Abstract
The anatomy and ultrastructure of root nodules of Anadenanthera peregrina var. falcata (Leguminosae‐Mimosoideae) were analysed, as was plant growth. To ensure that nodules developed, seedlings were inoculated with a mixture of six strains of rhizobia. Nodules were produced that differed in appearance—and probably also effectiveness—but their structure was similar and they showed characteristics typical of indeterminate nodules, such as persistent meristematic tissue and a gradient of cells at different stages of development. Many starch grains were present in inner cortex cells and interstitial cells of infected tissue. Infected cells were densely packed with bacteroids, which contained many poly‐β‐hydroxybutyrate granules. The high incidence of these granules, together with high levels of starch accumulation in interstitial cells, suggested low N2‐fixation efficiency of the rhizobia isolates used for inoculation. In the symbiosomes of early‐senescent infected cells, reticulum‐like structures, small vesicles and a fibrillar material were observed; these may be related to bacteroid degradation. In the cytoplasm of late‐senescent infected cells, many vesicles and membrane‐like structures were observed, probably associated with membrane degradation of bacteroids and peribacteroids. The total biomass of plants inoculated with rhizobia was low and their xylopodia and shoots had low levels of N compared with non‐inoculated plants fertilized with ammonium nitrate. However, inoculated plants did not show N‐deficiency symptoms and grew better than non‐inoculated plants without N fertilization. These growth results, together with ultrastructural observations of nodules, suggest that nitrogen fixation of rhizobia isolates associated with Anadenanthera peregrina var. falcata roots is poor.
Key words: Nodule anatomy, nodule ultrastructure, Anadenanthera peregrina Var. falcata, angico do cerrado, rhizobia, plant growth, N content, nodule development, nodule senescence, xylopodium, nitrogen fixation, Brazilian savanna
INTRODUCTION
Anadenanthera peregrina (L.) Speg. var. falcata (Benth.) Altschul (Leguminosae‐Mimosoideae) is an important woody leguminous tree that occurs in many regions of Brazilian savanna. Savanna accounts for 207 million ha in 16 Brazilian states (Vargas and Hungria, 1997), and occurs on a range of soil types (Reatto et al., 1998). A number of these soils are deficient in nutrients such as nitrogen, and the ability to fix this element may be important. The presence of symbiotic bacteria (rhizobia), localized in special cells of root nodules, can contribute to the nutrition of the host legume by fixing atmospheric nitrogen and may thus help the host to survive in the savanna. There is very little information on nitrogen fixation in relevant leguminous tree species (Sprent and Parsons, 2000), and the nodule structure and function of only a small number of these plants have been examined to date (Sprent, 2001). Anadenanthera peregrina var. falcata (popularly known as ‘angico do cerrado’) is economically important as timber and an extract from its bark is used in tanning (Lorenzi, 1994). The morphology and anatomy of its root nodules have been described by Cordeiro and Beltrati (1989), but no ultrastructural study was carried out. The aim of the present study was to examine aspects of the initial growth of angico do cerrado plants infected with rhizobia and to record ultrastructural characteristics of root nodules.
MATERIALS AND METHODS
Inoculum
Native rhizobia strains were isolated from root nodules collected from A. peregrina var. falcata plants growing in the Corumbataí Brazilian savanna reserve (22°15′S and 47°00′W, 810 m a.s.l.). The isolates were stored in the Rhizobial Bank of Unesp Rio Claro, SP, Brazil with provisional numbers 3b, 4b (slow‐growing), 5b and 6a (fast‐growing). The strains IBRC 143 and 144 (fast‐growing) of the Unesp Rio Claro Rhizobial Bank were also used to inoculate plants used in the experiment. Strains were grown on yeast extract mannitol agar at 28 °C.
Seeds, potting, fertilization, inoculation and treatments
Seeds of A. peregrina var. falcata obtained from Instituto Florestal of São Paulo State were surface sterilized and planted in 6 l pots containing 2 : 1 sand/vermiculite (v/v) as substrate (5·8 kg). All pots received the following basal nutrients prior to planting (in mg kg–1 substrate): potassium chloride (60), superphosphate (150), magnesium oxide (40), zinc sulfate (10), copper sulfate (10), boric acid (2), sodium molybdate (2), iron sulfate (2) and manganese sulfate (10). For plants receiving complete mineral fertilization, N was added as ammonium nitrate (60 mg kg–1 substrate). Nutrients were thoroughly mixed into the substrate, water was added to field capacity and the pots were incubated for 10 d in a glasshouse before seeds were planted. Additional nutrients (20 ml of solution) were added to the surface of each pot every 2 months. For the rhizobial treatments, seedlings were inoculated with an equal mixture (42 ml of suspension) of all six strains (see above) to ensure nodule development. There were three treatments, with ten replicate plants per treatment: non‐inoculated plants receiving mineral fertilization without N (control); non‐inoculated plants receiving complete mineral fertilization (ammonium nitrate); and inoculated plants (rhizobia) receiving mineral fertilization without N.
Growth conditions and harvesting
The experiment was conduced under natural daylight in a glasshouse between January and October 1999. Average maximum and minimum temperatures were 34 ± 5 and 15 ± 5 °C, respectively. Plants were harvested at 4 and 10 months. They were separated into roots, xylopodia (woody underground structures present in some Brazilian savanna plants) and shoots, and dry mass was recorded after drying for 72 h under a constant airflow.
