Abstract
Light, fluorescence and electron microscopy were used to analyse the structural properties of protoplasts obtained from established suspension culture of Solanum lycopersicoides Dun. composed of meristematic cell aggregates. Four types of protoplasts were distinguished immediately after isolation: (1) mononuclear; (2) polynuclear, (3) anuclear and (4) homogeneous protoplasts. Only mononuclear protoplasts were capable of complete cell wall regeneration and mitotic division. Other types of protoplasts were eliminated during culture. Three phases were distinguished in the developing protoplast culture: (1) the elimination phase during which protoplasts damaged during isolation underwent complete degradation; (2) a phase of intense division during which both mitotic cell division and amitotic nuclear division took place; and (3) a stabilization phase leading to the formation of suspension culture. The cell suspension culture obtained from protoplasts was capable of regenerating diploid plants.
Key words: Solanum lycopersicoides, protoplast culture, structure, ultrastructure, plant regeneration, genetic stability
INTRODUCTION
Many tissue propagation and plant regeneration techniques make use of the totipotential ability of plant cells (Smith and Drew, 1990) and are often used to improve cultivated plants. Solanum lycopersicoides is a wild species that could enrich domestic plants in the family Solanaceae because of its valuable characteristics; for example, its resistance to low temperature and to many bacterial and fungal diseases (Rick, 1987). Due to these traits, this species has been investigated with regards to regeneration of plants from cell suspension cultures (Handley and Sink, 1985a) and isolated protoplasts (Handley and Sink, 1985b; Tan et al., 1987; Lefrancois and Chupeau, 1993). However, these methods of plant regeneration have a low regeneration frequency and there are difficulties in maintaining a stable culture. Because of this, somatic hybridization between S. lycopersicoides and other plants is difficult (Handley et al., 1986).
Protoplast isolation and culture methods have contributed to the generation of somatic hybrids of many different species and genera, including species from the Solanaceae (e.g. Gavrilenko et al., 1992; Melchers et al., 1992; Ishige, 1995; Rooka et al., 1995; Chen and Adachi, 1998; Yemets et al., 2000). Despite the unquestionable achievements in this field, a number of difficulties are still encountered during protoplast culture, the causes of which are not completely understood. One difficulty is somaclonal variation (Peschke and Phillips, 1992), often appearing as an increase in the ploidy level of cells that have (Samoylov and Sink, 1996; Chen and Adachi, 1998) or have not (Jones et al., 1989; Zhang et al., 1998) undergone protoplast fusion. There are also some reports of a lack of increase in ploidy level in plants regenerated from protoplasts (Burza and Malepszy, 1995). The reason behind these differences has been variously ascribed to the effect of the genotype of the donor plants (Olin‐Fatich, 1996), the type of donor tissue (Shizukawa and Mii, 1997; Qiao et al., 1998), the method of culturing protoplasts and plant regeneration (Firoozabady, 1986), and the structural diversity of protoplasts (Korlach and Zoglauer, 1995; Laparra et al., 1997; Zhang et al., 1998).
This work presents the results of a complex analysis of structural and ultrastructural differentiation of S. lycopersicoides protoplasts in order to seek structural evidence for the causes of somaclonal variation. The results are discussed in relation to the ascertained genetic stability of the regenerated plants.
MATERIALS AND METHODS
Protoplast isolation and plant regeneration
Due to the difficulty in establishing a sharp time boundary between protoplasts and the cells derived from them, we use the term ‘protoplast’ for any structure that is formed in culture.
A suspension culture for protoplast isolation was established from root primordia culture of S. lycopersicoides Dun. (Tylicki et al., 2000b) by selecting aggregates that did not form primordial structures. The culture was stabilized on a modified liquid MS (Murashige and Skoog, 1962) medium (2,5D.E) containing 2375 mg l–1 KNO3, lacking NH4NO3, and supplemented with 2500 mg l–1 edamine and 1 mg l–1 2,4D (2,4‐dichlorophenoxyacetic acid). The medium was changed once a week. Culture on this medium is described below as the initial culture.
Protoplast isolation was carried out according to Burza and Malepszy (1995) for 20–22 h at 28 °C. Protoplasts were purified in CPW–0·25 solution (Orczyk and Malepszy, 1985) (a protoplast washing solution; Freason et al., 1973). The number of protoplasts was determined in a Fuchs–Rosenthal chamber. Protoplasts with a density of 1 × 105 ml–1 were plated on 60 mm Petri dishes in 2 ml 2,5D.E liquid medium supplemented with 0·25 m mannitol (2,5D.E0.25). In successive passages, the mannitol concentration was decreased (see Table 1). To initiate shoot organogenesis, tissue obtained from protoplasts was cultured on liquid MS medium containing 2 mg l–1 benzyloadenine (BA). Culturing took place under lights (54 µmol m–2 s–1) on a shaker (150 rpm), with a 16 h photoperiod, passaging every 2 weeks. To regenerate plants, cell aggregates with shoot primordia were transferred to solid mineral MS medium without growth regulators.
Table 1.
Successive lowering of the mannitol concentration in 2,5D.E medium during protoplast culture
| Culture time (d) | Dilution (v/v fresh 2,5D.E : culture) | Mannitol concentration in culture (m) |
| Initiation | 100 % 2,5D.E0.25 | 0·25 |
| 16 | 1 (2,5D.E0.15): 2 | 0·18 |
| 23 | 1 (2,5D.E): 1 | 0·09 |
| 30 | 2 (2,5D.E): 1 | 0·03 |
| 37 | 100 % 2,5D.E | 0·00 |
2,5D.E0.25, 2,5D.E0.15, 2,5D.E supplemented with 0·25 or 0·15 m mannitol, respectively.
