF.1998. Simultaneous highly selective MR water and fat imaging using a simple new type of spectral‐spatial excitation. Magnetic Resonance in Medicine 40: 194–202. [DOI] [PubMed] [Google Scholar]
- SchickF, Forster J, Machann J, Huppert P, Claussen CD.1997. Highly selective water and fat imaging applying multislice sequences without sensitivity to B‐1 field inhomogeneities. Magnetic Reson ance in Medicine 38: 269–274. [DOI] [PubMed] [Google Scholar]
- SrivastavaM, Saxena A, Baby P.1999. GC‐MS investigation and antimicrobial activity of the essential oil of Carum copticum Benth & Hook. Acta Alimentaria 28: 291–295. [Google Scholar]
- StarkDD, Bradley WG.1999. Magnetic resonance imaging. 3rd edn. London: Mosby. [Google Scholar]
- SunL, Aletras AH, Schmalbrock P, Skinner TE, Chakeres D, Irsik R, Robitaille PML.1994. Water and fat MR imaging with chemical shift selective 3D steady state methods. Magnetic Resonance in Medicine 31: 359–364. [DOI] [PubMed] [Google Scholar]
- TseTY, Spanswick RM, Jelinski LW.1996. Quantitative evaluation of NMR and MRI methods to measure sucrose concentrations in plants. Protoplasma 194: 54–62. [Google Scholar]
- WeisJ, Ericsson A, Hemmingsson A.1998. Chemical shift artifact‐free imaging – a new option in MRI. Magnetic Resonance Imaging 16: 839–844. [DOI] [PubMed] [Google Scholar]
- YoungSW.1988. Nuclear magnetic resonance imaging: basic principles. 2nd edn. New York: Raven Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.