Light and electron microscopy
Samples of nodules at different stages of development were collected from plants inoculated with rhizobia at 4 and 10 months. Nodules were fixed in 2·5 % glutaraldehyde with 0·1 m sodium phosphate buffer (pH 7·2). Samples were rinsed in phosphate buffer, post‐fixed in 1 % osmium tetroxide for 1 h, stained with 2 % uranyl acetate solution for 2 h, dehydrated in a graded acetone series and embedded in a series of Spurr’s resin (Spurr, 1969) and acetone 1 : 5, 1 : 3, 1 : 1, 3 : 1, 5 : 1 (v/v) for an extended period because these nodules are relatively difficult to infiltrate properly. The same samples were used for light microscopy (LM) and transmission electron microscopy (TEM). For LM, 1 µm sections were cut using a Sorvall MT2‐B ultra‐microtome and stained with 1·5 % methylene blue and 1·5 % azure II. Sections were viewed using an Olympus BX40 microscope and photographed on Ilford Pan F plus film asa 50. For TEM, ultra‐thin sections (60–90 nm) were stained with 2 % ethanolic uranyl acetate for 20 min and with aqueous lead citrate for 10 min and observed in a Philips CM 100 transmission electron microscope at 80 kV and photographed using Kodak Eastman Motion Picture film.
For scanning electron microscopy (SEM), nodules fixed in 2·5 % glutaraldehyde with 0·1 m sodium phosphate buffer were dehydrated in a graded acetone series to absolute, mounted on stubs with double‐sided tape, coated with gold and carbon in a Balzers SCD 050 sputter coater and viewed with a Philips SEM 505 at 12 kV using Fuji Neopan SS film to record the images.
Nitrogen content and statistical analysis
The N content of xylopodia and shoots was determined by micro Kjeldahl analysis (Sarruge and Haag, 1974). Data from roots, xylopodia, shoots and total biomass were analysed separately using ANOVA, as was the N content of xylopodia and shoots. The significance of differences between treatment means was determined by Duncan’s test, at P = 0·05, using the SAS System program package version 6·12 for Windows (SAS Institute Inc.).
RESULTS
Morphology, anatomy and ultrastructure of nodules
The general appearance of an inoculated angico do cerrado plant after 10 months’ growth under the study conditions is shown in Fig. 1A. Nodules were observed only on plants that had been inoculated and were localized mainly on the upper portion of the root system on lateral roots close to the xylopodium (Fig. 1A and B). Approximately 52 nodules collected from 4‐ and 10‐month‐old plants were analysed by light and electron microscopy. Young nodules were spherical, brown or white externally, and became enlarged, cylindrical and multi‐lobed with age (Fig. 1C). When they were cut in half, mature nodules showed three coloured zones: a narrow distal white zone was followed by a pink zone (nitrogen‐fixing zone) and then a large proximal green or light‐brown zone (Fig. 1D). However, this zonation was not always so discrete, evident and uniform, and some nodules were completely black, indicative of degeneration (Fig. 1D). In accordance with these three zones, light micrographs of longitudinal sections of mature nodules showed an invasion zone (white) in the distal part of the nodule which included recently infected cells derived from the meristem, followed by a N2‐fixing zone (pink) with mature infected cells, and a large senescent zone (green or light‐brown) with cells in various stages of degeneration (Fig. 2A). The anatomy and ultrastructural features described below refer to nodules with these three coloured zones. Nodules had an outer cortex that surrounded a tightly packed endodermis, and inside this an inner cortex surrounded the central infected tissue, which contained small interstitial uninfected cells and infected hypertrophied cells (Fig. 2A–C). Vascular bundles were observed in the inner cortex, and a continuous sequence of uninfected cells seemed to radiate from these bundles to the central tissue (Fig. 2B and C). The walls of endodermal cells were thick and contained abundant polyphenolic compounds, which stained dark‐blue/green with toluidine blue (Fig. 3A).
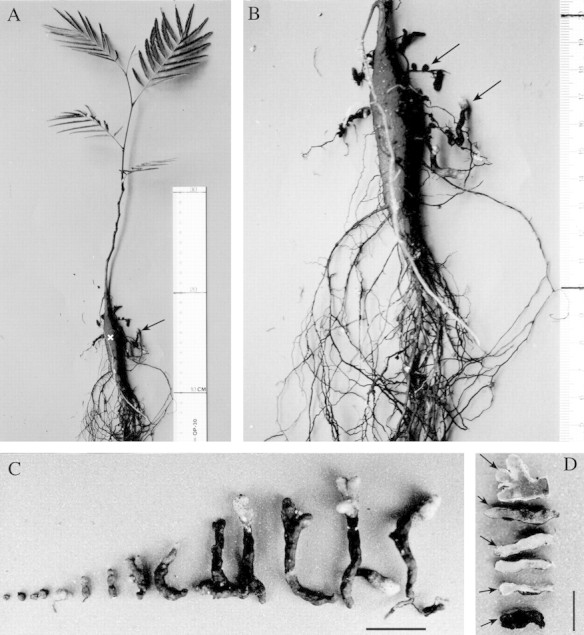
Fig. 1. Angico do cerrado plant inoculated with rhizobia, and its nodules. A, Typical morphology of a 10‐month‐old inoculated plant grown under experimental conditions. The shoot shows no symptoms of nitrogen deficiency and most of the nodules (arrow) are located near the xylopodium (x). B, Higher magnification of the same xylopodium. Note some lateral roots bearing nodules (arrows) that differ in morphology, colour and size. C, Nodules at different stages of development. Young nodules are white and brown, and round in shape. These become cylindrical and multi‐lobed as they mature, and older nodules have white tips. Bar = 10 mm. D, Nodules showing their inner pigmentation pattern as described in the text (small arrow). Sometimes this pattern was less evident and poorly delimited (small arrowheads). A section through the tip of a multi‐lobed nodule (large arrow) is shown; the pink colouration is due to leghaemoglobin. A black, degenerate nodule (bottom of picture) is also shown. Bar = 5 mm.