Analysis of the nuclear DNA content of the initial culture and regenerated plants was performed in a flow cytometer (Partec CAII; Münster, Germany) according to the procedure described by Malepszy et al. (1998).
The following material was taken for microscopic analysis [light, fluorescence and transmission electron microscopy (TEM)]: (1) fraction of small aggregates from the initial culture; (2) protoplasts directly after isolation and 12 h, 1, 2, 4, 8 and 16 d after isolation; and (3) cell aggregates obtained from protoplast culture.
Light microscopy
To obtain a morphological description of the protoplasts, they were fixed for 2 h in 2 % glutaraldehyde, pH 7·2 (0·1 m cacodylate buffer), supplemented with 0·25 m mannitol, and were stained with 1 % carmine acetate. To determine the mitotic index of the initial culture and the suspension culture obtained from protoplasts, the fixed material was subjected to the Feulgen reaction. Material for microscopic analysis was fixed as described above, dehydrated in ethanol and embedded in methacryl resin (Technovit 7100; Heraeus Kulzer, Wehrheim, Germany) (Tylicki et al., 2000a). Sections approx. 4 µm thick were cut using a microtome Mikrom HM 340E (Adamas Instrumenten BV, Leersum, Holland) and stained with 0·5 % toluidine blue. The preparations were observed and photographed using a light microscope NU (Zeiss, Jena, Germany).
Fluorescence microscopy
For fluorescent staining of the nuclei, fixed material was stained in toto for 10–15 min in 0·0001 % DAPI (4′,6′‐diamidino‐2‐phenylindole; Sigma), and cell walls were stained with 0·05 % calcofluor (Sigma) for 10–15 min. The preparations were examined using a Nikon fluorescence microscope at an excitation wavelength of 365 nm (maximum emission approx. 420 nm), and photographs were taken. The viability of fresh isolated protoplasts was determined by the fluorescein diacetate test according to Hunag et al. (1986), using fluorescence microscopy.
Electron microscopy
Material was fixed for 2 h in 2·5 % glutaraldehyde, pH 7·2 (0·1 m cacodylate buffer), with 0·25 m mannitol, and then for 12 h in 1 % OsO4. After fixation it was dehydrated in ethanol and embedded in a mixture of epoxide resins (Epon–Spurr) using propylene oxide. Ultra‐thin sections (approx. 80 nm) were cut on an LKB ultramicrotome (Bromma, Sweden), contrasted using uranyl acetate and lead citrate (Reynolds, 1963) and analysed in a TEM (JEOL JEM 100C, Akishima, Japan).
Calculations
To characterize the development of a protoplast culture the following calculations were made: (1) the proportion of the four types of protoplasts in culture were calculated directly after isolation and also after 1, 2, 4, 8, 16, 30 and 60 d. One thousand protoplasts were categorized on each date. (2) The mitotic index was calculated for 1000 cells of the initial culture and for 1000 protoplasts 4, 8, 16, 30 and 60 d after isolation. (3) The percentage of protoplasts with regenerated cell walls was calculated for 1000 protoplasts of each type 16 d after isolation. (4) Average numbers of mitochondria, plastids and dictyosomes were recorded on ultra‐thin sections prepared from suspension of protoplasts derived from three independent cultures 16 d after isolation. For each calculation three sections were taken. First, an ultra‐thin section was taken for calculation; five semi‐thin sections were then discarded, and the next ultra‐thin section was taken for calculation. Each ultra‐thin section included about 50 protoplasts. The average number of mitochondria, plastids and dictyosomes in mononuclear protoplasts and polynuclear protoplasts was compared statistically. Results were evaluated using the Shapiro–Wilk W‐test to check that the distribution was normal. When data were not normally distributed, the significance was determined using the Mann–Whitney U‐test, whereas a Student’s t‐test was used if the distribution was normal. In the case of all comparisons, the Levene L‐test showed that the variances were homoscedastic. All calculations were repeated at least three times on samples that derived from independent experiments.
RESULTS
Characterization of the initial culture and protoplasts immediately after isolation
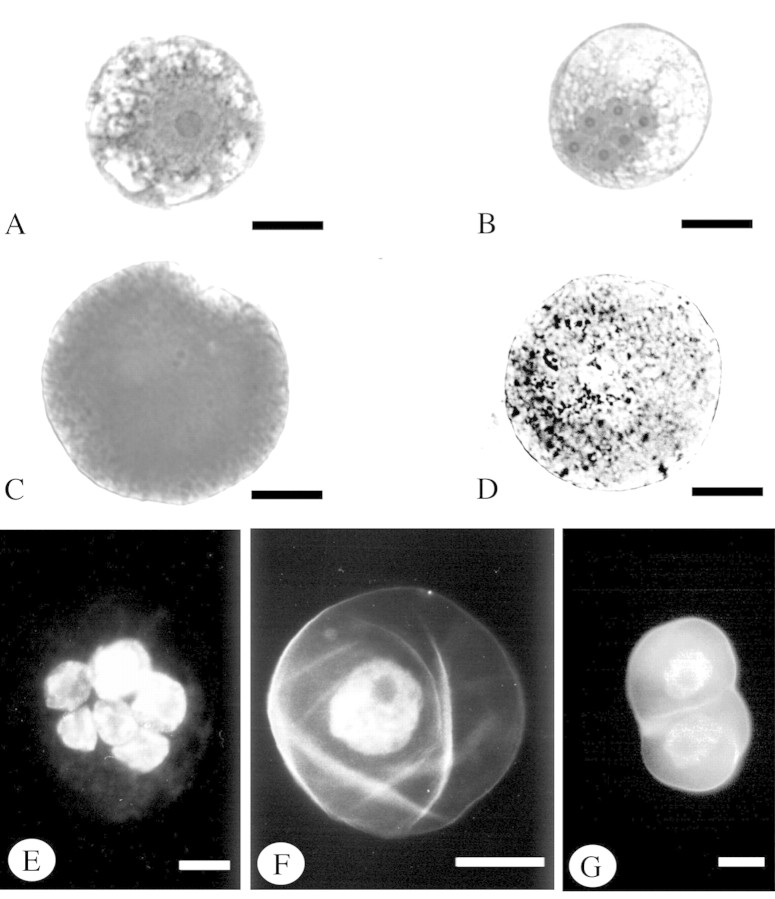
The initial culture used for protoplast isolation was composed of small aggregates of cells of typically meristematic character (Fig. 1A). Because of the structural similarity of all cells from these aggregates, the culture was considered to be homogeneous. The results of the ploidy measurements by flow cytometry indicated that the cells were mixoploid (Fig. 2A). From 12 ml of this suspension, 5–6 × 106 protoplasts were obtained. Of the fresh isolated protoplasts, 79 % were shown to be living. Directly after isolation and purification the protoplast population was non‐homogeneous. Four types could be distinguished: (1) mononuclear (52·2 %), (2) polynuclear (28·3 %), (3) anuclear (9·5 %) and (4) homogeneous protoplasts (10 %) (Figs 1C–F and 3A–D).