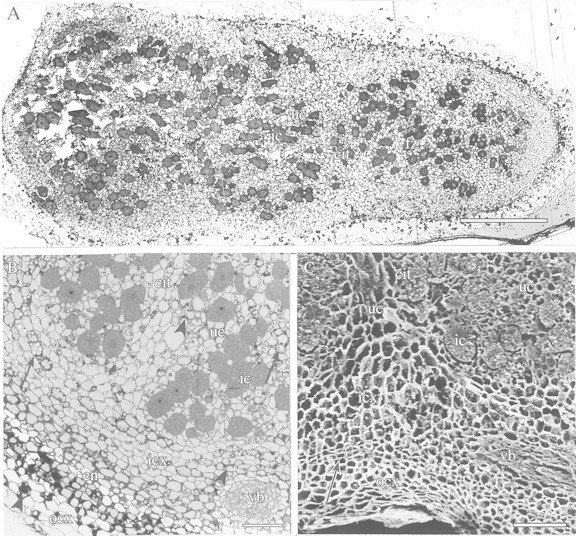
Fig. 2. Angico do cerrado nodule with the three zones of pigmentation (see text). A, LM of a nodule sectioned longitudinally. A distal invasion zone (iz) is followed by a fixation zone (fz) and then a large proximal senescent zone (sz). Note the high proportion of uninfected cells (uc) in relation to infected cells (ic) along the length of the central infected tissue (cit). Bar = 500 µm. B, LM of a longitudinal section of a nodule illustrating its structure. ocx, Outer cortex, en, endodermis; icx, inner cortex; vb, vascular bundle. Polyphenolic compounds (arrowheads) are present in cells of the outer and inner cortex and uninfected cells of the central tissue, and starch granules (arrows) are observed in uninfected cells and in the inner cortex. Bar = 100 µm. C, SEM of a small portion of a nodule sectioned longitudinally. Tightly packed endodermis (arrow) separates the outer (ocx) and inner cortex (icx) and a continuous sequence of uninfected cells (uc) apparently radiates from the vascular bundle (vb) to the central tissue (cit) and delimit groups of infected cells (ic). Bar = 100 µm.
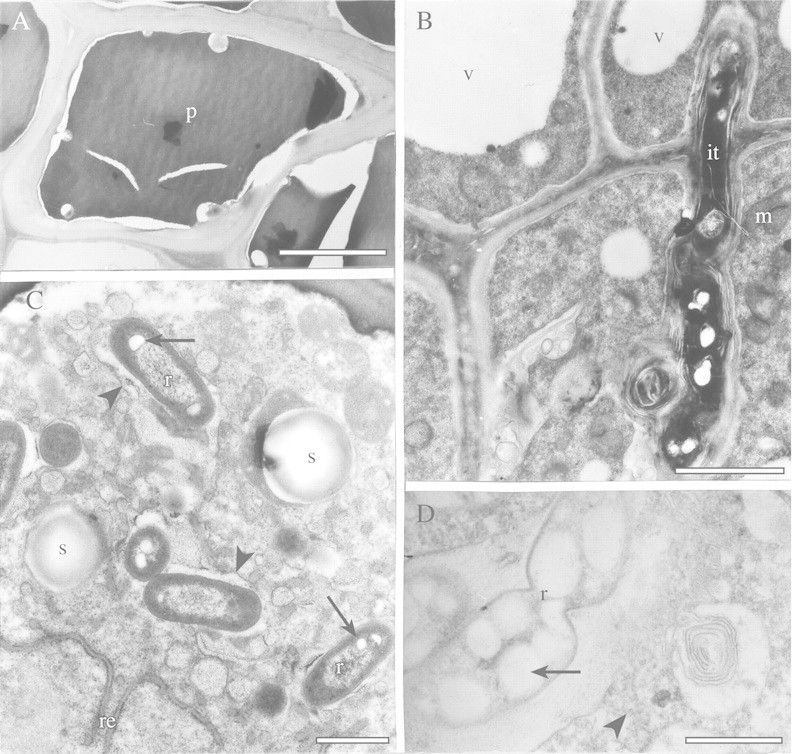
Fig. 3. TEM of endodermal and recently infected cells of angico do cerrado nodules. A, Typical endodermal cell with thick cell wall and polyphenolic compounds (p) in its interior. Bar = 5 µm. B, Infection thread (it) in cells near the nodule meristem. Vacuoles (v) and mitochondria (m) can be seen in the plant cells. Bar = 2 µm. C, The cytoplasm of recently infected cells contains some small starch grains (s) and rough endoplasmic reticulum (re). Rhizobia (r) with small poly‐β‐hydroxybutyrate granules (arrows) are delimited by a membrane (arrowhead). Bar = 1 µm. D, Division of a rhizobium (r), which contains large poly‐β‐hydroxybutyrate granules (arrow) in a symbiosome. Some polyribosomes (arrowhead) can be seen in the host cytoplasm. Bar = 1 µm.
The presence, in nodules, of a persistent meristem is typical of the indeterminate type of nodules. This structure allowed us to study cells at different stages of development and senescence in the same sample. Near the nodule meristem, infection threads traversed the cells (Fig. 3B) and released some bacteria (not shown), which were each surrounded by a peribacteroid membrane (Fig. 3C). There was an increase in cytoplasmic volume in these recently infected cells, and bacteria began to multiply by binary fission in the symbiosome (Fig. 3D). In infected cells of angico do cerrado nodules, many rhizobia (up to 16) were surrounded by a single peribacteroid membrane (Fig. 4A). In interstitial cells, a narrow band of peripheral cytoplasm, large vacuoles, polyphenolic deposits and round starch granules were observed (Fig. 4A), which were identified by fresh, freehand sections of nodules stained with 5 % (v/v) lugol solution (data not shown).
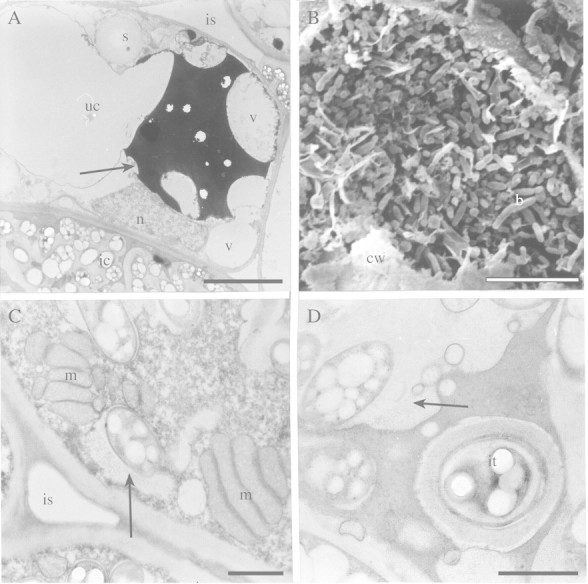
Fig. 4. Ultrastructure of uninfected and infected cells of angico do cerrado nodules. A, TEM of uninfected cell (uc) with vacuoles (v) containing polyphenolic compounds (arrow) occupying part of the cell that has thin peripheral cytoplasm with round starch granules (s). n, Nucleus. An infected cell with symbiosome containing many rhizobia (ic) and intercellular space (is) can also be seen. Bar = 5 µm. B, SEM of mature infected cell with bacteroids (b) occupying almost the entire cell. The remains of the cell wall (cw) are visible. Bar = 10 µm. C, TEM of mature infected cell with dense cytoplasm containing numerous mitochondria (m) near to an intercellular space (is). Note the size of the peribacteroid space (arrow) in the symbiosome. Bar = 1 µm. D, Infection thread (it) in an early senescent infected cell. Note the width of the peribacteroid space (arrow) in symbiosomes. Bar = 1 µm.
As development proceeds, infected cells become enlarged and densely packed with bacteroids (Fig. 4B), and some mitochondria were observed in their dense cytoplasm close to intercellular spaces (Fig. 4C). Bacteroids contained many granules, assumed to be poly‐β‐hydroxybutyrate (Figs 4A, C, D and 5A, B), suggesting a high rate of carbon accumulation in these microsymbionts. Even in early‐senescent infected cells, infection threads were observed (Fig. 4D), and in the symbiosomes of these cells the peribacteroid space was typically large (Figs 4A, D and 5A, B) and filled with small vesicles and a fibrillar material (Figs 4D and 5A, B). These vesicles in symbiosomes of infected cells in the earlier stages of senescence seemed to be associated with the reticulum‐like structure present in the interior of the symbiosome (Fig. 5A–C), and sometimes small vesicles and the fibrillar material were in direct contact with the bacteroid cytoplasm, since the inner and outer bacteroid membranes had apparently disappeared (Fig. 5D). At a later stage of senescence, the bacteroid had almost completely lysed inside the symbiosome (Fig. 5E). The host cytoplasm, which lacked conspicuous organelles, contained many vesicles and membrane‐like structures (fragments) probably associated with bacteroid and peri‐bacteroid membrane degradation (Fig. 5F).