Fig. 1. Light micrographs showing the structure of the initial culture cells and groups of protoplasts immediately after isolation. Material was fixed in 2 % glutaraldehyde, pH 7·2 (0·1 m cacodylate buffer). A, C, D–F, Stained with toluidine blue; B, stained with carmine acetate, photographed in toto. A, Section through cell aggregate of initial culture composed of small meristematic cells. B, Two protoplasts undergoing fusion. C, Section through mononuclear protoplast. Note the centrally located nucleus with nucleolus, surrounded by cell organelles. D, Section through polynuclear protoplast. In the centre of the protoplast is a group of five nuclei, three of which have a nucleolus. Note the numerous cell organelles in the cytoplasm. E, Section through an anuclear protoplast. A large vacuole occupies the central position, while disintegrating organelles can be seen in the cytoplasm. F, Section through homogeneous protoplast. Note the clearly visible plasmalemma and the thick, almost completely homogeneous, cytoplasm. Bars = 10 µm.
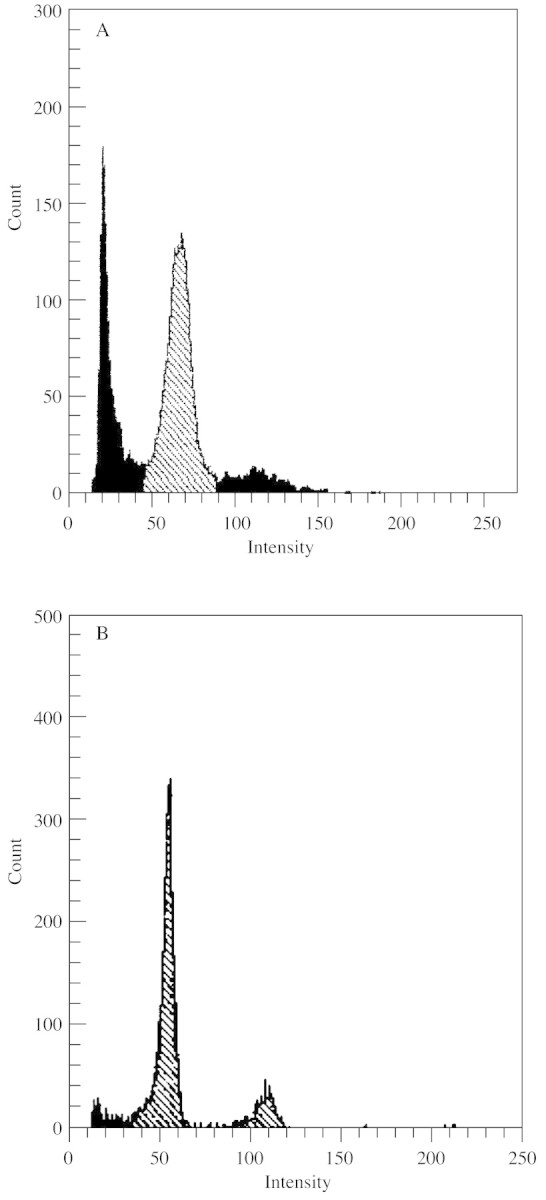
Fig. 2. Flow cytometric analysis of the nuclear DNA content of the initial culture for protoplast isolation (A) and of regenerated plants (B).
Fig. 3. Morphology of fresh isolated protoplasts and of a protoplast during cell wall regeneration. Material fixed in 2 % glutaraldehyde, pH 7·2 (0·1 m cacodylate buffer), photographed in toto. A–D, Light micrographs, material stained with carmine acetate. E–G, DAPI + calcofluor staining, images viewed in a fluorescence microscope. Bar = 10 µm. A, Mononuclear protoplast. B, Polynuclear protoplast. C, Homogeneous protoplast. D, Anuclear protoplast. E, Polynuclear protoplast without cell wall fluorescence. Six nuclei are visible. F, Mononuclear protoplast after cell wall regeneration; note fluorescence of nucleus and cell wall. G, Aggregate of two cells formed after mitotic division of a mononuclear protoplast; cell walls and nuclei are clearly visible.