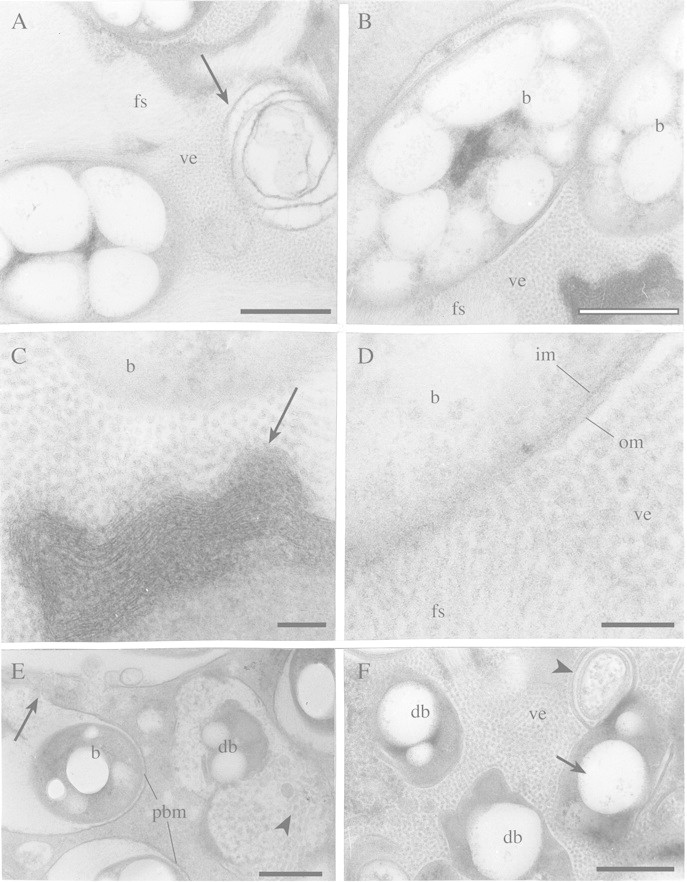
Fig. 5. TEM of infected cells at different stages of senescence of angico do cerrado mature nodule. A, Detail of the peribacteroid space with fibrillar substance (fs) and small vesicles (ve) apparently associated with a reticulum‐like structure (arrow). Bar = 0·5 µm. B, Low magnification of a symbiosome containing bacteroids (b), small vesicles (ve), fibrillar substance (fs) and a different kind of reticulum‐like (arrow) structure (shown in higher magnification in Figs 5C and D). Bar = 0·5 µm. C, Small vesicles may be associated with the reticulum‐like structure (arrow) and with bacteroid (b) cytoplasm lysis in the symbiosome. Bar = 0·1 µm. D, Small vesicles (ve) and fibrillar substance (fs) are in direct contact with bacteroid (b) cytoplasm and may be related to its disorganization. The disappearance of the outer (om) and inner bacteroid membrane (im) can also be seen. Bar = 0·1 µm. E, Bacteroid in advanced stage of degeneration (db) in a symbiosome that contains many vesicles (arrowhead). In other symbiosomes, in earlier stages of degeneration, bacteroids (b) are more intact but the peribacteroid membrane (pbm) that is evident in some cells is disorganized in others (arrow). Bar = 0·5 µm. F, Even later stages of senescence. Degenerating bacteroids (db) still have poly‐β‐hydroxybutyrate granules (arrow). Many vesicles (ve) and membrane‐like structures (arrowhead) can be seen in the host cytoplasm but the peribacteroid membrane is inconspicuous. Bar = 0·5 µm.
Plant growth
Growth of A. peregrina var. falcata, as measured by dry matter and N content, was affected by rhizobia inoculation and ammonium nitrate fertilization. Inoculated plants accumulated significantly more biomass than the control (non‐inoculated plants without ammonium nitrate), but accumulated significantly less biomass than non‐inoculated plants supplemented with ammonium nitrate (Fig. 6). Figure 7 shows the effect of the treatments on N accumulation in xylopodia and shoots of plants fertilized with ammonium nitrate and those inoculated with rhizobia. The total N content of inoculated plants was much lower than that of ammonium nitrate‐fertilized plants (Fig. 7), but no deficiency symptoms were seen in their shoots (Fig. 1A).
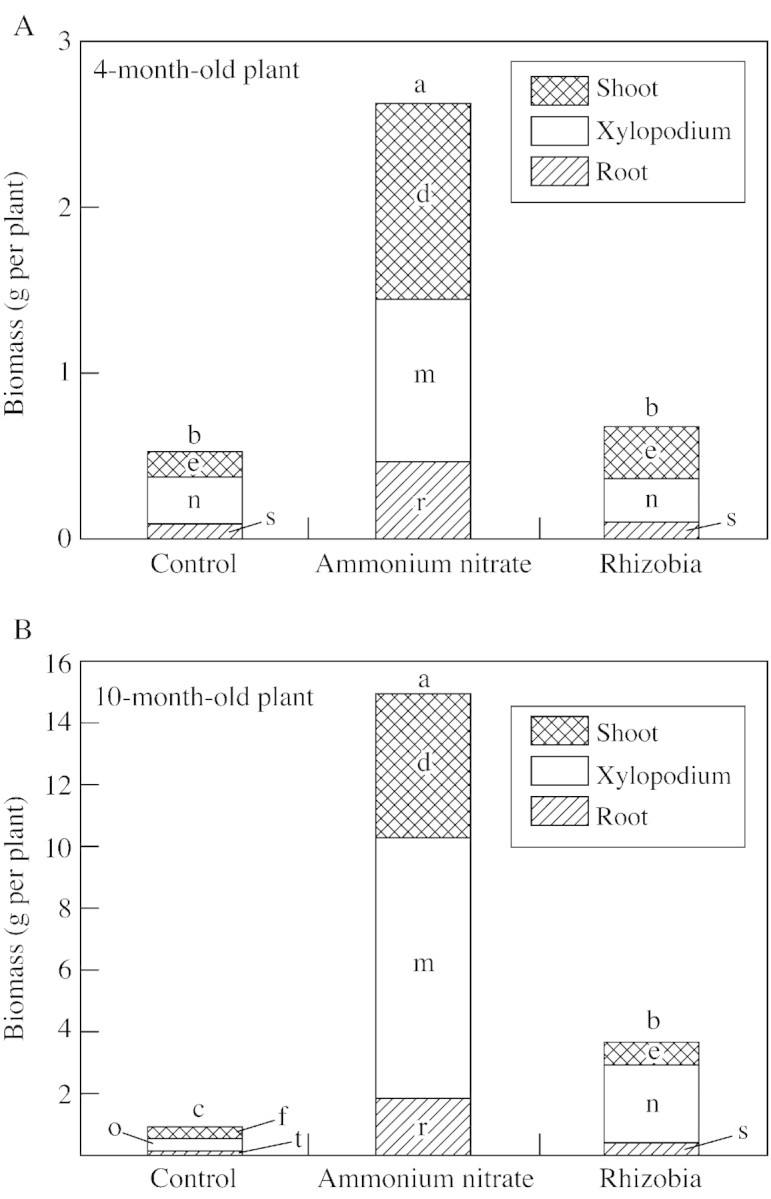
Fig. 6. Total biomass of roots, xylopodia and shoots of 4‐ and 10‐month‐old A. peregrina var. falcata plants. Lower case letters (a, b, c, for total biomass; d, e, f, for shoot; m, n, o, for xylopodium; r, s, t, for root) indicate differences between treatments are significantly different (P < 0·05) within each age class.