Mononuclear protoplasts had one large nucleus, most commonly with a nucleolus (Figs 1C and 3A). Their cytoplasm was dense and contained vacuoles, free ribosomes, a well‐developed endoplasmic reticulum, active dictyosomes and small plastids with starch grains (Fig. 4A and B). Mitochondria of these protoplasts were characterized by a dense matrix with clearly visible and numerous cristae (Fig. 4B).
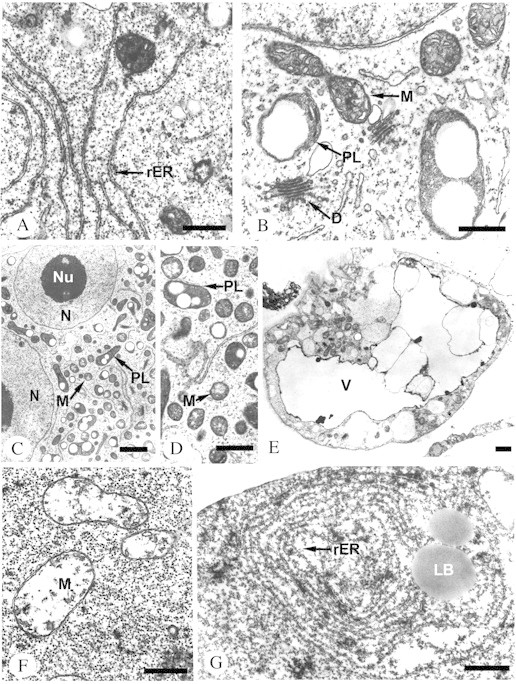
Fig. 4. Ultrastructural organization of protoplast types. Material fixed in 2 % glutaraldehyde, pH 7·2 (0·1 m cacodylate buffer), and 1 % osmium tetroxide; embedded in epoxide resin mixture (Epon–Spurr); ultra‐thin sections contrasted with uranyl acetate and lead citrate and analysed in a TEM. A, Fragment of mononuclear protoplast. Note the numerous rough endoplasmic reticulum (rER) cisternae lying parallel to each other in the cytoplasm. B, Fragment of mononuclear protoplast with typical cell organelles: active dictyosomes (D), mitochondria (M) with numerous cristae, some of them undergoing division, plastids (PL) with starch grains. C, Fragment of polynuclear protoplast containing numerous cell organelles: small plastids (PL), mitochondria (M) and two clearly visible nuclei (N) with nucleoli (Nu). D, Higher magnification of the cytoplasm of a polynuclear protoplast. Plastids (PL) with starch grains and mitochondria (M) with central electron‐transparent clearings are clearly visible. E, Section through an anuclear protoplast. Strongly vacuolized cytoplasm (V) with numerous vesicles. Local grouping of cell organelles undergoing disintegration. F, Fragment of the cytoplasm of a homogeneous protoplast containing mitochondria (M) with a degenerated system of cristae, an electron‐transparent matrix and a distinct double membrane. G, Concentric rER systems and lipid bodies (LB) in the cytoplasm of a homogeneous protoplast. Bars = 0·5 µm (A, B, D, F and G), 1 µm (C and E).
Polynuclear protoplasts were formed as a result of spontaneous fusions during isolation (Fig. 1B). They had two to several centrally located nuclei (Figs 1D and 3B), and a large number of mitochondria, plastids and dictyosomes (Table 2), which seemed to be smaller than the organelles in the mononuclear protoplasts (compare Fig. 1C and D). These protoplasts had a thick cytoplasm with numerous plastids and mitochondria (Fig. 4C). The central part of the mitochondria was characteristically electron‐transparent (Fig. 4D).
Table 2.
Number of mitochondria, plastids and dictyo somes in mono‐ and polynuclear protoplasts 16 d after isolation
| Protoplast type | Mitochondria | Plastids | Dictyosomes |
| Mononuclear | 47·5 ± 4·8* | 31·0 ± 6·1* | 8·5 ± 1·3† |
| Polynuclear | 98·7 ± 8·2* | 59·2 ± 4·4* | 11·7 ± 2† |
Data are means ± s.d.
* Mann–Whitney U‐test, P < 0·01.
† Student’s t‐test, P < 0·05.
As shown by in toto observation under a light microscope, anuclear protoplasts did not have nuclei (Fig. 3D). Their strong vacuolization was visible in sections (Figs 1E and 4E). The cytoplasm of anuclear protoplasts was diluted and contained degenerated cell organelles that often occurred in clusters (Fig. 4E).
Homogeneous protoplasts had very dense cytoplasm and the cell organelles were difficult to distinguish under a light microscope (Figs 1F and 3C). Using an electron microscope, they were shown to contain almost exclusively free ribosomes. Sporadically degenerating mitochondria were found with a very thin matrix, almost totally devoid of cristae (Fig. 4F), concentric systems of endoplasmic reticulum cisternae, and large, frequently fusing lipid bodies (Fig. 4G).
Structure and ultrastructure of protoplasts entering the division phase
Twelve hours after isolation, significant changes took place in protoplast ultrastructure. Groups of several dictyosomes often formed in the cytoplasm of a mononuclear protoplast (Fig. 5A). An increased number of vesicles was also observed around the dictyosomes (compare Figs 4B and 5A). In the cytoplasm multi‐vesicular bodies appeared (Fig. 5B), which were transported outside the plasmalemma where they participated in the formation of the cell wall (Fig. 5C). Sixteen days after isolation, 79 % of the protoplasts had a cell wall; mononuclear protoplasts predominated among these (Fig. 3F). Polynuclear protoplasts were less capable of regenerating a cell wall (Fig. 3E), and homogeneous and anuclear protoplasts rarely formed one (Fig. 6).
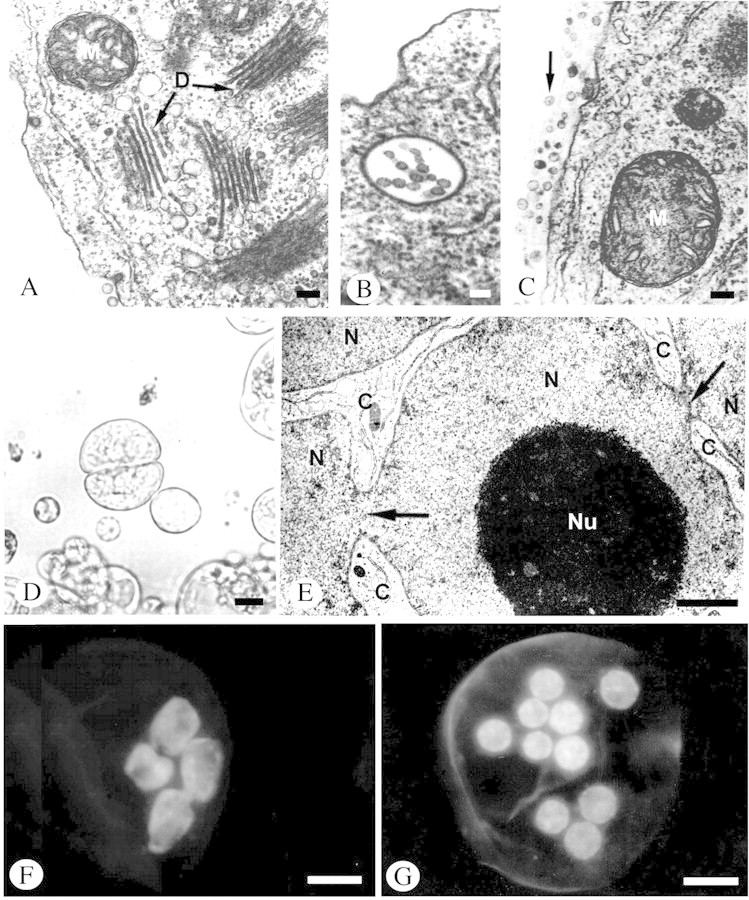
Fig. 5. Changes in the structure and ultrastructure of protoplasts during regeneration of the cell wall, mitotic cell division and amitotic nuclear fragmentation. A–C and E, Material prepared in the standard way for TEM analysis (as in Fig. 4); D, F and G, material in toto: micrographs of live material viewed under a light microscope (D), and in a florescence microscope following DAPI staining (F and G). A, Fragment of protoplast 12 h after isolation showing cluster of dictyosomes (D) and small mitochondrion (M) with clearly visible cristae. B, Multivascular body in protoplast 12 h after isolation. C, Numerous vesicles (marked with arrow) located on the outer side of the protoplast plasmalemma 12 h after isolation. D, Aggregate of two cells formed after division of a mononuclear protoplast 2 d after isolation. E, Constrictions (arrows) in strongly folded nucleus (N) leading to amitotic division. Large nucleolus (Nu) of this nucleus and narrow streaks of cytoplasm (C) are visible between the nuclear fragments. F, Amitotic nuclear divisions in protoplast. G, Nuclei of polynuclear protoplast formed as a result of amitotic divisions. Bars = 0·1 µm (A, B and C), 1 µm (E), 10 µm (D, F and G).
Fig. 6. Percentage of each type of protoplast with regenerated cell walls 16 d after isolation.
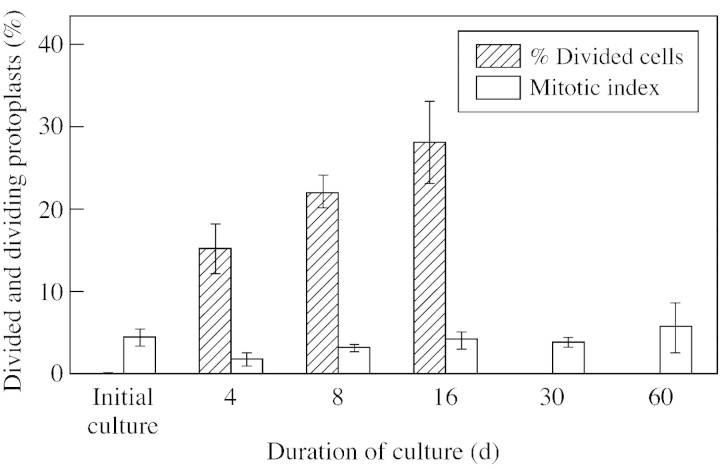
The ability to regenerate a cell wall correlated with mitotic divisions, which took place only in mononuclear protoplasts. The effects of mitotic division, in the form of two‐cell aggregates, were noted 2 d after isolation (Figs 3G and 5D). After 16 d, 28 % of the protoplasts had already undergone division and the mitotic index was 3·9 %, which corresponded approximately to the value of the mitotic index of the initial culture (Fig. 7). The results of the divisions were cell aggregates forming a suspension.
Fig. 7. Percentage of dividing protoplast and mitotic index.
Concomitantly with mitotic division, significant changes occurred in the shape of the protoplast nuclei between the fourth and eighth day after isolation. Some of the nuclei changed shape from spherical to irregular and strongly folded. This led to the formation of very deep constrictions within them (Fig. 5E). The consequence of these changes was amitotic nuclear divisions (Fig. 5F). Eight days after isolation, the number of polynuclear protoplasts (Fig. 5G) increased (up to 50 %), and then, after the eighth day of culture, decreased again (together with a decrease in mannitol content in the medium; Fig. 8).