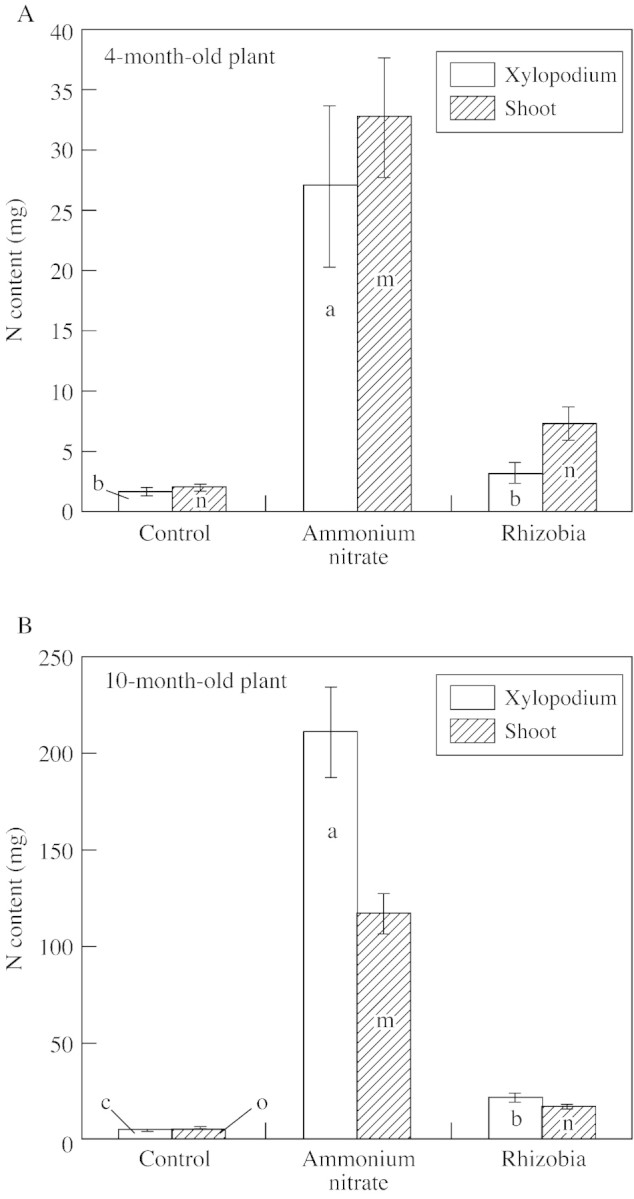
Fig. 7. Total N content of xylopodia and shoots of 4‐ and 10‐month‐old A. peregrina var. falcata plants. Lower case letters (a, b, c, for xylopodium; m, n, o, for shoot) indicate differences between treatments are significant (P < 0·05) within each age class.
DISCUSSION
The long cylindrical, branched shape of A. peregrina var. falcata nodules probably arises from the distribution and duration of their mitosis while the lobes are derived from dichotomous division of their persistent meristem. Morphological and anatomical characteristics of this nodule result in it being classified as astragaloid (Corby, 1981) or caesalpinioid (Sprent, 2001). The observations on angico do cerrado nodule anatomy presented in this study are in agreement with those reported previously by Cordeiro and Beltrati (1989) for the same tree species. The structure of this nodule is similar to that of indeterminate nodules in general (Newcomb, 1981; Hirsch, 1992), showing a longitudinal zonation similar to that observed in temperate legumes such as pea (Newcomb, 1976; Brewin, 1991) or alfalfa (Vasse et al., 1990; Hirsch, 1992). However, the colour distribution pattern was not uniform and was sometimes less evident in angico do cerrado nodules. This could be explained by the random distribution of infected cells at different stages of development and senescence in the central infected tissue. The pink zone of angico do cerrado nodules is probably due to the presence of leghaemoglobin and could also be influenced by polyphenolic compounds present in interstitial cells. This N2‐fixing zone was sometimes relatively small when compared with the large senescent zone, suggesting a limited area for N2‐fixation. The central infected tissue of angico do cerrado nodules had a high incidence of uninfected cells, and nodules could thus be characterized as ineffective (Newcomb, 1981). However, nodules did not show premature degeneration and were not smaller than A. peregrina var. falcata nodules collected for us in the Corumbataí Brazilian savanna reserve. These nodules were pink inside with a few interstitial cells (data not shown). Nodule appearance indicated a range of effectiveness, due, probably, to the six different strains of inocula. This might be reflected in growth of the plants. In real situations, plants are likely to have access to a wide range of rhizobia and therefore to produce nodules varying in effectiveness (see, for example, references in Sprent, 2001).