Fig. 8. Proportion of the four types of protoplasts throughout culture.
Phases of protoplast culture
Structural changes during protoplast culture allowed three phases to be distinguished: (1) elimination, (2) intense division activity and (3) stabilization.
The elimination phase (up to 48 h after isolation) was marked by a tendency for the proportion of mononuclear protoplasts to increase and for the remaining types of protoplasts to decrease (Fig. 8). This was caused by the progressive elimination of anuclear, homogeneous and polynuclear protoplasts.
The phase of intense division activity (from 48 h to 8 d) was marked by an initial decrease in the proportion of mononuclear protoplasts and a considerable increase in the proportion of polynuclear protoplasts (Fig. 8). This was caused by an intensification of the process of amitotic nuclear divisions. At this stage, the process of cell wall regeneration in mononuclear protoplasts was ending, which initiated the intense mitotic divisions. Homogeneous protoplasts were totally eliminated (Fig. 8).
In the stabilization phase (from the eighth day until the formation of a fine aggregate suspension culture), a considerable increase in the proportion of mononuclear cells occurred, and the final anuclear and polynuclear protoplasts were eliminated (Fig. 8). This phase led to the reformation of a small aggregate suspension capable of plant regeneration.
Plant regeneration
During the 2 months from initiation of the protoplast culture, an established cell suspension was obtained composed of small aggregates and single cells (Fig. 9A). The aggregates forming in culture did not show structural differentiation and resembled aggregates of the initial culture (compare Figs 1A and 9B). This culture was passaged on liquid MS medium containing 2 mg l–1 BA and cultured under light to initiate shoot regeneration. After 1 week, shoot primordia appeared on the surface of aggregates (Fig. 9C). Further culture on solid MS medium without organic substances (with the exception of sucrose), led to the formation of plants with a frequency of approx. 76 % (Fig. 7D). As shown by flow cytometry, regenerated plants were characteristically diploid (Fig. 2B).

Fig. 9. Regeneration of plants from protoplast‐derived, small aggregate suspension culture. A, C and D, Material in toto without staining. A, Inverted microscope; C, stereomicroscope; D, Nikon camera. B, Material prepared according to the procedure used for light microscopy (as in Fig. 1). A, Aggregates of a suspension culture obtained from isolated protoplasts 2 months after isolation. B, Micrograph of a section of aggregates from A. C, Shoot primordia derived from isolated protoplasts 2 weeks after passaging on solid regeneration medium. D, Plant regenerated from shoot primordia shown in C (natural size). Bars = 10 µm (A and B), 1 mm (C).
DISCUSSION
In our experiments, diploid plants that were characterized by profound structural differentiation were obtained from a protoplast culture. The plants were stable with respect to ploidy levels. This result led us to investigate the causes of the formation of a heterogeneous protoplast culture and its transformation into a population of homogeneous plants. Our results proved that from a structurally uniform initial culture, a protoplast population was formed that was highly heterogeneous both morphologically and physiologically. The heterogeneity of the culture may be due to the fact that protoplasts are liberated gradually from cell aggregates in a centripetal direction as a result of the surface action of enzymes digesting cell walls. Therefore, protoplasts that are liberated first are exposed for much longer to the enzymatic solution which may be the cause of structural and functional perturbations. Moreover, it is known that the isolation procedure favours the formation of polynuclear protoplasts through the process of spontaneous fusions (Grambow et al., 1972; Fowke et al., 1973; Carlberg et al., 1984). An additional factor affecting the heterogeneity of the protoplast population is amitoses (Novak, 1981; Carlberg et al., 1984). Our results clearly indicate that the amitotic process takes place only in a defined culture phase (intense division activity). The occurrence of additional variability due to amitoses besides that due to spontaneous protoplast fusions indicates that the elimination of spontaneous fusions (e.g. by using a two‐step procedure for protoplast isolation) is insufficient for complete prevention of non‐homogeneity in the protoplast culture. The heterogeneous protoplast culture obtained from a homogeneous initial culture contained four types of protoplasts. Polynuclear protoplasts had an increased number of cell organelles in comparison with mononuclear ones (Fowke et al., 1973). The variability in the number of organelles could be the result of progressive degradation of cell organelles not correlated with the degradation of nuclei. As a result of extensive changes in cells of the initial culture, most probably linked to the long isolation process, homogeneous protoplasts were formed. The elimination of these structures took place in the early stage of culture. Anuclear protoplasts were present in the culture for longer. As they were not capable of division it should be assumed that they were formed de novo. Their source could have been polynuclear protoplasts undergoing degradation. This hypothesis is supported by the increase in the proportion of anuclear protoplasts concomitant with a decrease in the proportion of polynuclear protoplasts during the stabilization phase. Mononuclear protoplasts survived the isolation procedure without serious structural changes.
Protoplast heterogeneity is one of the causes of somaclonal variation (Peschke and Phillips, 1992). Protoplasts obtained from different sources constitute a homogeneous or heterogeneous population, depending on the type of donor tissue. A homogeneous protoplast population is often obtained from leaf mesophyll protoplasts and leads to the regeneration of genetically stable plants (Firoozabady, 1986; Shizukawa and Mii, 1997), although this occurs less frequently in the case of protoplasts from a suspension culture (Qiao et al., 1998) where genetically altered plants may be obtained (e.g. Handley and Sink, 1985b; Jones et al., 1989; Yamagishi et al., 1997). Despite the isolation of protoplasts from a suspension culture and the heterogeneity of protoplasts after isolation, only diploid plants were regenerated in our work. This situation indicates the induction of processes eliminating anomalies during the culture, which guarantee a return to the state of structural homogeneity. One such process was the regeneration of the cell wall. The process of cell wall regeneration starts 2–4 h after isolation, and after 3 d 60–70 % of protoplasts have regenerated cell walls (Zhao et al., 1995). Similar results were obtained in our experiment; however, only mononuclear protoplasts were capable of complete cell wall regeneration. The remaining groups of protoplasts (polynuclear, anuclear and homogeneous) are characterized by serious structural perturbations and, in general, they did not regenerate cell walls. The gradual decrease in mannitol concentration in the culture medium was a very important factor in the progressive elimination of protoplasts incapable of cell wall regeneration. Cell divisions were inseparably linked to cell wall regeneration. Therefore, of all the types of protoplast, only mononuclear protoplasts were capable of cell division. In contrast to the results of Korlach and Zoglauer (1995), polynuclear protoplasts did not undergo cytokinesis in our experiments. The first divisions of mononuclear protoplasts were observed between the second and fourth days of culture, as documented for protoplast cultures of other species from the family Solanaceae (Handley and Sink, 1985b; Cardi et al., 1990; Latif et al., 1993; Hossain et al., 1995). As a result of mononuclear protoplast division, a fine aggregate suspension culture was formed whose aggregates were composed of meristematic cells. Appropriate culture conditions produced a selection process the consequence of which was the return of the culture to a homogeneous state and which led to the regeneration of genetically stable diploid plants.
ACKNOWLEDGEMENTS
We thank Tomasz Suchowolec and Jan Czerniecki for help in preparation of figures; Piotr Jadwiszczak for statistical evaluation and Kirk Palmer for language improvement.
Supplementary Material
Received: 21 March 2002; Accepted: 15 May 2002
References
- BurzaW, Malepszy S.1995. In vitro culture of Cucumis sativus L. XVIII. Plants from protoplasts through direct somatic embryogenesis. Plant Cell, Tissue and Organ Culture 41: 259–266. [Google Scholar]
- CardiT, Puite KJ, Ramulu KS.1990. Plant regeneration from mesophyll protoplasts of Solanum commersonii Dun. Plant Science 70: 215–221. [Google Scholar]
- CarlbergI, Glimelius K, Eriksson T.1984. Nuclear DNA connect during the initiation of callus formation from isolated protoplasts of Solanum tuberosum L. Plant Science Letters 35: 225–230. [Google Scholar]
- ChenLZ, Adachi T.1998. Protoplast fusion between Lycopersicon esculentum and L. peruvianum‐complex: somatic embryogenesis, plant regeneration and morphology. Plant Cell Reports 17: 508–514. [DOI] [PubMed] [Google Scholar]
- FiroozabadyE.1986. Rapid plant regeneration from Nicotiana mesophyll protoplasts. Plant Science 46: 127–131. [Google Scholar]
- FowkeLC, Bech‐Hansen CW, Gamborg OL, Shyluk JP.1973. Electron microscopic observations of cultured cells and protoplasts of Ammi visnaga American Journal of Botany 60: 304–312. [Google Scholar]
- FreasonEM, Power JB, Coocking EC.1973. The isolation, culture and regeneration of Petunia leaf protoplasts. Developmental Biology 33: 130–137. [DOI] [PubMed] [Google Scholar]
- GavrilenkoTA, Barbakar NI, Pavlov AV.1992. Somatic hybridization between Lycopersicon and non‐tuberous Solanum species of the Eutuberosa species. Plant Science 86: 203–214. [Google Scholar]
- GrambowHJ, Kao KN, Miller RA, Gamborg OL.1972. Cell division and plant development from protoplasts of carrot cell suspension cultures. Planta 103: 348–355. [DOI] [PubMed] [Google Scholar]
- HandleyLW, Sink KC.1985a Plant regeneration of Solanum lycopersicoides Dun. from stem explants, callus and suspension cultures. Plant Cell, Tissue and Organ Culture 5: 129–138. [Google Scholar]
- HandleyLW, Sink KC.1985b Plant regeneration of protoplasts isolated from suspension cultures of Solanum lycopersicoides Plant Science 42: 201–207. [Google Scholar]
- HandleyLW, Nickels RL, Cameron M, Moore PP, Sink KC.1986. Somatic hybrids between Lycopersicon esculentum and Solanum lycopersicoides Theoretical and Applied Genetics 71: 691–697. [DOI] [PubMed] [Google Scholar]
- HossainM, Imanishi S, Egashira H.1995. An improvement of tomato protoplasts culture for rapid plant regeneration. Plant Cell, Tissue and Organ Culture 42: 141–146. [Google Scholar]
- HunagCN, Cornejo MJ, Bush DS, Jones RL.1986. Estimating viability of plant protoplasts using double and single staining. Protoplasma 135: 80–87. [Google Scholar]