Thick‐walled cells containing polyphenolic substances formed a tightly packed endodermal layer which may provide a mechanical barrier against oxygen influx to the nitrogen‐fixing tissue. The polyphenolic compounds observed in the endodermis, in the outer and inner cortex cells and in interstitial cells of angico do cerrado nodules were mainly tannic acid (C. V. de Almeida, pers. comm.). Polyphenolic compounds are present in varying quantities and forms in plants tissues and have been suggested to have many functions in defending plants against damage caused by parasites or other stress factors (Nicholson and Hammerschmidt, 1992; Bussotti et al., 1998), especially when present in the outer cortex cells. One hypothesis to explain the presence of polyphenolics in uninfected interstitial cells could be as a means by which the plant controls the rhizobia population of the infected central tissue. Alternatively, the presence of these compounds in nodules could be related to a biosynthetic route usual for angico do cerrado, a tannin‐producing tree. Polyphenolic compounds have been observed in outer cortex cells of Lonchocarpus muelhbergianus (Cordeiro et al., 1996), Andira spp (Faria et al., 1986), Acacia spp and Prosopis spp (Räsänen et al., 2001), and in interstitial cells of Campsiandra sp., Moldenhawera floribunda, Melanoxylon brauna, Dhalstedtia sp. and Dimorphandra exaltata nodules (Faria et al., 1987).
Starch accumulation occurred throughout the infected tissue and inner cortex but only in uninfected cells, and accumulation did apparently not decrease in the N2‐fixing zone, as occurs in effective indeterminate nodules (Hirsch, 1992). This abundance of starch grains in uninfected cells could indicate underconsumption of energy (Postma et al., 1990) and therefore low functional activity of angico do cerrado nodules in our experiment. Low N2‐fixing activity was also indicated by low N accumulation in the xylopodium and shoots of inoculated plants.
The arrangement of uninfected cells from vascular bundles towards infected tissue observed in A. peregrina var. falcata nodules has been reported previously. This arrangement could form a integrated network through which products of nitrogen fixation may diffuse to be exported (Selker, 1988). Gordon et al. (1992) proposed that sucrose could also travel through the same network but in the opposite direction to provide a continuous supply of energy to the nodule.
Vacuoles and starch grains were not found in infected cells of nodules in our experiment. Vacuoles were observed in mature infected cells of pea (Newcomb, 1981), alfalfa (Vasse et al., 1990) and chickpea (Lee and Copeland, 1994), but not in infected cells of determinate nodules (Streeter, 1991) or in indeterminate nodules of Lonchocarpus muelhbergianus (Cordeiro et al., 1996). The presence of starch granules in mature infected cells of alfalfa, pea and chickpea (Vance et al., 1980; Newcomb, 1981; Lee and Copeland, 1994) contrast with their absence in angico do cerrado. This may be explained by a carbon drain (denoted by the massive presence of poly‐β‐hydroxybutyrate granules) from bacteroids or by an incapacity of infected cells to synthesize starch.
The symbiosome of angico do cerrado nodules contained many bacteroids that were relatively uniform in their shape (rod‐like), even in later stages of development. The cytoplasm of bacteroids was mostly occupied by poly‐β‐hydroxybutyrate reserves. These reserves are indicative of the low metabolic activity of bacteroids, and this probably affected biological nitrogen fixation and was consequently reflected in the low values for total biomass and N content of inoculated plants (see Figs 6 and 7). However, when the dry mass and total N content of inoculated plants were compared with those of the control (non‐inoculated plants without ammonium nitrate), results demonstrate that nitrogen fixation took placed.
Mitochondria were commonly observed near intercellular spaces in mature infected cells, probably related to a need to maintain mitochondrial respiration (Werner, 1992). Cortical microtubules may be associated with these mitochondria positions (Whitehead et al., 1998; Davidson and Newcomb, 2001)
Infection threads containing bacteria were not only present in the invasion zone of angico do cerrado nodules, but also in the senescent zone. A similar observation was made by Vance et al. (1980) in alfalfa and by Cordeiro et al. (1996) in Lonchocarpus muelhbergianus nodules. The appearance of intact infections threads containing bacteria in cells of the senescent zone contrasted with the initial disintegration of symbiosomes and cytoplasm of these same cells. We agree with Vance et al. (1980) that this could be an adaptive characteristic to re‐establish bacteroids in new nodule cells, since angico do cerrado is a perennial plant whose growth and nodule activity is influenced by the dry and wet seasons of the Brazilian savanna.
The small vesicles, fibrillar material and reticulum‐like structures observed in symbiosomes of senescent infected cells may be related to bacteroid degeneration. In some situations these vesicles and fibrillar substances were in direct contact with the bacteroid cytoplasm and could be associated with its disorganization. No cytochemical tests were carried out, but it is possible that the small vesicles have a lytic function and that the fibrillar material is the remains of cytoplasm, outer and inner membranes and bacteroids. The reticulum‐like structure could be producing the small lytic vesicles. The origin, composition and function of the reticulum‐like structure, small vesicles and fibrillar substance are unknown, but these observations reinforce the relatedness of symbiosomes and lysosomes proposed by Mellor and Wiemken (1988).
The ultrastructural characteristics of the nodules confirmed the limited effectiveness of rhizobia isolates in N2‐fixation, as suggested by the low growth of inoculated plants under the experimental conditions.
ACKNOWLEDGEMENTS
We thank Dr Joaquim Werner for the fertilization formula. This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) process no 98/09517‐4.
Supplementary Material
Received: 22 February 2002; Returned for revision: 23 April 2002; Accepted: 16 May 2002
References
- BrewinN.1991. Development of the legume root nodule. Annual Review of Cell Biology 7: 191–226. [DOI] [PubMed] [Google Scholar]
- BussottiF, Gravano E, Grossoni P, Tani C.1998. Occurrence of tannins in leaves of beech trees (Fagus sylvatica) along an ecological gradient, detected by histochemical and ultrastructural analyses. New Phytologist 138: 469–479. [Google Scholar]
- CorbyHDL.1981. The systematic value of leguminous root nodules. In: Polhill RM, Raven PH, eds. Advances in legume systematics part 2 Kew: Royal Botanic Gardens, 657–669. [Google Scholar]
- CordeiroL, Beltrati, CM.1989. Estrutura e desenvolvimento de nódulos radiculares de Anadenanthera falcata Speg. Revista Brasileira de Botânica 12: 61–70. [Google Scholar]
- CordeiroL, Sprent JI, McInroy SG.1996. Some developmental and structural aspects of nodules of Lonchocarpus muelhbergianus Hassl. Naturalia 21: 9–21. [Google Scholar]
- DavidsonAL, Newcomb W.2001. Organization of microtubules in developing pea root nodule cells. Canadian Journal of Botany 79: 777–786. [Google Scholar]
- FariaSM, McInroy SG, Sprent JI.1987. The occurrence of infected cells, with persistent infection threads, in legume root nodules. Canadian Journal of Botany 65: 553–558. [Google Scholar]
- FariaSM, Sutherland JM, Sprent JI.1986. A new type of infected cell in root nodules of Andira spp (Leguminosae). Plant Science 45: 143–147. [Google Scholar]
- GordonAJ, Thomas BJ, Reynolds PHS.1992. Localization of sucrose synthase in soybean root nodules. New Phytologist 122: 35–44. [DOI] [PubMed] [Google Scholar]
- HirschAM.1992. Developmental biology of legume nodulation. New Phytologist 122: 211–237. [DOI] [PubMed] [Google Scholar]
- LeeHS, Copeland L.1994. Ultrastructure of chickpea nodules. Protoplasma 182: 32–38. [Google Scholar]
- LorenziH.1994. Árvores brasileiras. Nova Odessa: Plantarum. [Google Scholar]
- MellorRB, Wiemken A.1988. Peribacteroid organelles as organ‐specific forms of lysosomes. In: Bothe H, De Bruijn FJ, Newton WE, eds. Nitrogen fixation: hundred years after. Stuttgart: G. Fischer, 528. [Google Scholar]
- NewcombW.1976. A correlated light and electron microscopic study of symbiotic growth and differentiation in Pisum sativum root nodules. Canadian Journal of Botany 54: 2163–86. [Google Scholar]
- NewcombW.1981. Nodule morphogenesis and differentiation. Inter national Review of Cytology 13: 247–291. [Google Scholar]
- NicholsonRL, Hammerscmidt R.1992. Phenolic compounds and their role in disease resistance. Annual Review of Phytopathology 30: 369–389. [Google Scholar]
- PostmaJG, Jager D, Jacobsen E, Feenstra WJ.1990. Studies on a non‐fixing mutant of pea (Pisum sativum L.). I. Phenotypical description and bacteroid activity. Plant Science 68: 151–161. [Google Scholar]
- RäsänenLR, Sprent JI, Lindström K.2001. Symbiotic properties of sinorhizobia isolated from Acacia and Prosopis nodules in Sudan and Senegal. Plant and Soil 235: 193–210. [Google Scholar]
- ReattoA, Correia JR, Spera, ST.1998. Solos do Bioma Cerrado. In: Sano SM, Almeida, SP, eds. Cerrado: ambiente e flora Planaltina: Embrapa‐CPAC, 47–86. [Google Scholar]
- SarrugeJR, Haag HP.1974. Análises químicas em plantas. Piracicaba: Esalq/Usp. [Google Scholar]
- SelkerJML.1988. Three‐dimensional organization of uninfected tissue in soybean root nodules and its relation to cell specialization in the central region. Protoplasma 147: 178–190. [Google Scholar]
- SprentJI.2001. Nodulation in legumes. Kew: Royal Botanic Gardens. [Google Scholar]
- SprentJI, Parsons R.2000. Nitrogen fixation in legume and non‐legume trees. Field Crops Research 65: 183–196. [Google Scholar]
- SpurrAR.1969. A low‐viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research 26: 31–43. [DOI] [PubMed] [Google Scholar]
- StreeterJG.1991. Transport and metabolism of carbon and nitrogen in legume nodules. Advances in Botanical Research 18: 129–187. [Google Scholar]
- VanceCP, Johnson LEB, Halvorsen G, Heichel H, Barnes DK.1980. Histological and ultrastructural observations of Medicago sativa root nodule senescence after foliage removal. Canadian Journal of Botany 58: 295–309. [Google Scholar]
- VargasMAT, Hungria M.1997. Biologia dos solos dos cerrados. Planaltina: Embrapa‐CPAC. [Google Scholar]
- VasseJ, Billy F, Camut S, Truchet G.1990. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. Journal of Bacteriology 171: 4295–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WernerD.1992. Physiology of nitrogen‐fixing legume nodules: compartments and functions. In: Stacey G, Burris RH, Evans HJ, eds. Biological nitrogen fixation New York: Chapman and Hill, 399–431. [Google Scholar]
- WhiteheadLF, Day DA, Hardham AR.1998. Cytoskeletal arrays in the cells of soybean root nodules: the role of actin microfilaments in the organisation of symbiosomes. Protoplasma 203: 194–205. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