- IshigeT.1995. Somatic cell fusion between diploid potato (Solanum tuberosum) lines using transformed antibiotic selection markers. Plant Science 112: 231–238. [Google Scholar]
- JonesH, Karp A, Jones MGK.1989. Isolation, culture, and regeneration of plants from potato protoplasts. Plant Cell Reports 8: 307–311. [DOI] [PubMed] [Google Scholar]
- KorlachJ, Zoglauer K.1995. Developmental patterns during direct somatic embryogenesis in protoplast culture of european larch (Larix decidua Mill.). Plant Cell Reports 15: 242–247. [DOI] [PubMed] [Google Scholar]
- LaparraH, Bronner R, Hahne G.1997. Histological analysis of somatic embryogenesis induced in leaf explants of Helianthus smithii Heiser. Protoplasma 196: 1–11. [Google Scholar]
- LatifM, Mumtaz N, Davey MR, Power JB.1993. Plant regeneration from protoplasts isolated from cell suspension culture of the wild tomato, Lycopersicon chilense Dun. Plant Cell, Tissue and Organ Culture 32: 311–317. [Google Scholar]
- LefrancoisC, Chupeau Y.1993. Standard conditions for plant regeneration from leaf protoplasts of several Lycopersicon species. Journal of Plant Physiology 141: 629–632. [Google Scholar]
- MalepszyS, Sarreb DA, Mackiewicz HO, Narkiewicz M.1998. Triploids in cucumber. I. Factors Influencing embryo rescue efficiency. Gartenbauwissenschaft 63: 34–37. [Google Scholar]
- MelchersG, Mohri Y, Watanabe K, Wakabayashi S, Harada K.1992. One‐step generation of cytoplasmic male sterility by fusion of mitochondrial‐inactivated tomato protoplasts with nuclear inactivated Solanum protoplasts. Proceedings of the National Academy of Sciences of the USA 89: 6832–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MurashigeT, Skoog F.1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15: 473–479. [Google Scholar]
- NovakFJ.1981. Chromosomal characteristics of long term callus cultures of Allium sativum L. Cytologia 46: 371–397. [Google Scholar]
- Olin‐FatichM.1996. The morphology, cytology, and C‐banded karyotypes of Brassica campestris, B. oleracea, and B. napus plants regenerated from protoplasts. Theoretical and Applied Genetics 93: 414–420. [DOI] [PubMed] [Google Scholar]
- OrczykW, Malepszy S.1985. In vitro culture of Cucumis sativus L. V. Stabilising effect of glycine on leaf protoplasts. Plant Cell Reports 4: 269–273. [DOI] [PubMed] [Google Scholar]
- PeschkeVM, Phillips RL.1992. Genetic implications of somaclonal variation in plants. Advances in Genetics 30: 41–75. [Google Scholar]
- QiaoJ, Kuroda H, Hayashi T, Sakai F.1998. Efficient plantlet regeneration from protoplasts isolated from suspension cultures of poplar (Populus alba L.). Plant Cell Reports 17: 201–205. [DOI] [PubMed] [Google Scholar]
- ReynoldsES.1963. The use of lead citrate at high pH as an electronopaque stain in electron microscopy. Journal of Cell Biology 17: 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RickCM.1987. Genetic resources in Lycopersicon In: Nevis DJ, Jones RA, eds. Tomato biotechnology New York: Alan R. Riss Inc., 17–26. [Google Scholar]
- RookaVM, Valkonen JPT, Pehu E.1995. Production and characterization of haploids derived from somatic hybrids between Solanum brevidens and S. tuberosum through anther culture. Plant Science 112: 85–95. [Google Scholar]
- SamoylovVM, Sink KC.1996. The role of irradiation dose and DNA content of somatic hybrid calli in producing asymmetric plants between an interspecific tomato hybrid and eggplant. Theoretical and Applied Genetics 92: 850–857. [DOI] [PubMed] [Google Scholar]
- ShizukawaY, Mii M.1997. A simple and efficient plant regeneration system for leaf protoplasts of Nierembergia repens by inducing single shoots on the microcolonies. Plant Cell Reports 16: 545–549. [DOI] [PubMed] [Google Scholar]
- SmithKM, Drew RA.1990. Current applications of tissue culture in plant propagation and improvement. Australian Journal of Plant Physiology 17: 267–289. [Google Scholar]
- TanMMC, Boerrigter HS, Kool AJ.1987. A rapid procedure for plant regeneration from protoplasts isolated from suspension cultures and leaf mesophyll cells of wild Solanum species and Lycopersicon pennelli Plant Science 49: 67–72. [Google Scholar]
- TylickiA, Burza W, Kuraś M, Malepszy S.2000a A new way of achieving plant regeneration of Solanum lycopersicoides Dun. from root primordia culture. Plant Cell Reports 19: 837–844. [Google Scholar]
- TylickiA, Burza W, Kuraś M, Dziadczyk E, Malepszy S.2000b Structural and ultrastructural analysis of root primordia in vitro cultures (RPC) of Solanum lycopersicoides Dun. Plant Science 156: 73–83. [DOI] [PubMed] [Google Scholar]
- YamagishiM, Itoh K, Koba T, Sukekiyo Y, Shimamoto K, Shimada T.1997. Characteristics of genetic variation in the progenies of protoplasts‐derived plants of rice, Oryza sativa cv Nipponbare. Theoretical and Applied Genetics 94: 1–7. [DOI] [PubMed] [Google Scholar]
- YemetsAI, Kundel’chuk OP, Smertenko AP, Solodushko VG, Rudas VA, Gleba YY, Blume YB.2000. Transfer of amiprophosmethyl resistance from a Nicotiana plumbaginifolia mutant by somatic hybridisation. Theoretical and Applied Genetics 100: 847–857. [Google Scholar]
- ZhangYJ, Qian YQ, Mu XJ, Cai QG, Zhou YL, Wei XP.1998. Plant regeneration from in vitro‐cultured seedling leaf protoplasts of Actinidia eriantha Benth. Plant Cell Reports 17: 819–821. [DOI] [PubMed] [Google Scholar]
- ZhaoKN, Bittisnich DJ, Halloran GM, Whitecross MI.1995. Studies of cotyledon protoplasts cultures from Brassica napus, B. campestris and B. oleracea I. Cell wall regeneration and cell division. Plant Cell, Tissue and Organ Culture 40: 59–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.